Back to Journals » Veterinary Medicine: Research and Reports » Volume 13
A Comparative Study on Pathological Changes in the Small Intestine of Sheep and Goat Experimentally Infected with Trichostrongylus colubriformis
Authors Tafere A, Terefe G, Mamo G , Kaba T, Shiferaw J
Received 28 March 2022
Accepted for publication 19 August 2022
Published 2 September 2022 Volume 2022:13 Pages 213—233
DOI https://doi.org/10.2147/VMRR.S365549
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Young Lyoo
Arega Tafere,1 Getachew Terefe,2 Gezahagne Mamo,2 Tamirat Kaba,2,3 Jirata Shiferaw2
1Wolaita Sodo University School of Veterinary Medicine, Wolaita Sodo, Ethiopia; 2Addis Ababa University College of Veterinary Medicine and Agriculture, Bishoftu, Ethiopia; 3Department of Animal and Veterinary Science, College of Agricultural Science, Arba Minch University, Arba Minch, Ethiopia, Arba Minch, Ethiopia
Correspondence: Jirata Shiferaw, Addis Ababa University College of Veterinary Medicine and Agriculture, Bishoftu, Ethiopia, Email [email protected]
Background: Trichostrongylus colubriformis, also called hairworm, is a genus of parasitic roundworm affecting gastro-intestinal tracts of a ruminant. Gross and microscopic lesion characterizations and comparing its effect in the small intestine of sheep and goats experimentally infected with T. colubriformis were undertaken in the study.
Methods: During the study period, 13 sheep and 14 goats were included in the experiment. The larvae of T. culibriformis were obtained from abattoirs and larvae were recovered by Bearmann techniques. The infective larvae of T. culibriformis (L3) as a single dose of 10,000 per-animal was administered orally to infected groups of sheep and goats. Blood was collected for hematological and serum biochemical analysis. Tissues for gross and histopathologic lesions characterization were collected from killed infected animals at 56 days.
Results: From the infected group, the total recovered mean worm burden was recorded as higher in goats (P< 0.05) than sheep, with an establishment rate of 50.16% and 34.46%, respectively. The total mean PCV, Hb, and albumin values recorded in the infected groups of sheep and goats were significantly (P< 0.05) lower than non-infected control of both animal groups. In goats, the total serum protein was significantly (P< 0.05) lower in the infected group than the non-infected control group. Gross lesions found were enteritis with petechial hemorrhages, edema, hyperemia, and mucosal slough, which were marked in the duodenum (62.69%) and jejunum (33.33%) in sheep and 47.05% duodenum and 45.09% jejunum in goats. The microscopic lesions developed by T. colubriformis were subtotal villus atrophy, hemorrhage, straightened and elongated dilated crypts, loss of epithelium, mucosal erosion, and infiltration of inflammatory cells.
Conclusion: The present study showed that T. colubriformis infection caused physiological and pathological changes of the small-intestine in sheep and goats, with more severe infection in goats than sheep, although they were under the same management condition.
Keywords: control, experimental infection, lesion, sheep and goat, T. colubriformis
Background
Parasitic diseases are a worldwide concern and considered as an obstacle in the health and production performance of animals.1 Animals are impressed by multifarious gastrointestinal parasites such as nematodes, trematodes, and cestodes.2 Gastrointestinal parasitic infections are a worldwide problem for small and large scale farmers, but their effect is greater in sub-Saharan Africa in general and Ethiopia in particular due to a wide range of agro-ecological factors which are suitable for diversified hosts and parasitic species.3 Domestic ruminants are frequently exposed to multiple parasitic infections throughout their life.4,5 In the field, while sharing common pasture, animals are exposed to a variety of parasites among which are gastrointestinal nematodes that cause considerable animal health problems in many parts of the world and are regarded as an important health and economic problem of sheep and goat production.6,7
Infection caused by GI nematodes (Trichostrongylus, Haemonchus, Teladorsagia, Bunostomum, Oesophagostomum, and Chabertia) can be similar to a nutritional disease, since the presence of worms usually induces a decrease in appetite and digestibility of the food and diversion of nutrients from production sites toward the repair of tissue damage caused by parasites.8,9 Most infected sheep and goats remain sub-clinically infected; others show gastrointestinal pathologies whose intensity depends on the worm burden, the species of nematode, their life cycle (if there are exclude tissue phases), and their localization. Pathological conditions of the animals can be assessed by examination of hematological and biochemical analysis of blood.10
Hematology has been widely used to provide information about health status in animals. A deviation of certain blood parameters from their normal limits might serve as a guide for diagnosis of diseases including parasitism.11 Although general aspects of morphology, biology, epidemiology, and control of gastrointestinal nematodes of small ruminants are generally known, others, such as the pathogenic role of these parasites in sheep and goats regarding pathology are less known.9 Peculiarly small intestine lesions caused by GI nematodes such as T. colubriformis and its effect on hematology and biochemical parameters in sheep and goats have not been studied in detail, and studies on host–parasite interaction in sheep and goats remain few and dispersed.9 Thus, the aims of the study were; a) to characterize gross and microscopic lesions in the small intestine of sheep and goats experimentally infected with T. colubriformis and b) to compare the effect of T. colubriformis on hematological and serum biochemical parameters in experimentally infected sheep and goats.
Materials and Methods
Study Area and Period
The study was conducted on sheep and goats in Addis Ababa University College of veterinary medicine and agriculture, Bishoftu, Ethiopia from October 2019 to May 2020. Bishoftu is located 47 km south east of Addis Ababa. Geographically, the area is located at 9°N latitude and 40°E longitude at an altitude of 1,850 meters above sea level. It has an annual average rainfall of 892 mm, of which 84% is in the long rainy season (June to September). The dry season extends from October to February and the average annual temperature in Bishoftu is 20°C with a mean relative humidity of 61.3%.12
Experimental Design and Groups
A complete randomized study design was used by forming four groups. The study at the beginning involved 14 sheep and 14 goats aged between 12 and 18 months but, after 2 weeks of the trial period, one experimental animal from the non-infected group of sheep was resigned due to being unfit for the experiment as a result of persistent stress. Thus, 13 sheep and 14 goats were included during the experimental period. From the 14 goats, seven were infected (G4), whereas the remaining half were kept as uninfected controls (G3). In sheep, seven and isx animals were categorized as infected (G2) and uninfected controls (G1), respectively. The animals were acclimatized for 4 weeks, during which time they were monitored for helminthes parasitism and the presence of any abnormality that potentially precludes them from the study. All healthy animals were randomized in such a way that each group contains an approximately similar mean body weight. All animals were handled and managed according to guiding principles of animal welfare.13 They were provided with grass hay and water following supplementary feeding.
