Back to Journals » Clinical Epidemiology » Volume 15
β2-Adrenoceptor Agonists in Asthma or Chronic Obstructive Pulmonary Disease and Risk of Parkinson’s Disease: Nested Case-Control Study
Authors Paakinaho A , Tiihonen M, Koskela H, Koponen M, Tiihonen J , Hartikainen S, Tolppanen AM
Received 31 January 2023
Accepted for publication 22 May 2023
Published 12 June 2023 Volume 2023:15 Pages 695—705
DOI https://doi.org/10.2147/CLEP.S405325
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Henrik Sørensen
Anne Paakinaho,1 Miia Tiihonen,1 Heikki Koskela,2,3 Marjaana Koponen,1,4 Jari Tiihonen,5,6 Sirpa Hartikainen,1 Anna-Maija Tolppanen1
1School of Pharmacy, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland; 2Unit for Medicine and Clinical Research, Pulmonary Division, Kuopio University Hospital, Kuopio, Finland; 3School of Medicine, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland; 4Centre for Medicine Use and Safety, Faculty of Pharmacy and Pharmaceutical Sciences, Monash University, Parkville, Victoria, Australia; 5Department of Forensic Psychiatry, University of Eastern Finland, Niuvanniemi Hospital, Kuopio, Finland; 6Department of Clinical Neuroscience, Karolinska Institutet, and Center for Psychiatry Research, Stockholm City Council, Stockholm, Sweden
Correspondence: Anne Paakinaho, School of Pharmacy, Faculty of Health Sciences, University of Eastern Finland, P.O. Box 1627, Kuopio, FI-70211, Finland, Tel +358 405408490, Email [email protected]
Introduction: Although β 2-adrenoceptor (β 2AR) agonists have been associated with a lower risk of Parkinson’s disease (PD), the findings are inconclusive and may reflect confounding by indication. We studied the association between inhaled β 2AR agonists and risk of PD in persons with asthma or chronic obstructive pulmonary disease (COPD).
Methods: The nested case-control study was conducted within a register-based Finnish Parkinson’s disease study (FINPARK) and included 1406 clinically verified PD cases diagnosed during 1999– 2015, who also had asthma/COPD > 3 years before PD. PD cases were matched with up to seven controls by age, sex, duration of asthma/COPD, pulmonary diagnosis, and region (N = 8630). Cumulative and average annual exposure to short- and long-acting β 2AR agonists before a 3-year lag period was assessed with quartiles of defined daily doses (DDDs). Adjusted odds ratios (aORs) were calculated with 95% confidence intervals (CIs) using conditional logistic regression.
Results: Cumulative exposure to either short- or long-acting β 2AR agonists was not associated with a risk of PD. With average annual exposure, a decreased risk was observed only for the highest quartile of long-acting β 2AR agonists (aOR 0.75; 95% CI 0.58– 0.97). In the stratified analysis the lowest risk estimates were observed among those with both asthma and COPD diagnoses. The suggestion of an inverse association was seen for the highest quartile of long-acting β 2AR agonists in asthma.
Discussion: Higher levels of exposure to β 2AR agonists were not consistently associated with a reduced risk of PD. The inverse association in the highest category of average annual exposure to long-acting β 2AR agonists may be explained by unmeasured confounding, such as disease severity or smoking.
