Back to Journals » Risk Management and Healthcare Policy » Volume 17
A China-Based Cost-Effectiveness Analysis of Novel Oral Anticoagulants versus Warfarin in Patients with Left Ventricular Thrombosis
Authors Tian S , Zhong H, Yin M, Jiang P, Liu Q
Received 8 January 2024
Accepted for publication 5 April 2024
Published 13 April 2024 Volume 2024:17 Pages 945—953
DOI https://doi.org/10.2147/RMHP.S454463
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Haiyan Qu
Shuo Tian,1,2,* Haitao Zhong,3,4,* Mengyue Yin,5 Pei Jiang,3 Qiao Liu1
1Department of Pharmacy, The Second Xiangya Hospital of Central South University, Central South University, Changsha, Hunan, People’s Republic of China; 2Department of Clinical Pharmacy, Jining First People’s Hospital, Shandong First Medical University, Jining, Shandong, People’s Republic of China; 3Translational Pharmaceutical Laboratory, Jining First People’s Hospital, Shandong First Medical University, Jining, Shandong, People’s Republic of China; 4Institute of Translational Pharmacy, Jining Medical Research Academy, Jining, Shandong, People’s Republic of China; 5The Affiliated Taian City Central Hospital of Qingdao University, Taian, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiao Liu, Department of Pharmacy, The Second Xiangya Hospital of Central South University, Central South University, Changsha, Hunan, People’s Republic of China, Email [email protected]
Purpose: This study aims to conduct a comprehensive cost-effectiveness comparison between novel oral anticoagulants (NOACs) and warfarin in Chinese patients with left ventricular thrombosis (LVT). By incorporating the impact of volume-based procurement (VBP) policy for pharmaceuticals in China, this analysis intends to provide crucial insights for informed healthcare decision-making.
Patients and Methods: A Markov model was employed to simulate the disease progression of LVT over a 54-week time horizon, using weekly cycles and six mutually exclusive health states. The model incorporated transition probabilities between health states calculated based on clinical trial data and literature sources. Various cost and utility parameters were also included. Additionally, a series of sensitivity analyses were conducted to address parameter variations and associated uncertainties.
Results: The study finding suggest that from the perspective of Chinese healthcare, the majority of brand-name drug (BND) NOACs generally lack cost-effectiveness when compared to warfarin. However, when considered the VBP policy, NOACs, particularly rivaroxaban, prove to be more cost-effective than warfarin. Rivaroxaban provided an additional 0.0304 quality-adjusted life years (QALYs) per patient and reduced overall medical costs by 9095.73 CNY, resulting in an incremental cost-effectiveness ratio (ICER) of − 298,786.20 CNY/QALY. Sensitivity analysis indicated a 78.4% probability of any NOACs being more cost-effective compared to warfarin. However, specifically considering NOACs under the VBP policy, the likelihood of them being more cost-effective approached 90%.
Conclusion: Taking into account Chinese pharmaceutical procurement policies, the findings highlight the superior efficacy of NOACs, especially rivaroxaban, in enhancing both the quality of life and economic benefits for Chinese LVT patients. NOACs present a more cost-effective treatment option, improving patient quality of life and healthcare cost efficiency compared to warfarin.
Keywords: cost-effectiveness, novel oral anticoagulants, left ventricular thrombosis, thrombus resolution
Introduction
Left ventricular thrombosis (LVT) presents a significant challenge in cardiovascular disease management due to its complicated clinical presentation and treatment requirements.1 Patients with LVT face a 10–22% risk of thromboembolic events within the first three months.2,3 Current guidelines for myocardial infarction or stroke management recommend the use of anticoagulants in patients with LVT to mitigate the risk of stroke or systemic embolic events.2,4 Historically, warfarin has served as the mainstay in anticoagulation therapy for LVT, providing a standard but not necessarily the most optimal solution. Recently, non-vitamin K antagonist oral anticoagulants (NOACs) have emerged as a practical alternative to warfarin,5 primarily due to their convenience and the absence of a requirement for frequent laboratory monitoring.