Experimental Parasite Inoculation and Method of Infection
To generate the required number of infective larvae for the experimental infection, an ample number of adult female T. colubriformis were collect from Elfora export abattoirs and crushed with a mortar and pestle to liberate the eggs. The eggs were cultured on parasite egg-free animal feces to produce a pathogenic stage of larvae (L3). After 14 days of culturing at room temperature under sufficient moisture, the larvae were recovered by the modified Baermann technique (Annex II).
After letting the L3 attain its full infective potential for 3 weeks at +4°, they were drenched to two donor sheep. One month later, up on confirmation of a reasonable fecal egg count set, a large volume of fecal sample was collected daily for further culturing to produce the required number of L3 for the 14 experimental animals. A total of 140,000 L3 were required for the experiment. Recovered L3 after 3 weeks of maturity was given to each experimental animal at a single dose of 10,000 L3/animal through oral route according to the method described by Terefe et al.14 After experimental infection, samples were collected at days 0, 7, 14, 21, 28, 35, 42, 49, and 56. Following development of infection, all animals were killed after 2 months of infection for worm recovery and gross and hisopathologic sampling was undertaken (Supplementary Material-Annex II).
Sample Collection and Processing
Blood Sample for Hematological and Biochemical Examination
Blood samples were collected from the jugular vein of infected animals; 5 mL of the blood was stored in blood sample bottles containing ethylene di-amine tetra acetic acid (EDTA) for hematology, while another 6 mL were placed into anticoagulant-free tubes and allowed to clot at room temperature for about 3 hours. The serum samples were later stored at a temperature of −20°C for biochemical analysis, as described by Schalm et al.15 For hematological and biochemical analysis, blood samples were similarly obtained in six sheep and seven goats that served as experimental controls for comparison with the infected groups. For samples on the lesions, recovered worms, hematological, and serum biochemical data during research, the recording sheets are attached as Supplementary Materials (Annex III).
The Total Red Blood Cell Count
The total red blood cell (RBC) count was performed in 1:200 dilution of blood in Haym’s solution. The blood was taken up to 0.5 marks with a RBC diluting pipette and suck the Haym’s solution up to mark 101. Mechanically the pipette was shaken thoroughly by holding the pipette between the index finger and thumb. A third of the contents of the pipette was discarded and wiped off the tip. The counting chamber and cover slip were cleaned and the cover slip placed in position over the counting chamber by gentle pressure. A drop of blood was added to the counting chamber by holding the pipette at an angle of 45°.
The cells are settled for 1–2 minutes and total RBCs in each mm3 area were counted under low magnification (10x) and were determined according to Feldman et al.16
Hemoglobin Determination
The hemoglobin (Hb) concentration was evaluated by matching acid hematin solution against a standard colored solution found in Sahl’s hemoglobin meter.17 The Sahl’s method is based on converting hemoglobin to acid hematin (brown color) and then visually matching its color against a solid glass standard. Diluted hydrochloric acid was mixed into a graduated cylinder with 20 µL of blood sample and distilled water was added until the color of the diluted blood sample matches the glass standard and the dilution was determined based on the hemoglobin level of the blood sample according to Philippe.18
Packed Cell Volume and Blood Indices
Packed Cell Volume (PCV) was measured using a microhematocrit reader from microhematocrit capillary tubes three quarters filled with blood, sealed, and centrifuged at 3,000 rpm for 3 minutes. The Mean Corpuscular volume (MCV) and Mean Corpuscular Hemoglobin Concentration (MCHC) were calculated from total RBC count, PCV, and Hb as described by Ibrahim.19
Total Leucocytes Count
Total leucocyte count (TLC) was also determined by taking the fresh blood up to 0.5 levels in a WBC diluting pipette. Glacial acetic acid was sucked up to 11 marks. Mechanically the pipette was rotated gently by holding the pipette between the index finger and thumb. One-third of the contents of the pipette was discarded and wiped off the tip. The counting chamber of the hemocytometer and cover slip were cleaned. The cover slip was placed over the counting chamber. A drop of blood was added to the counting chamber by holding the pipette at an angle of 45° and the cells were allowed to settle for 2 minutes. Finally, total white blood cells in each millimeter area were identified under low magnification (10x); and the total white blood cell count was determined. Total blood eosinophil was also determined by using Fast Read 102 Counting Chambers according to Sharma and Singh.20
Serum Protein and Enzyme Analysis
Collection of 5 mL blood for biochemical analysis was carried out from the jugular vein and serum was separated after centrifugation at 3,000 rpm for 5 minutes and stored in a deep freeze at −20°C until used. The serum total protein and albumin and enzyme activities of aspartate aminotransferase and alkaline phosphatase were measured using an automatic protein and enzyme analyzer machine (AU_protein and vitros 250 for enzymes).
Necropsy Techniques and Tissue Sampling
Gross Pathological Examination and Lesion Characterization
Animals were killed on day 56 after infection by intravenous barbiturate over dose followed by severing the jugular vein. A longitudinal incision at the midline starting from the xiphoid cartilage to the inguinal region was made. The intestines and other abdominal organs were exposed by cutting the abdominal and paracostal muscles along the midline incision. The small intestine of each slaughtered animal were tied at both ends and separated from the other organs with minimal manipulation. The intestinal portions were opened separately and examined for gross pathological lesions before their mucosal surfaces were scraped to remove embedded worms.21
The duodenum, proximal jejunum, distal jejunum, and the ileum were demarcated on the basis of their distances from the pylorus for the assessment of the small intestine gross lesion. The duodenum represents an average length of 84.23 cm and 72.5 cm in sheep and goats, respectively. From the pylorus, the proximal jejunum extends for 9 m from the distal end of the duodenum while the distal jejunum extends for 11 m from the distal end of the proximal jejunum in sheep and goats. The ileum extends up to the ileocecal junction with an average length of 32.42 cm in sheep and 25.85 cm in goats.22 A checklist format (Supplementary Material, Annex III) of gross pathological lesions was made as intestinal wall thinning, mucus hyper-secretion, hyperemia, petechial hemorrhage, edema, ulceration, necrosis, and erosion, and thus compared to the different experimental groups and intestinal segments.