Keywords: Parkinson disease, adrenergic beta-2 receptor agonists, asthma, chronic obstructive pulmonary disease, risk factors
Introduction
Better understanding of risk factors for Parkinson’s disease (PD) could help in elucidating the causes and disease process of PD. Medications targeting the β2-adrenoceptor (β2AR) have been studied in this context, but despite the mechanistic evidence,1 the results from observational epidemiological studies are not as conclusive.2–4
The β2ARs can regulate α-synuclein gene (SNCA) expression by epigenetic mechanisms and β2AR agonists (eg salbutamol) decreased, and conversely β2AR antagonists increased SNCA expression in different experimental models.1 After the initial epidemiological study that showed a decreased risk of PD for salbutamol users,1 other epidemiological studies have investigated the association between β2AR agonists and risk of PD.2,4–10 Also recently β2AR agonist combination products (specifically, formoterol combined with budesonide) were associated with a decreased risk of PD in a study that aimed to identify candidates for repurposing with a machine learning-based signal detection approach.11
Due to inconsistent findings in previous studies, the implied protective effect of β2AR agonists on PD development is being debated.3,12 The inconsistency in previous literature might be due to differences in study populations and settings. Most of the previous studies have simultaneously investigated the associations of β2AR agonists and antagonists, and due to different indications for β2AR antagonists, these studies were not restricted to people with pulmonary diseases.2,4,7–9 As β2AR agonists are used to treat asthma and chronic obstructive pulmonary disease (COPD), the lower risk of PD among β2AR agonist users might be explained by confounding by indication. Asthma and COPD are chronic diseases with an inflammatory process involvement, and airway obstruction as hallmark, although in asthma the obstruction is reversible and in COPD mainly irreversible.13 Smoking has been suggested as one possible explanation for the protective association for β2AR agonists.3 Smoking has been linked to a lower risk of PD14 and it is the strongest risk factor for COPD for which β2AR agonists are frequently prescribed.15
Confounding by indication could be minimized by restricting a study to persons with asthma or COPD. This could also reduce the influence of smoking, especially when restricting to COPD, because smoking is not directly recorded in the register data. However, there are only two previous indication-restricted studies, both conducted with a nested case-control design. A study restricted to persons with COPD did not demonstrate an association between β2AR agonist use and risk of PD.5 In another study of persons with asthma or COPD with exposure to β2AR agonists measured as months exposed, an increasing use of short-acting but not long-acting β2AR agonists were associated with a lower risk of PD.6
We evaluated the association between inhaled β2AR agonists and risk of PD in a nationwide nested case-control study restricted to people with asthma or COPD. We also investigated whether there is a dose-response relationship between short- and long-acting β2AR agonists and risk of PD. To control for reverse causality (for example, PD cases having higher contact with prescribers due to prodromal symptoms and consequently higher likelihood for medication changes), exposure that had occurred at least three years before the outcome was considered.
Methods
Study Population and Data Sources
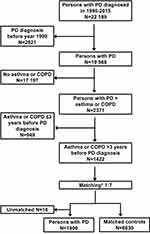
The Finnish Parkinson’s disease study (FINPARK) is a nested case-control study within the population of Finland. FINPARK includes 22,189 community-dwelling residents of Finland who received special reimbursement for PD drugs during 1996–2015. These persons with clinically verified PD were identified from the Special Reimbursement Register that contains information on entitlements to higher reimbursements for drugs due to chronic diseases. Special reimbursement for PD drugs is granted if predefined criteria for PD diagnosis are fulfilled and diagnoses must be confirmed by a neurologist. Initially, FINPARK included 29,942 persons with reimbursement for PD drugs, but 25.9% of them were excluded to increase the validity of PD diagnosis. Exclusions have been described in detail earlier,16 and the proportion of excluded persons corresponds to the estimated proportion of people with a false PD diagnosis.17,18 Up to seven age, sex, and region of residence matched comparison persons without PD (N = 148 009) were identified for cases from the Social Insurance Institution database.
Personal identification numbers enable linkage across nationwide Finnish registers. Data on chronic diseases is extracted from the Special Reimbursement Register and reimbursed prescription drugs from the Prescription Register which are both maintained by the Social Insurance Institution of Finland. Information on hospitalizations was obtained from the Care Register for Health Care maintained by the Finnish Institute for Health and Welfare. Cancer history was extracted from the Finnish Cancer Registry maintained by the Cancer Society of Finland and occupational social class from Statistics Finland.