Although NOACs were originally developed and widely acclaimed for their effectiveness in atrial fibrillation (AF) anticoagulation therapy,6,7 they have also demonstrated potential in the treatment of LVT. In comparison to vitamin K antagonists (VKAs), NOACs have demonstrated non-inferior efficacy and safety in managing LVT, as evidenced by several randomized clinical trials (RCTs) and meta-analyses.8–10 This evolving landscape is evident in the latest American Heart Association scientific statement (2022), which suggests NOACs as a viable alternative to warfarin, despite the limited scope of randomized data.11 Furthermore, the 2023 European Society of Cardiology (ESC) guidelines on Acute Coronary Syndromes (ACS) recommend considering oral anticoagulation (warfarin or NOACs) for 3–6 months with diagnosed LVT, providing a guideline recommendation endorsing the use of NOACs for LVT.12 Although numerous studies have investigated the cost-effectiveness of NOACs compared to warfarin, particularly in patients with AF,13–15 a significant gap in the literature persists, specifically addressing their cost-benefit analysis in the treatment of LVT. This gap becomes particularly conspicuous in light of the implications of the Chinese volume-based procurement (VBP) policy, which markedly affects drug pricing and availability,16 subsequently influencing the overall cost-effectiveness of different therapeutic options.
This study endeavors to provide a comprehensive evaluation of the cost-effectiveness of NOACs, particularly rivaroxaban, within the context of Chinese healthcare, for the treatment of LVT. By incorporating the latest clinical evidence and advances in diagnostic methodologies, the study aims to provide insights that are both clinically relevant and economically sound, thus addressing the existing knowledge gap within China’s healthcare system and pharmaceutical policies. The significance of this research resides in its potential to influence clinical practices towards more efficient and effective management strategies for LVT in China. The implications of this research are multifaceted, extending from patient care to healthcare policy, and promise to significantly enhance the quality of life for patients suffering from LVT.
Materials and Methods
Overview
For this economic evaluation, Tree Age Pro software (version 2022, https://www.treeage.com/) was utilized for mathematical modeling. The study investigated the cost-effectiveness of novel oral anticoagulants (NOACs) compared to warfarin in the treatment of left ventricular thrombosis (LVT) from the perspective of the Chinese healthcare system.
Efficacy data, specifically the thrombolysis rates of NOACs and warfarin, were sourced from an observational study17(ClinicalTrials.gov identifier: NCT05006677, hereinafter referred to as the E-DISSOLVE study). Adverse reaction rates were obtained from a comprehensive meta-analysis,18 ensuring a thorough and reliable assessment of treatment safety. The use of these publicly available data sources meant that the study was exempt from ethical review by the Clinical Ethics Committee (EC) of Jining First People’s Hospital, in accordance with the Measures for Ethical Review of Life Science and Medical Research Involving Humans (2023).
This economic evaluation was conducted in line with the Chinese guidelines for pharmacoeconomic evaluation (2020),19 ensuring that the methodologies and analytical approaches employed were consistent with current best practices in the field of pharmacoeconomic.
Model Construction
For this analysis, a Markov model was utilized due to its efficacy in depicting the progression and transition of chronic conditions such as LVT. The model is especially suitable for cost-effectiveness research, owing to its ability to accurately represent the sequential events and health states transitions over time. Our Markov model consisted of six distinct health states: left ventricular thrombosis (LVT), thrombus resolution (TR), systemic embolism (SE) or stroke, adverse event (AE) including major bleeding and clinically relevant non-major bleed (CRNMB), and death (Figure 1).