Intestinal Worm Recovery
From euthanized animals, the small intestine was sampled and tied at both ends, cut, and placed in a labeled tray. The small intestine of each animal was opened longitudinally and the contents were gently washed into collecting jars and filtered through the strainer at an aperture of 250 μm, capable of retaining the adult worms; the contents were then washed into a bucket under running water and the total volume was made up to 2 L. A sample of 200 mL was transferred to a labeled plastic container and preserved in 10% formalin; 20 mL of the sub-sample was taken onto a petridish, and parasites examined under a steromicroscope. The number of worms found in 20 mL x100 gave the total number of worms found in the small intestine, as described by Hansen and Perry.23
Histopathological Techniques
A 4 cm square of tissue sample was taken from the lesion and unaffected part of the small intestine and fixed in 10% buffered formalin for histopathological examination. The preserved tissue samples were trimmed and processed in an automatic tissue processor in different chambers containing different alcohol concentrations (70, 95, and 100%, 100%, 100%), cleared in xylene and embedded in paraffin for preparation into fine blocks (Supplementary Material, Annex II). Blocks were sectioned at 5 μm, dewaxed, rehydrated (with decreasing concentration of different alcohols), and stained using hematoxyline and eosin (H&E) stain. Then, it was dehydrated again with different increasing concentrations of alcohol according to Bancroft and Gamble.24 Xylene I, II, and II were used to clear alcohols (Supplementary Material-Annex I). The slides were mounted with Canada balsam and allowed to drybefore examination under a 40x and 100x magnification light microscope.
Statistical Analysis
Data was recorded, checked, and coded on a Microsoft Excel spreadsheet (Microsoft Corporation) and R version 3.5.1 statistical software was used for descriptive analysis to describe the data. Chi square (χ2) test was used to determine the lesion frequency between intestinal segments of infected animal groups. The hematological and biochemical means were compared between groups of animals by using the t-test (mean comparison test) at 5% significance level. At 95% confidence level, P-values less than or equal to 0.05 are considered as significant, as described by Mohammed et al.25
Results
Worm Load
In the feces of infected animal groups (sheep (G2) and goat (G4)) T. colubriformis eggs were detected beginning at the third week post-experimental infection. No eggs were detected in uninfected controls during the entire experimental period. The groups mean adult worm load detected were 3,446 and 5,016 T. colubriformis, which corresponded to the establishment rate of 34.46% and 50.16% of the infective larvae in sheep and goats, respectively. In goats, worm burden ranged from 1,980 to 6,080 and six animals recorded more than 5,000 adult parasites while, in sheep, more than 5,000 adult parasites were counted in one animal and the range was from 2,000 to 5,510 adult worms. Higher mean worm burden with an establishment rate was registered in goats than sheep with a significant difference (P<0.05) (Table 1).
 |
Table 1 Total Worm Burden (Group Mean) and Worm Establishment Rate in T. colubriformis Infected Group of Sheep and Goats |
Clinical Responses and Body Weight Changes
At 2 weeks of post-experimental infection in both species of infected animal groups, two sheep and four goats showed alterations in feces, eliminating agglomerated pellets with a “grape bunch” aspect, which had a variable consistency from semi-solid to pasty. The other sheep and goats of the infected group showed normal defecating characteristics throughout the experimental period. This change continued in all individuals of the infected experimental group of animals until the end of the experiment. A few infected animals showed signs of depression, discomfort, and a minimal decrease in body condition. In contrast, the control group of animals had normal fecal consistency. Control animals, both sheep and goats, showed a progressive gain in body weight throughout the trial period. On the other hand, animals in the T. colubriformis nematode infected sheep, after 4 weeks and goats after 3 weeks of exposure showed a slight reduction in body weight, with no significant difference compared to the non-infected controls until the end of the trial (Figure 1). The differences oin live weight between the infected and non-infected control sheep and goats were found to be non-significant (P>0.05).
 |
Figure 1 Controlled and infected Body weight in goats and sheep. Abbreviations: w, week; Kg, kilogram. |
Hematological and Biochemical Analysis
Hematological Examination
Blood indices were evaluated through RBC counts to indicate the evidence of anemia. The mean of PCV and Hb value was decreased in the infected group of sheep and goats compared to the uninfected groups, with a significant difference (P<0.05). PCV and Hb results were lower from the third week of infection up to the sixth week of the trial period and began to rise to the end of the experiment in both species of experimentally T. colubriformis infected groups. The overall mean of RBC count was shown to be lower in the experimentally infected sheep and goats by T. colubriformis than the non-infected groups and found to be non-significant (Figures 2–4).
 |
Figure 2 PCV value in T. colubriformis infected and controlled sheep and goats. |
 |
Figure 3 Hemoglobin level registered in T. colubriformis infected and controlled sheep and goats. |
 |
Figure 4 RBC count in T. colubriformis infected and controlled goats and sheep. |
Similarly, MCV and MCH mean were decreased in the experimentally infected animals, except MCHC, as compared to the control groups in sheep and goats. The overall mean RBC count, PCV, and Hb value for infected sheep were 11.78±0.29x106/µL, 24.45±1.15%, and 10.27±0.22 g/dL, respectively, while the overall means for infected goats were 12.24±0.58x106/µL, 21.60±1.78%, and 8.32±0.48 g/dL, respectively. For the control group, these results were: sheep (RBCs 12.50±0.26x106/µL, PCV 29.42±0.38%, Hb 12.43±0.36 g/dL) and goats (RBCs 13.65±0.41x106/µL, PCV 30.03±0.60%, Hb 11.44±0.21 g/dL) (Table 2).