Identification of Cases and Controls for This Study
Cases who were diagnosed with PD during 1999–2015 and diagnosed with asthma or COPD >3 years before PD diagnosis were selected (N = 1422). Cases diagnosed in 1996–1998 were excluded because Prescription Register data are available since 1995, and a 3-year lag was applied in drug exposure assessment. Thus, the drug exposure assessment period ranged between 1–17 years. The lag period of three years stems from a previous study on the FINPARK cohort showing that the incidence of muscle relaxant use, a sign of prodromal motor symptoms, began to increase among PD cases already three years before the diagnosis.19
Formation of the study population is described in Figure 1. Asthma and COPD were defined from the Finnish Care Register for Health Care (1972–2012) using ICD-10, ICD-9, and ICD-8 codes and from the Special Reimbursement Register (1972–2012) using ICD-10 and ICD-9 codes (Supplementary Table 1). The diagnosis date for asthma or COPD was determined either as the earliest date for the hospitalization or specialized healthcare outpatient visit, or as the date for the entitlement to reimbursement for drugs that are used in the treatment of asthma or COPD, depending on which occurred first.
Each PD case (N = 1422) was matched up to seven controls who were diagnosed with asthma or COPD but had no PD diagnosis from the controls of the entire FINPARK study. Controls were matched on the date of PD diagnosis for the case (index date). Controls were matched according to age (± 2 years) on index date, same sex, and pulmonary diagnosis type (asthma, COPD, or both), time since asthma or COPD diagnosis on index date (± 3 years) and university hospital district, which could be the same or neighboring district. The exclusion criteria for cases and controls were the same except that the controls were not allowed to have dementia in PD (ICD-10 code F02.3). Cases (N=16) that no controls were found were excluded and the final study population comprised of 1406 PD cases and 8630 matched controls.
Drug Exposure
Exposure to β2AR agonists since 1995 was obtained from the Prescription Register. The Prescription Register covers all reimbursed prescription drug purchases and drugs used in the hospitals are not included. Drugs are categorized according to Anatomical Therapeutic Chemical (ATC) classification codes and for each drug the register includes information such as the dispensing date, number of packages and defined daily dose (DDD) per purchase. The DDD is the assumed average maintenance dose per day for a drug used for its main indication for adults.20
Use of β2AR agonists were defined with ATC codes and were categorized as short-acting (salbutamol, terbutaline, fenoterol) and long-acting β2AR agonists (salmeterol, formoterol, indacaterol, olodaterol, and vilanterol) (Supplementary Table 2). The combinations of β2AR agonists with corticosteroids and anticholinergics were included. Additionally, use of inhaled corticosteroids and anticholinergics were extracted separately (Supplementary Table 2).
Exposure was extracted until the beginning of the 3-year lag period and categorized as use/no use before the 3-year lag. The earliest date of purchase was determined. The lag period was applied to minimize protopathic bias ie the likelihood that prodromal symptoms or diagnostic process of PD could affect drug exposure. After applying the 3-year lag period, possible drug exposure assessment period was 10.8 years on average.
The dose-response analyses were restricted to those who used β2AR agonists at least 3 years before index date. Due to matched design, those PD cases and controls who were unmatched after the exclusion of nonusers were also excluded. Cumulative exposure was calculated as cumulative sum of DDDs. We estimated in how many distinct years in relation to index date the user had carried out purchases. Cumulative DDDs were divided by the sum of years with purchase to get an average exposure per year (later briefly annual exposure). Continuous variables, cumulative DDDs, and annual exposure were categorized into quartiles in the main analyses and into tertiles in sensitivity analyses. These analyses were performed for short- and long-acting β2AR agonists separately and on any β2AR agonist level when use of either short- and/or long-acting β2AR agonists was considered.