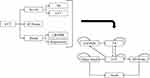 |
Figure 1 Diagram of Markov model. Abbreviations: LVT, left ventricular thrombus; AE, adverse event; TR, thrombus resolution; SE, Systemic embolism; CRNMB, clinically relevant no major bleed. |
Patients in the model began in the LVT state. The model was programmed to operate over 54 cycles, each representing a week, to align with the E-DISSOLVE study’s finding that thrombus completely resolves by day 378. Given that the total duration of the model was approximately one year, this study opted not to apply an annual discount rate for costs or outcomes.
Economic evaluation parameters were established in accordance with the 2020 Chinese guidelines for pharmacoeconomic evaluations. The willingness-to-pay (WTP) threshold was established at three times the per capita GDP of China in 2022. The cost-effectiveness of NOACs compared to warfarin was evaluated by calculating cumulative costs, quality-adjusted life years (QALYs), and the incremental cost-effectiveness ratio (ICER). A treatment was deemed cost-effective if its ICER fell below the WTP threshold.
Survival and Health State Utilities
Transition probabilities between health states were derived from the data from the E-DISSOLVE study and relevant meta-analyses pertaining to AE rates. The weekly transition probabilities in the model were calculated using a specific formula, owing to varying follow-up durations across referenced studies regarding thrombus resolution and the occurrence of adverse events.
Where P represents the probability of occurrence within the follow-up time, r denotes the occurrence rate within a specific time, and t signifies the specified time, which in this study is one week. This formula was utilized to standardize the probability of events occurring within different follow-up periods to a consistent weekly rate.
The thrombus resolution rates for NOACs and warfarin, as obtained from the E-DISSOLVE study, were integrated at various checkpoints: 6 weeks, 12 weeks, 26 weeks, and 52 weeks. The resolution rates were applied as follows: for the first 6-week cycle, the 6-week resolution rate was used; for the 6–12 weeks cycle, the resolution rate was calculated based on the number of patients experiencing thrombus resolution between 6 and 12 weeks divided by the number of patients who did not resolve in the first 6 weeks; similar calculations were applied for subsequent cycles. Tables S1 and S2 provide these data in detail.
As the E-DISSOLVE study did not encompass research on quality of life, the utility values for this project were obtained from a study based on the EuroQOL-5D (EQ-5D) 3-level utility data,20 which was aligned with the ICD-10 categorization. This approach enabled us to match the health states in our model with corresponding utility values according to the ICD-10 codes. By employing this method, we successfully incorporated comprehensive and relevant quality-of-life measures into our model, in spite of the absence of such data in the E-DISSOLVE study itself. Detailed information regarding the utility values used in the model and their corresponding ICD-10 codes can be found in Tables S3.
Costs
In this model, direct medical costs were rigorously calculated, adhering to the Chinese government’s Diagnosis-Related Groups (DRG) policy.21 This policy, informed by the ICD-10 categorization, imposes an upper limit on direct medical costs for various diseases, requiring all tertiary hospitals in China to adhere to these cost ceilings. For each health state within our model, the corresponding cost limits were determined via the ICD-10 directory, guaranteeing a standardized approach to cost inclusion, comparable to the integration of utility values.
Drug costs, a critical component of this analysis, were obtained from the 2022 Chinese Drug Bidding Database. Given China’s centralized drug procurement policy, typically only one NOAC holds a dominant market share in each province. In most provinces, the dominant NOAC is rivaroxaban, primarily in the form of generic drugs produced by Chinese pharmaceutical companies. Therefore, the typical winning bids for these dominant NOACs were selected for inclusion in our study. This approach enabled us to compare the costs of using these commonly procured NOACs, often generic versions of rivaroxaban, with the costs of using brand-name drug (BND) anticoagulant therapies. The NOACs analyzed in our study included rivaroxaban, apixaban, edoxaban, and dabigatran, along with the price of warfarin. The cost of anticoagulation therapy over the treatment period was determined based on the regimens outlined in the E-DISSOLVE study and relevant meta-analyses. All costs in this study are presented in Chinese Yuan (CNY).