 |
Table 2 Blood Parameters (Mean±SE) of Control and T. colubriformis Infected Sheep and Goats |
The total mean leukocyte counts were significantly higher in the infected groups of both sheep (11.99±0.49x103/µL) and goats (12.58±0.52x103/µL) as compared to the control experimental groups (10.52±0.21x103/µL sheep and 11.03±0.44x103/µL goat), with a P<0.05 (Table 2). The mean WBC count was elevated in the first week of T. colubriformis infection and the peak level was recorded on week 3 in sheep post-infection. The infected goat demonstrated a little peak at week 2 and a higher peak was recorded at week 3, which then declined until the end of the trial period (Figure 5). Similarly the infected sheep gradually declined up to the end of the experiment after a peak at week 3. Both uninfected control sheep and goats showed little fluctuation throughout the trial period (Figure 5).
 |
Figure 5 WBC count in T. colubriformis infected and controlled sheep and goats. |
Blood Eosinophil
The weekly mean blood eosinophil number (Table 3) was increased significantly (P<0.05) in the infected sheep (week 5 =72.28x104/mL; week 6 =69.71x104/mL) and goats (week 5 =138.28x104/mL; week 6 =153.42x104/mL) than that of the uninfected groups (week 5 =11x104/mL; week 6 =11.33x104/mL, and week 5 =37.71x104/mL; week 6 =26.28x104/mL, respectively).
 |
Table 3 Weekly Blood Eosinophil Count (Mean±SE) in T. colubriformis Infected and Control Groups of Sheep and Goats |
Peak blood eosinophil numbers were recorded in week 7 for sheep and in week 6 for goats infected with T. colubriformis (Figure 6). Toward the end of the experiment, a gradual fall in the level of eosinophil number was seen in all infected groups of animals. The mean blood eosinophil in all control groups maintained a normal level throughout the experimental weeks. The total mean eosinophil numbers of infected (53.74x104/mL sheep; 61.57x104/mL goat) and controls (15.40x104/mL sheep; 28.31x104/mL goat) were determined. Significantly higher results were found in experimentally infected animals than controls.
 |
Figure 6 Blood eosinophil count in T. colubriformis infected and controlled sheep and goats. |
Biochemical Findings
The mean values with the serum biochemical parameters, including total protein, albumin, aspartate aminotransferase (AST), and alkaline phosphatase (ALP), in controls and T. colubriformis infected sheep and goats are presented in Table 4. From the experiment, the T. colubriformis infected group had statistically reduced (P<0.05) total mean of serum protein (4.21±0.36 g/dL) and albumin (1.49±0.15 g/dL) than the control group (5.70±0.28 g/dL, total protein; 2.35±0.09 g/dL, albumin) of goats. The values of total albumin serum concentrations resulted as mean (1.87±0.9 g/dL) and statistically significant (P<0.05), while total protein was mean (4.98±0.27 g/dL) and non-significant (P>0.05) in the infected group, which were lower than in the control group (2.38±0.07 g/dL, albumin; 8.35±2.10 g/dL protein) in sheep. The mean values of both ALP and AST were statistically non-significant between infected and control animals of both sheep and goats.
 |
Table 4 Biochemical Indices Analysis (Mean±SE) of T. colubriformis Challenged and Unchallenged Sheep and Goats |
Regarding the T. colubriformis infected group of sheep and goats, the overall mean PCV, Hb, blood indices, total protein, and albumin values were lower in infected goats than infected sheep, while total leukocyte and blood eosinophil count were slightly higher in the infected group of goats than sheep (Table 5). Hemoglobin and albumin values were statistically significant (P<0.05).
 |
Table 5 Hematological and Biochemical Values (Mean±SE) of T. colubriformis Infected Sheep and Goats |
Necropsy of Small Intestine
Gross Pathological Changes
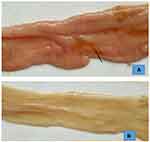
The postmortem examination of T. colubriformis infected sheep and goats revealed gross lesions such as enteritis and mucus hypersecretion, together with petechial hemorrhages, hyperemia, edema and mucosal slough which were especially marked in the duodenum (62.69%) and proximal jejunum (33.33%) in sheep and 47.05% and 45.09% in goats, respectively (Figure 7). Occurrences of total gross pathological lesions identified were significantly (P<0.05) higher in T. colubriformis infected goats (40.48%) than sheep (21.43%) (Table 6).
 |
Table 6 Characterization of Gross Pathological Lesions Presented in Segments of Small Intestine of Sheep and Goats Experimentally Infected with T. colubriformis |
From a total of gross pathological lesions examined, petechial hemorrhage in sheep (11; 40.74%) and goat (17; 33.33%) were recorded from the small intestine infected with T. colubriformis (Figure 8). Macroscopic lesions constituted (Table 6) were significantly higher (P<0.05) in the proximal part of the small intestine than the distal part of the small intestine in both T. colubriformis exposed groups of animals. Furthermore, areas with superficial erosions which were rounded or irregular and characterized by breaks in the mucosa, were observed in the small intestine of infected group of sheep and goats (Figure 9). The frequency of gross pathological lesions presented on each segment of the small intestine and the total gross pathological changes present (Table 7) were statistically significant (P<0.05).
 |
Table 7 Frequency of Gross Lesions Presented in Segments of Small Intestine of Sheep and Goats Experimentally Infected with T. colubriformis |
Histopathological Changes
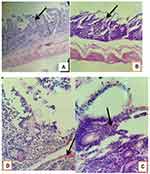
The microscopic lesions (Figures 10–14) established by T. colubriformis were characterized as subtotal villus atrophy, loss of epithelium, petechial hemorrhages, straightened and dilated crypts, erosions, mucosal secretion, and inflammatory cells infiltration at the mucosal surface of small intestine were observed in T. colubriformis infected goat and sheep jejunum. The villus atrophy lesion was observed in T. colubriformis infected goat jejunum, while fused villi and widespread loss of epithelium were seen in experimental infected sheep jejunum. T. colubriformis infected sheep duodenum showed mucosal secretion, total villus atrophy, and elongated intestinal crypts, while in infected goats, the duodenum showed total villus atrophy. In both infected experimental animals duodenum, the surface of mucosa showed leaks and erosions which were sometimes found to be disrupting the integrity of the epithelium.