Confounders
History of comorbid conditions comprised of cardiovascular diseases, diabetes, stroke, substance abuse and traumatic brain injury and were identified from the Finnish Care Register for Health Care, the Special Reimbursement Register or the Prescription Register. Additionally, history of cancer was obtained from the Cancer Registry. All comorbid conditions were measured until the start of the 3-year lag period and data sources, specific codes, coding systems, and time periods are described in more detail in Supplementary Table 3. Information on the highest occupational socioeconomic position in 1972–1994, was derived from Statistics Finland data and grouped according to classification by Statistics Finland. Students, long-term unemployed, other positions not elsewhere classified, socioeconomic status unknown or missing where classified into the ‘Others’ class.
Statistical Analyses
Characteristics of PD cases and controls and different exposure groups were compared with a Chi-Square test for categorical variables. T-test was applied for continuous variables that were normally distributed and Mann–Whitney U-test for nonnormally distributed variables. Use of inhaled β2AR agonists, corticosteroids and anticholinergics was compared between cases and controls. The associations between use of β2AR agonists and PD were investigated with conditional logistic regression due to the matched design. The analyses were adjusted with potential confounders. In addition, sensitivity analyses with additional adjustment for inhaled corticosteroids and anticholinergics were performed.
For categorical variables cumulative DDDs and annual exposure, the lowest quantile was used as reference. To evaluate whether the statistically significant associations were modified by pulmonary diagnoses, a model with exposure*pulmonary diagnosis interaction term was fitted and if there was evidence of modification (P for interaction <0.1), stratified analyses according to pulmonary diagnosis were performed. The Kruskal–Wallis test was applied to estimate differences in continuous variables, cumulative DDD, and annual exposure between pulmonary diagnosis types. Statistical analyses were conducted with SAS 9.4.
Results
Characteristics of 1406 PD cases and 8630 controls are presented in Table 1. Mean age was 73 years ranging from 33 to 95 years in the study population and 51% of both cases and controls were men. The median duration of asthma/COPD on index date was 12.4 years for controls and 12.9 for cases. The majority of the cases and controls had only asthma (>74%), approximately 15% had both asthma and COPD and less than 10% had only COPD. The history of comorbid conditions before the 3-year lag period were similar between cases and controls, except for cancer history which prevalence was higher among controls. Cardiovascular diseases were the most common comorbidities.
 |
Table 1 Description of PD Cases and Controls with Asthma/COPD |
Distribution of inhaled β2AR agonist exposure was similar between cases and controls (Table 1). Over 83% had purchased short-acting and 48% long-acting β2AR agonists. Neither short- nor long-acting β2AR agonists were associated with risk of PD when any use was compared to nonuse before the 3-year lag (adjusted odds ratio (aOR) 1.13; 95% CI 0.95–1.33 and 1.01; 0.89–1.14, respectively). Exposure to inhaled corticosteroids and anticholinergics was comparable between cases and controls (Supplementary Table 4), and additional adjustment for them did not affect the association of any short- or long-acting β2AR agonist use before the 3-year lag in sensitivity analyses (1.12; 95% CI 0.93–1.36 and 0.99; 0.87–1.13, respectively).
Altogether, 7376 users of short-acting, 2889 users of long-acting and 7851 users of any β2AR agonists were included in the dose-response analyses. Cumulative DDDs were not associated with risk of PD in any of the analyses (Table 2). There was no association between annual exposure and risk of PD for short-acting β2AR agonists or for any β2AR agonists. The highest quartile for annual exposure for long-acting β2AR agonists was associated with a decreased risk of PD (aOR 0.75; 95% CI 0.58–0.97) (Table 2). The results of sensitivity analyses with tertile categorization were similar to main analyses, with narrower 95% CIs resulting from borderline association between the highest cumulative exposure to any β2AR agonist and lower risk of PD (aOR 0.85; 95% CI 0.72–1.00). In addition, the association of the highest tertile of annual exposure for long-acting β2AR agonists was weaker (aOR 0.82; 95% CI 0.65–1.02, Supplementary Table 5) than the association of the highest quartile in the main analysis. The results from sensitivity analysis with further adjustment with inhaled corticosteroids and anticholinergics for quartile categorization were similar to the main analysis (Supplementary Table 6).