The detailed values for all the aforementioned costs can be found in Table S4–S6 for thorough review and analysis.
Sensitivity Analysis
To verify the robustness of our model against uncertainties in the model parameters, both one-way deterministic sensitivity analyses (DSA) and probabilistic sensitivity analysis (PSA) were performed. In the DSA, the impact of varying individual model parameters on the cost-effectiveness outcomes was evaluated. The ranges for these parameters were sourced from published studies where available or established at ±10% of the base-case values to reflect plausible variations. The PSA was employed to evaluate the combined effect of simultaneous variation in model parameters on the cost-effectiveness results. This was accomplished through a Monte Carlo simulation, executing 1000 iterations to yield a spectrum of 1000 ICER estimates for the competing treatment options. The specific ranges and distributions used for each parameter in both DSA and PSA are thoroughly detailed in Table S1–S6.
Results
Base-Case Analysis
In our 54-week study evaluating the treatment of LVT with NOACs versus warfarin, we assessed the efficacy of all NOACs as a collective group, without differentiating between BND and VBP drugs. The overall incremental effectiveness of NOACs over warfarin was measured at 0.0304 QALYs (0.6238 for NOACs vs 0.5933 for warfarin). However, a significant economic advantage was observed when using VBP NOACs over warfarin. Specifically, employing rivaroxaban under the VBP policy led to a substantial cost reduction of 9095.73 CNY (483,507.12 for rivaroxaban vs 492,602.85 for warfarin), resulting in an ICER of −298,786.20 CNY/QALY. These findings not only establish VBP rivaroxaban as a cost-effective alternative to warfarin but also as a predominantly dominant strategy in terms of cost-effectiveness. It was also observed that most BND NOACs did not demonstrate cost-effectiveness compared to warfarin, in contrast to VBP drugs, which consistently showed cost-effectiveness. Detailed comparisons of the costs between different NOACs and their corresponding BND are elaborated in Table 1.
 |
Table 1 Cost-Effectiveness Analysis Results |
Sensitivity Analysis
The results of the DSA for our model are illustrated in Figure 2, through a tornado diagram. In our analysis, the ICER was significantly influenced by price fluctuations due to the relatively small incremental effectiveness values observed. The diagram revealed that the most impactful parameters on the ICER were the direct treatment costs associated with LVT and post-thrombus resolution, while the impact of NOACs prices ranked fourth in terms of influence. In the DSA, considering the prices of all NOACs in the Chinese market, the black line in the diagram represents the ICER generated under the average price of all NOACs, which amounted to −31,803.06 CNY/QALY. Generally, within the defined range of model parameters, the ICER fluctuated significantly, predominantly driven by variations in treatment costs and NOACs pricing.
The cost-effectiveness acceptability curve, as depicted in Figure 3 of our study, illustrates the probability of NOACs being cost-effective compared to warfarin across different willingness-to-pay (WTP) thresholds. Interestingly, even at a WTP threshold of 0 CNY, the probability of NOACs being cost-effective was marginally higher (55.4%) compared to warfarin (44.6%). This indicates that NOACs are more likely to be cost-effective despite the lack of a specific WTP threshold. Furthermore, as the WTP threshold increased, the probability of NOACs being a more cost-effective option also rose continuously.
 |
Figure 3 Acceptability curve. Abbreviation: NOACs, non-vitamin K antagonist oral anticoagulants. |
Additionally, the scatter plot derived from Monte Carlo simulations, showcased in Figure 4, provides further insight. Out of 1000 simulations, the probability of the ICER being less than 257,094 CNY (3 times the per capita GDP of China) was found to be 78.4%, when considering all NOACs available in the Chinese market for the simulation. However, when we specifically simulated scenarios using VBP NOACs, the probability of achieving an ICER below 257,094 CNY nearly reached 90%: 95.0% for rivaroxaban, 93.1% for apixaban, 89.1% for edoxaban, and 82.5% for dabigatran. (Figure S1–S4).