 |
Figure 14 Histologically, hemorrhages at the surface of mucosa (A) and in sub mucosa (B) of T. colubriformis infected goat jejunum. |
Discussion
This study was carried out to assess hematological, serum biochemical, and pathological alterations in indigenous Ethiopian highland sheep and goats experimentally infected with T. colubriformis. The experimental infection of T. colubriformis in sheep and goats showed an influence on values of hematological and biochemical parameters and intestinal pathological conditions. The depression and decrease in appetite were minimal and not the main disorder resulting from T. colubriformis infected sheep and goat. This may relate with the number of infective larvae given during the study period. According to the previous reports of Steel et al26 and Symons,27 severe consumption disorders presented in young lambs were as a result of infection with a large number of T. colubriformis larvae. The reduced appetite of animals infected with this nematode may be due to an increase in the plasma concentration of cholecystokinin (CCK) hormone which was stimulated and elevated in T. colubriformis infected sheep.28
The hematological results revealed a decrease in PCV, hemoglobin, and RBC values with a significant difference in infected animals than the non-infected control group of sheep and goats. This is probably as a result of hemorrhagic lesions in intestinal tissues, as observed from histological analyses and tumor necrosis factor-α (TNF-α) has also been implicated in suppressing hematopoietic progenitors during inflammation, resulting in decreased erythrocyte production and decreased erythrocyte survival.29 Pathological conditions and laboratory results may differ depending on the factors such as the intensity of infection and species of nematodes. Thus, changes in PCV and RBC values are more common in parasitism by species of nematodes, such as H. contortus.30 The reports of Roy et al31 also described that the severity of pathological establishment was higher when 30,000 infective larvae of T. colubriformis were administered compared to 15,000 larvae.
The overall hemoglobin and total RBC count observed for both infected and control goats and sheep were within the normal reference interval stated in veterinary medicine, a textbook of the disease of cattle, sheep, goats, pigs, and horses,32 suggesting the absence of development of significant anemia. However, the infected sheep and goats groups recorded lower PCV, hemoglobin, and total RBC counts than uninfected controls (normal reference range). These results (29.42±0.38, control sheep; 24.45±1.15, infected sheep) were in agreement with the report of Cardia et al33 and the PCV mean of the infected group (29.80±0.49) was significantly lower (P<0.05) than the PCV of the control group (32.70±0.79) in lambs with experimental T. colubriformis infections. Other researchers such as Horton et al34 also reported mild increases in Hb, erythrocyte, and hematocrit values in non-infected lambs and the lowest for infected lambs, which is consistent with this finding. Radostits et al32 stated that hematological differences were affected by host and species factors, while lower PCV and hemoglobin values were recorded in T. colubriformis infected goats than sheep.
The Mean Corpuscular Volume (MCV) and Mean Corpuscular Hemoglobin (MCH) blood indices were decreased, except Mean Corpuscular Hemoglobin Concentration (MCHC) which was unchanged between the infected and healthy animal groups. The present findings of blood indices were supported by Horton et al.34 In infected experimental groups of the current study, mild decreases were observed in MCV in the non-infected groups,which disagrees with the report of Horton et al,34 but it was in the normal reference level indicated by Radostits et al.32 Reduction in MCV may be due to a decrease in PCV level which may be attributed to the dilution of blood.35 Blood parameter alterations can also be associated with inhibition of mineral and vitamin absorption such as vitamin E, selenium, and iron from the intestinal mucosa as a result of T. colubriformis infection. Horak et al36 reported hematological changes were observed in lambs infected with T. colubriformis, and Hoekstra37 described that selenium has been shown to have a specific enzyme function in sheep red blood cells. Horton et al34 also added that Hb, erythrocyte, and PCV values were lowest for T. colubriformis infected lambs receiving vitamin E and Se than uninfected lambs fed with vitamin E and Selenium, but MCV was not affected by either T. colubriformis infection or supplementation of Vitamin E and Selenium.
The mean of total leukocyte count revealed a significant increase in both the infected group of sheep and goats than non-infected controls. The observed leukocytosis in infected sheep in the current study is consistent with the finding of Horton et al,34 and leucocyte counts were higher in infected lambs than in the non-infested groups. According to the reports of Furlanello et al,38 leukocytosis occurred due to maturation of lymphocytes and neutrophils. Intestinal nematode damage intestinal mucosa which leads to activation of macrophages that release pro-inflammatory cytokines (Th2 associated cytokines), including interlukin (IL4, IL5) and tumor necrosis factor (TNF) and interlukin-4 and 5 causes the proliferation of lymphocytes. The later TNF is also important for activation of blood mononuclear cells such as lymphocytes which are responsible for lymphocytosis.39
The mean blood eosinophil count was increased significantly in the infected group of sheep and goats compared to the control group. Similar results were found in the reports of Cardia et al.33 The blood eosinophil count between the T. colubriformis infected group of sheep and goats of the present study was non-significant. The eosinophillia may serve as an indicator of the host’s responsiveness to T. colubriformis infection.40 Dawkins et al40 noted marked eosinophilia in the high responder lambs following challenge with T. colubriformis. Moreover, ovine gastrointestinal nematode infections cause a lot of factors that induce migration of blood eosinophils according to Wildblood et al.41 Evidence from different studies suggested that eosinophils have been viewed as an important indicator of helminth infection and pathogenesis.42 The registered eosinophilia was as a result of animal’s sensitivity to the foreign protein of a parasite which may be a part of an immune phenomenon.16
The major alterations also occurred in the mean values of total serum protein and albumin concentration in both infected groups of experimental animals. Greater total serum protein and albumin loss were observed in T. colubriformis infected goats than sheep. This is probably due to a decrease in protein absorption and albumin loss into the intestinal lumen through the lesions developed by the worm load in the intestinal epithelium, as evidenced by the increased worm burden registered in the infected group of goats compared to sheep. Lower serum concentration of albumin and protein values were also recorded in infected animals of sheep and goats than non-infected controls. Related results with the present study were reported by Cardia et al.33 Horton et al34 also evidenced that total serum protein and albumin concentrations were depressed by about 14% (P<0.001) in T. colubriformis infested lambs as compared to non-infested animals. In the present study, the infected group of sheep and goats presented the lowest albumin serum concentration than uninfected controls at the end of the experimental period. Cardia et al33 observed that mean values of albumin serum concentrations were significantly lower in the infected group than uninfected control lambs which supports the current results. The rejection of T. colubriformis incoming larvae by immune animals is accompanied by an intestinal inflammatory response involving the secretion of biogenic amines with a concurrent plasma albumin loss. This is the major factor responsible for the cause of decreased blood protein and albumin level in sheep infected with T. colubriformis.26 The findings of the present study regarding the activities of aspartate aminotransferase and alkaline phosphatase were non-significant (P<0.05) in infected and control groups of sheep and goats.
The current results were inconsistent with the reports of Horton et al34 that aspartate aminotransferase values were significantly (P<0.001) higher in lambs infected with T. colubriformis than controls. This disagreement between previous and present report may be associated to the experimental infective T. colubriformis larvae doses which were 100,000 in the previous while 10,000 in the current study. Kumar et al43 also described in their clinicopathological studies of gastrointestinal tract disorders in sheep with parasitic infection that the activity of AST and ALP were significantly increased in GIT parasitic infected sheep, which disagrees with the present finding.
According to Kumar et al,43 specific hepatic functions are greatly affected by a wide variety of pathological conditions of extra hepatic origin, especially gastrointestinal origin. These enzymes have their function and greatest concentration within the cell, thus the increase in enzymatic activities reflects cellular abnormalities which are directly related to hepatocytes damage, intestinal pathological lesions, and cardiac infarction,44 which were not evidenced in the present finding. This difference between the previous and current reports in the development of pathological changes may be associated with the species and burden of parasites.
The gross and microscopic intestinal pathology and their characteristics which are consistent with the injuries caused by T. colubriformis infection have been previously studied in small ruminants, but no more comparable recent data between sheep and goats are obtainable. The predominant characteristics of gross intestinal T. colubriformis induced lesions in sheep and goats of the present study includes enteritis and mucus hypersecretion, together with edema, hyperemia, petechial hemorrhages, and mucosal slough, which were markedly observed in the duodenum and proximal jejunum. The above described most pathological lesions in the present study were frequently investigated in the proximal part of the small intestine, duodenum, and proximal jejunum which was supported by Beveridge et al45 and Roy et al's31 reports in the proximal part of the small intestine, 2 meters from the pylorus.
The overall macroscopic lesion percentages were greater in the infected group of goats than sheep. This result may relate with the number of worm burden found in the site of infection, as it was greater in goats than sheep. Trapani et al46 reported that the severity of the lesion correlates with the local density of worms. According to the reports of Abebe and Esayas47 in arid and semiarid zones of eastern Ethiopia, the prevalence of T. colubriformis in goats (89.90%) at post-mortem examination was greater than in sheep (87.15%), which were supported with the present study of worm establishment rate in goats (50.16%) than in sheep (34.46%). Williams et al48 also reported that rams were able to resist the T. colubriformis larval challenge effectively and registered the small total worm count evidenced the present finding of lower worm burden in sheep than goats. This worm burden and pathological difference between T. colubriformis infected sheep and goats may be associated with factors such as species and breed resistance of the host.
The microscopic lesions observed in the duodenum and proximal jejunum of T. colubriformis infected sheep that is villus atrophy and crypt hyperplasia of the current study were less severe than the total atrophy described by Barker49 in clinical trichostrongylosis, but were comparable to previous descriptions in sheep infected at a subclinical level with T. colubriformis45,50 and in experimental infections of sheep with T. colubriformis on nematode distributions, numbers, and on pathological changes.31 Trapani et al46 observed a similar degree of villus atrophy with the present finding in goats naturally infected by T. colubriformis. Fused villi with widespread loss of intestinal epithelium were identified in the present study in experimental T. colubriformis infected goats, which were consistent with the reports of Trapani et al46 in natural T. colubriformis infected goats. Erosions of intestinal epithelium of the current findings were also comparable with the results of Barker49 in his study of intestinal pathology associated with T. colubriformis infection in sheep but less severe than the current finding of experimental T. colubriformis infection in sheep. Beveridge et al45 also added related reports with the present study that sloughed epithelial cells are present in the lumen as well as a significant number of inflammatory cells. The severity of pathological changes can be associated with the total numbers of larvae administered and the total numbers of worms which establish at site of infection. The changes may be due to a mechanical effect of the parasite or due to chemical secretions from the worms.
Similar to the reports by Trapani et al46 and Paciello et al51 in goats, this study indicated that the immune response to T. colubriformis infection in sheep and goats was characterized by an increased rate of infiltrating inflammatory cells in the intestinal mucosa. Trapani et al46 also noted that, in the lamina propria, there were inflammatory cells consisting of lymphocytes, plasma cells, eosinophils, macrophages, and globule leukocytes in T. colubriformis naturally infected goats. In most affected gut, inflammatory cells were located at submucosal compartments. In some cases mild or moderate increases of intraepithelial lymphocytes and moderate dilatation of the lymphatic vessels were observed.45 The precise role of infiltrating inflammatory populations during infection with the parasite and the mechanisms by which these cells may influence the sheep and goat immune responses are important issues that remain to be elucidated.
Conclusions
The result obtained in the current study described the effect of T. colubriformis experimental infection on hematological parameters, biochemical indices, and pathological changes in the small intestine of sheep and goats. The study found T. colubriformis adult worm burden and its pathological effect were greater in goats than sheep. Also, it affects blood parameters, biochemical indices, and caused pathological alterations of the small intestine in sheep and goats. Thus, in the present study, T. colubriformis infection was not a significant cause of anemia in sheep and goats, even though a slight reduction of blood parameters was observed in infected animals than controls. Generally, this experimental study concluded that pathological, hematological, and biochemical effects of T. colubriformis infection were greater in goats than sheep and even statistically it was non-significant.