 |
Figure 4 Scatter plot for all NOACs. Abbreviations: NOACs, non-vitamin K antagonist oral anticoagulants; WTP, Willingness-to-pay. |
Discussion
While VKAs remain the primary treatment for LVT and are recommended in most guidelines, there has been an increase in studies exploring the use of NOACs for LVT treatment in recent years. Over the past decade, NOACs have been shown to be at least as effective as VKAs in preventing systemic embolism, with the added benefits of higher safety and lower bleeding risk.14,22 Notably, the 2023 ESC guidelines on ACS providing a clinical guideline basis for the utilization of NOACs for LVT.12 However, a 2022 review in the Journal of the American College of Cardiology (JACC) comparing VKAs and NOACs for LVT treatment highlighted inconsistent results across studies,11 with contradictory findings on efficacy and a not clearly established reduction in bleeding risk when NOACs are used in LVT patients. Despite these inconsistencies, the majority of recent meta-analyses and RCTs indicate that NOACs are non-inferior to VKAs for LVT treatment, and may even pose a lower risk of bleeding.18,23,24
At present, there are no specific guidelines in China for the diagnosis and treatment of LVT, except for a brief mention in the 2019 Chinese guidelines for the treatment of acute ST-segment elevation myocardial infarction.25 In Chinese clinical practice, treatment for LVT primarily depends on foreign guidelines or past experiences. This circumstance has prompted many Chinese researchers to investigate this area. Studies by Huang26 have demonstrated that NOACs can significantly reduce bleeding events and stroke risks in LVT patients compared to VKAs, with similar overall mortality rates. Research by Yang27 reported a high thrombus resolution rate with acceptable safety using rivaroxaban. Furthermore, the cost-effectiveness of using NOACs for treating atrial fibrillation and preventing venous thrombosis in China has been well-established.13–15 Given this backdrop, our study was inspired to conduct a cost-effectiveness analysis of NOACs compared to warfarin in the treatment of LVT, underscoring a pivotal area of research in the Chinese healthcare context.
Our study exhibits several key strengths that enhance its validity and applicability. Firstly, the thrombus resolution rates in our model are derived from the E-DISSOLVE study,17 a 10-year retrospective study specifically conducted on Chinese LVT patients. This offers a highly precise depiction of real-world thrombus resolution rates in China. Secondly, aligning our cost analysis with China’s DRG policy and the Chinese Drug Bidding Database enables us to present a comprehensive and realistic evaluation of the economic burden associated with various treatments for LVT. All costs are compiled and presented within the context of the Chinese healthcare system, providing practical insights for healthcare decision-makers. Thirdly, although VBP drugs dominate the Chinese market, our study encompasses the prices of all NOACs available, particularly brand name drugs, which often represent the highest costs. This approach ensures a comprehensive analysis of the economic implications across a range of treatment options. Finally, while our study indicates that the efficacy of the two treatment options is similar, aligning with previous efficacy research, this finding could offer valuable references for clinical practice or guideline development in China.
However, our study is not without certain limitations. Firstly, the cost estimates in our model are based on China’s government-mandated DRG payment standards, which only represent a limit. In actual clinical practice, there can be variation in patient expenses, either falling below or exceeding this limit.28 Secondly, although the thrombus resolution rates in our study are derived from the E-DISSOLVE study,17 the incidence rates of adverse reactions have been sourced from a meta-analysis. This implies that the probabilities used in our model are based on data from various studies. Despite DSA results indicating a minimal impact of adverse reaction rates on the ICER, there remains a possibility of introducing variability in our model. Additionally, due to the absence of quality-of-life data in the E-DISSOLVE study, we resorted to using utility values from other literature sources,20 a factor that could potentially affect our final results. Lastly, even though VBP drugs have been officially certified by the government as passing the generic drug consistency evaluation, a potential discrepancy in efficacy between these VBP drugs and their brand-name counterparts in actual clinical practice still exists.29 However, our study does not consider this potential difference in efficacy. This oversight might affect the accuracy of our cost-effectiveness analysis, given that treatment effectiveness is a crucial determinant of its overall value.