Abbreviations
IL, Interlukin; TNF, tumor necrosis factor; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PCV, packed cell volume; GIT, gastro-intestinal tract; AST, aspartate aminotransfer; ALT, alanine aminotransfare.
Data Sharing Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All the experiments regarding sheep and goats were performed in accordance with the Addis Ababa University College of Veterinary Medicine research ethics and animal welfare guide for the care and use of animals. All animal experimental protocols were revised and approved by AAU-CVMA (Ref. No. VM/ERC/05/10/019, 16/11/2019).
Acknowledgments
The authors would like to thank the Office of the Vice President for Research and Technology Transfer of the Addis Ababa University for financial support and staffs of the Ethiopian Animal Health Institute at Sebeta for technical support. This paper is based on the thesis of Arega Tafere. It has been published on the institutional website: http://213.55.95.56/bitstream/handle/123456789/23047/Arega%202020.pdf
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Addis Ababa University (AAU) graduate students’ grant and the Thematic Research project entitled “Biological control of nematode parasites in ruminants” funded by the AAU.
Disclosure
Dr Tamirat Kaba reports grants from Addis Ababa University, personal fees from Arba Minch University, during the conduct of the study. The authors declare that they have no other competing interests.
References
1. Horal IG. Paramphisomiasis of domestic ruminants. In: Dawes B, editor. Advance in Parasitology. New York: Academic Press; 2006:33–70.
2. Sykes AR. The effect of subclinical parasitism in sheep. Vet Res. 2004;102:32–34.
3. Fikru R, Teshale S, Reta D, Yosef K. Epidemiology of gastrointestinal parasites of ruminants in Western Oromia, Ethiopia. Intl J Appl Res Vet Med. 2006;4:451–457.
4. Clark IA. Heterologous immunity revisited. Parasitology. 2001;122:851–859. doi:10.1017/S003118200100734X
5. Cox FEG. Concomitant infections, parasites and immune responses. Prasitology. 2001;112:823–838.
6. Waller PJ, Rudby-Martin L, Ljungstrom BL, Rydzik A. The epidemiology of abomasal nematodes of sheep in Sweden, with particular reference to over-winter survival strategies. Vet Parasitol. 2004;122(3):207–220. doi:10.1016/j.vetpar.2004.04.007
7. Rinaldi L, Veneziano V, Cringoli G. Dairy goat production and the importance of gastrointestinal strongyle parasitism. Trans R Soc Trop Med Hyg. 2007;101:745–746. doi:10.1016/j.trstmh.2007.03.010
8. Hoste H, Jackson F, Athanasiadou S, Thamsborg SM, Hoskin SO. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006;22:253–261. doi:10.1016/j.pt.2006.04.004
9. Hoste H, Sotiraki S, Landau SY, Jackson F, Beveridge I. Goat-nematode interactions: think differently. Trends Parasitol. 2010;26:376–381. doi:10.1016/j.pt.2010.04.007
10. Chirkena K, Getachew S, Beyene G, Dinede G. Hematological parameters of sheep: an aid in the diagnosis of gastrointestinal (GIT) and respiratory diseases. Nat Sci. 2016;14(5):97–102.
11. Mal G, Suchitra Sena D, Kumar R, Sahani MS. Haemoatological and mineral profile of bacterian and dromedary camel. Indian J Anim Sci. 2001;71:1162–1163.
12. ADARDO. Ada‘a district agricultural and rural development office; 2007.
13. Hewson CJ. What is animal welfare? Common definitions and their practical consequences. Can Vet J. 2003;44(6):496–499.
14. Terefe G, Yacob HT, Grisez C, et al. Haemonchus contortus egg excretion and female length reduction in sheep previously infected with Oestrus ovis (Diptera: oestridae) larvae. Vet Parasitol. 2005;128:271–283. doi:10.1016/j.vetpar.2004.11.036
15. Schalm OW, Jain NC, Carol EJ. Veterinary Hematology.
16. Feldman BF, Zinkl JG, Jain NV. Schalm’s Veterinary Hematology.
17. Dein FJ. Laboratory Manual of Avian Haematology. Association of Avian Veterinarian, East North Port; 1984.
18. Philippe. Clinical & biomedical sciences of tropical diseases. Stichting van Openbaar Nut. 2009;2:701.
19. Ibrahim A. Hematological and some biochemical values of indigenous chickens in Al-Ahsa, Saudi Arabia during summer season. Asian J Poult Sci. 2013;6:138–145.
20. Sharma IJ, Singh HS. Student’s Laboratory Manual of Veterinary Physiology. Kalyani Publishers; 2000:12–35.
21. Boray JC. Studies on intestinal Paramphistomosis in cattle. Aust Vet J. 2004;35:282–287. doi:10.1111/j.1751-0813.1959.tb08480.x
22. Gutte MM, Lambate SB. Comparative Gross Anatomical and Histomorphological Studies on Small Intestine in Sheep (Ovis Aries) and Goat (Capra Hircus). MAFSU, Nagpur: Indian agricultural research institute; 2017:21–22.
23. Hansen J, Perry B. The Epidemiology, Diagnosis and Control of Helminth Parasites of Ruminants, a Handbook. Nairobi: International Laboratory for Research on Animal Diseases; 1994:17–171.
24. Bancroft JD, Gamble M. Theory and Practice of Histological Techniques.
25. Mohammed H, AZizollah K, Seyed M, Saeed N. Hematological and serum Biochemical analyses in experimental caprine coccidiosis. J Parasit Dis. 2013;38(1):116–123.
26. Steel JW, Symons LEA, Jones WO. Effects of level of larval intake on the productivity and physiological and metabolic responses of lambs infected with Trichostrongylus colubriformis. Aust J Agric Res. 1980;31:821–838. doi:10.1071/AR9800821
27. Symons LEA. Plasma zinc and inappetence in sheep infected with Trichostrongylus colubriformis. J Comp Path. 1983;93:547–550. doi:10.1016/0021-9975(83)90061-0
28. Symons LEA, Hennessy DR. Cholecystokinin and anorexia in sheep infected by the intestinal nematode Trichostrongylus colubriformis. Int J Parasitol. 1981;11:55–58. doi:10.1016/0020-7519(81)90025-4
29. Boulter N, Hall R. Immunity and vaccine development in the bovine theilerioses. Adv Parasitol. 2000;44:41–97.
30. Shakya KP, Miller JE, Horohov DW. A Th2 type of immune response is associated with increased resistance to Haemonchus contortus in naturally infected Gulf Coast Native lambs. Vet Parasitol. 2009;163:57–66. doi:10.1016/j.vetpar.2009.03.052
31. Roy EA, Hoste H, Beveridge I. The effects of concurrent experimental infections of sheep with Trichostrongylus colubriformis and T. vitrinus on nematode distributions, numbers and on pathological changes. Parasite. 2004;11:293–300. doi:10.1051/parasite/2004113293
32. Radostits OM, Gay CC, Hinchcliff KW, Constable PD. Veterinary Medicine, a Textbook of the Disease of Cattle, Sheep, Goat, Pig and Horse.
33. Cardia DFF, Rocha-Oliveira RA, Tsunemi MH, Amarante AFT. Immune response and performance of growing Santa Ines lambs with artificial Trichostrongylus colubriformis infections. Vet Parasitol. 2011;182:248–258. doi:10.1016/j.vetpar.2011.05.017
34. Horton GMJ, Owen NC, Horak IF, Schroder J. Haematological changes caused by trichostrongylus colubriformis in lambs fed a dystrophogenic diet. J S Afr Vet Assoc. 1977;48(2):99–103.
35. Schetters TP, Kleuskens JA, Van De Crommert J, et al. Systemic inflammatory response in dogs experimentally infected with Babesia canis, a hematological study. Vet Parasitol. 2009;162:7–15. doi:10.1016/j.vetpar.2009.02.012
36. Horak LG, Clark R, Gray RS. The pathological physiology of helminth infestations. III. Trichostrongylus colubriformis. Onderstepoort J Vet Res. 1968;5:195.
37. Hoekstra WG. Biochemical Role of Selenium in Trace Element Metabolism in Animals. Baltimore: University Park Press; 1974:61–77.
38. Furlanello T, Fiorio F, Caldin M, Lubas G, Solano-Gallego L. Clinico-pathological Findings in naturally occuring cases of babesiosis caused by large form Babesia from dogs of northeastern Italy. Vet Parasitol. 2005;134:77–85. doi:10.1016/j.vetpar.2005.07.016
39. Duque AG, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi:10.3389/fimmu.2014.00491
40. Dawkins HJS, Windon RG, Eagleson GK. Eosinophil responses in sheep selected for high and low responsiveness to Trichostrongylus colubriformis. Int J Parasitol. 1989;19:199–205. doi:10.1016/0020-7519(89)90008-8
41. Wildblood LA, Kerr K, Clark A, Cameron A, Turner OG, Jones DG. Production of eosinophil chemoattractant activity by ovine gastrointestinal nematodes. Vet Immunol Immunopathol. 2005;107:57–65. doi:10.1016/j.vetimm.2005.03.010
42. Nickdel MB, Roberts F, Brombacher F, Alexander J, Roberts CW. Counter-protective role for interleukin-5 during acute Toxoplasma gondii infection. Infection Immunity. 2001;69:1044–1052. doi:10.1128/IAI.69.2.1044-1052.2001
43. Kumar S, Jakhar KK, Singh S, Potliya S, Kumar K, Pal M. Clinico-pathological studies of gastrointestinal tract disorders in sheep with parasitic infection. Vet World. 2015;8(1):29–32. doi:10.14202/vetworld.2015.29-32
44. Purohit K, Bhowmik MK, Roy S, Singh AS, Mukhopadhayay SK. Some biochemical studies on Garole sheep infected with amphistome parasites. Indian J Anim Sci. 2003;73(10):1120–1122.
45. Beveridge I, Pullman AL, Phillips PH, Martin RR, Barelds A, Grimson R. Comparison of the effects of infection with Trichostrongylus colubriformis, T. vitrinus and T. rugatus in Merino lambs. Vet Parasitol. 1989;32(2–3):229–245. doi:10.1016/0304-4017(89)90123-4
46. Trapani F, Paciello O, Papparella S, Rinaldi L, Cringoli G, Maiolino P. Histopathological, histochemical and immunohistochemical findings of the small intestine in goats naturally infected by Trichostrongylus colubriformis. Vet Parasitol. 2013;191:390–393. doi:10.1016/j.vetpar.2012.09.017
47. Abebe W, Esayas G. Survey of ovine and caprine gastro-intestinal helminthosis in eastern part of Ethiopia during the dry season of the year. Revue Med Vet. 2001;152(5):379–384.
48. Williams AR, Palmer DG, Williams IH, Vercoe PE, Karlsson LJ. Faecal dry matter, inflammatory cells and antibodies in parasite-resistant sheep challenged with either T colubriformis or T circumcincta. Vet Parasitol. 2010;170:230–237. doi:10.1016/j.vetpar.2010.02.033
49. Barker IK. Intestinal pathology associated with Trichostrongylus colubriformis infection in sheep: histology. Parasitology. 1975;70:165–171. doi:10.1017/S0031182000049623
50. Roy EA, Hoste H, Fuller P, Tatarczuch L, Beveridge I. Development of morphological changes and ileal glucagon gene expression in the small intestine of lambs infected with Trichostrongylus colubriformis. J Comp Pathol. 1996;115:441–453. doi:10.1016/S0021-9975(96)80077-6
51. Paciello O, Veneziano V, Schioppi M, Morgoglione ME, Papparella S. Histopathology and immunohistochemical findings in the abomasum, intestine and mesenteric lymph nodes in goats naturally infected with gastrointestinal nematodes. Parassitologia. 2004;46:122.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.