Conclusion
Within the framework of the Chinese healthcare system, our study clearly establishes the superiority of NOACs over warfarin for the cost and treatment of LVT, particularly in the context of the Chinese VBP policy. These insights are of considerable value in guiding clinical decision-making for the management of LVT in China. By highlighting the advantages of NOACs, our study provides a solid foundation for potential shifts in treatment protocols and healthcare policies.
Abbreviations
NOAC, Non-vitamin K antagonist oral anticoagulants; VKA, Vitamin K antagonists (warfarin); LVT, Left ventricular thrombus; AE, Adverse event; SE, Systemic embolism; TR, Thrombus resolution; CRNMB, Clinically relevant no major bleed; ICER, Incremental cost-effectiveness ratio; DSA, Deterministic sensitivity analysis; PSA, Probabilistic sensitivity analysis; DRG, Chinese government’s Diagnosis-Related Groups (DRG) policy; BND, Brand name drug; VBP, Drugs under Chinese volume-based procurement policy; CNY, Chinese Yuan; WTP, Willingness-to-pay.
Data Sharing Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the first author (E-mail address: [email protected]).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Key R&D Program of Jining (Grant numbers 2023YXNS205 and 2021YXNS011).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Levine GN, McEvoy JW, Fang JC. et al. Management of patients at risk for and with left ventricular thrombus: a scientific statement from the American Heart Association. Circulation. 2022;146(15):e205–e223. doi:10.1161/CIR.0000000000001092
2. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. doi:10.1161/STR.0000000000000024
3. Vallabhajosyula S, Kanwar S, Aung H, et al. Temporal trends and outcomes of left ventricular aneurysm after acute myocardial infarction. Am J Cardiol. 2020;133:32–38. doi:10.1016/j.amjcard.2020.07.043
4. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–425. doi:10.1161/CIR.0b013e3182742c84
5. Altiok E, Marx N. Oral Anticoagulation. Deutsches Arzteblatt Int. 2018;115(46):776–783. doi:10.3238/arztebl.2018.0776
6. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50(5):e1–e88. doi:10.1093/ejcts/ezw313
7. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e151. doi:10.1161/CIR.0000000000000665
8. Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American Perspective: 2021 Update. Circulation. 2021;143(6):583–596. doi:10.1161/CIRCULATIONAHA.120.050438
9. Lopes RD, Hong H, Harskamp RE, et al. Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention: a network meta-analysis of randomized controlled trials. JAMA Cardiol. 2019;4(8):747–755. doi:10.1001/jamacardio.2019.1880
10. Robinson AA, Trankle CR, Eubanks G, et al. Off-label use of direct oral anticoagulants compared with warfarin for left ventricular thrombi. JAMA Cardiol. 2020;5(6):685–692. doi:10.1001/jamacardio.2020.0652
11. Camaj A, Fuster V, Giustino G, et al. Left ventricular thrombus following acute myocardial infarction: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(10):1010–1022. doi:10.1016/j.jacc.2022.01.011
12. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes: developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2023;44(38):3720–3826. doi:10.1093/eurheartj/ehad191
13. Li Y, Chen P, Wang X, et al. Methods for economic evaluations of novel oral anticoagulants in patients with atrial fibrillation: a systematic review. Appl Health Econ Health Pol. 2023;1:2.
14. Sun KX, Cui B, Cao SS, et al. Cost-effectiveness analysis of direct oral anticoagulants versus vitamin k antagonists for venous thromboembolism in China. Front Pharmacol. 2021;12:716224. doi:10.3389/fphar.2021.716224
15. Zhou H, Nie X, Jiang M, Dong W. Cost-effectiveness of anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation in mainland China. J Clin Pharm Ther. 2022;47(4):523–530. doi:10.1111/jcpt.13575
16. Zhu Z, Wang Q, Sun Q, Lexchin J, Yang L. Improving access to medicines and beyond: the national volume-based procurement policy in China. BMJ Global Health. 2023;8(7):e011535. doi:10.1136/bmjgh-2022-011535
17. Yang Q, Lang X, Quan X, Gong Z, Liang Y, Mansour A. Different oral antithrombotic therapy for the treatment of ventricular thrombus: an observational study from 2010 to 2019. Int J Clin Pract. 2022;2022:7400860. doi:10.1155/2022/7400860
18. da Silva Ferreira H, Lima Lopes J, Augusto J, et al. Effect of direct oral anticoagulants versus vitamin K antagonists or warfarin in patients with left ventricular thrombus outcomes: a systematic review and meta-analysis. Revista Portuguesa De Cardiol. 2023;42(1):63–70. doi:10.1016/j.repc.2021.11.013
19. Yue X, Li Y, Wu J, Guo JJ. Current development and practice of pharmacoeconomic evaluation guidelines for universal health coverage in China. Value in Health Regional Issues. 2021;24:1–5. doi:10.1016/j.vhri.2020.07.580
20. Falk Hvidberg M, Hernández Alava M. Catalogues of EQ-5D-3L health-related quality of life scores for 199 chronic conditions and health risks for use in the UK and the USA. PharmacoEconomics. 2023;41(10):1287–1388. doi:10.1007/s40273-023-01285-4
21. Liu R, Shi J, Yang B, et al. Charting a path forward: policy analysis of China’s evolved DRG-based hospital payment system. Int Health. 2017;9(5):317–324. doi:10.1093/inthealth/ihx030
22. Humbert M, Simonneau G, Pittrow D, et al. Oral anticoagulants (NOAC and VKA) in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2022;41(6):716–721. doi:10.1016/j.healun.2022.02.002
23. Alcalai R, Butnaru A, Moravsky G, et al. Apixaban vs. warfarin in patients with left ventricular thrombus: a prospective multicentre randomized clinical trial‡. Eur Heart J Cardiovasc Pharmacother. 2022;8(7):660–667. doi:10.1093/ehjcvp/pvab057
24. Shrestha DB, Dawadi S, Dhakal B, et al. Direct oral anticoagulants (DOAC) versus vitamin K antagonist in left ventricular thrombus: an updated meta-analysis. Health Sci Rep. 2023;6(11):e1736. doi:10.1002/hsr2.1736
25. Hao Y, Zhao D, Liu J, et al. Performance of management strategies with class i recommendations among patients hospitalized with ST-segment elevation myocardial infarction in China. JAMA Cardiol. 2022;7(5):484–491. doi:10.1001/jamacardio.2022.0117
26. Huang L, Tan Y, Pan Y. Systematic review of efficacy of direct oral anticoagulants and vitamin K antagonists in left ventricular thrombus. ESC Heart Fail. 2022;9(5):3519–3532. doi:10.1002/ehf2.14084
27. Yang Q, Quan X, Zhang Y, et al. An exploratory study of effectiveness and safety of rivaroxaban in patients with left ventricular thrombus (R-DISSOLVE). J Thromb Thrombol. 2023;55(4):649–659. doi:10.1007/s11239-023-02790-1
28. He AJ. Scaling-up through piloting: dual-track provider payment reforms in China’s health system. Health Policy Plann. 2023;38(2):218–227. doi:10.1093/heapol/czac080
29. Yang Y, Hu R, Geng X, et al. The impact of National Centralised Drug Procurement policy on the use of policy-related original and generic drugs in China. Int J Health Plann Manag. 2022;37(3):1650–1662. doi:10.1002/hpm.3429
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