 |
Table 2 Dose-Response Associations Between Use of Inhaled β2AR Agonists and PD |
There was evidence for different association per pulmonary diagnosis type in this dose-response analysis of annual exposure for long-acting β2AR agonists (P for interaction 0.07). The lowest aORs were observed among those with both asthma and COPD (N = 670) with the third quartile of annual exposure (aOR 0.48; 95% CI 0.27–0.87), but not the fourth quartile, being associated with a decreased risk of PD compared with the lowest quartile (Figure 2). For asthma (N = 2089), the highest quartile showed a slight protective association (aOR 0.74; 95% CI 0.55–1.01). No associations were seen among those with only COPD (N = 130). People with asthma and COPD had the highest annual exposure for long-acting β2AR agonists (median 240 DDD/year; interquartile range (IQR) 141–316) than asthma (median 200 DDD/year; IQR 99–309) and COPD (median 173 DDD/year; IQR 90–300), p<0.0001 (Table 3).
 |
Table 3 Comparison of Cumulative DDD and Average Annual Exposure (DDD/Year) for β2AR Agonists Between Different Pulmonary Diagnosis Types. Results are Reported as Median (IQR) |
Discussion
Findings of this nested case-control study of people with asthma/COPD suggest that the use of inhaled β2AR agonists is not associated with risk of PD and higher cumulative exposure is not consistently associated with lower risk of developing PD. An association with a decreased risk was observed only for the highest quartile of annual exposure for long-acting β2AR agonists. This was modified by pulmonary diagnosis type, with the lowest aORs among those with both asthma and COPD diagnosis. This might suggest that regular and long-term use of long-acting β2AR agonists is more common among those with more severe disease such as persons with both asthma and COPD, and the protective association might be confounded by lifestyle factors such as smoking.
Although direct comparisons to earlier studies are challenging due to differences in methods and study populations, our findings are supportive of those from a nested case-control study restricted to persons with COPD. In that study, the use of short- or long-acting β2AR agonists was not associated with risk of PD.5 The exposure was assessed every six months for a 2-year window and categorized into regular, irregular use and no use. After applying a 2- or 3-year lag period, neither regular nor irregular use was associated with risk of PD compared to nonuse.5 Another nested case-control study of persons with asthma/COPD studied short- and long-acting β2AR agonists separately, and reported an association between short-acting β2AR agonist and lower risk of PD (OR 0.90; 95% CI 0.86–0.96 per additional month of exposure),6 while we did not observe any association with short-acting β2AR agonist use. Additionally, a cohort study which investigated the association between asthma and risk of subsequent PD, reported no association for inhaled β2AR agonists compared to persons without asthma,21 a result similar to our findings.
We did not observe a consistent protective association in the dose-response relationship analyses. Association was observed only for long-acting β2AR agonists in annual exposure analysis. Previous dose-response studies have mostly focused on salbutamol in study populations not restricted on asthma/COPD,1,2 which complicates the comparison. Also, follow-up and exposure assessment periods are not directly comparable to our study. Only one study reported dose-response results for long-acting β2AR agonist formoterol comparing average daily dose quartiles to nonuse and quartiles were associated with a decreased risk of PD except for the lowest quartile.4 In that same study, salbutamol average daily dose quartiles were associated with a decreased risk except for the highest quartile which was no longer associated with risk of PD compared to nonuse. As for salbutamol, findings from other two dose-response studies are varying.1,2 In a previous cohort study, the second and third tertile of cumulative DDDs for salbutamol (including both inhaled and systemic) showed protective association for risk of PD compared to nonuse.1 Categorization of cumulative DDDs in that study (<60, 60 to 180, ≥180 DDDs) are not comparable with ours since we had a longer exposure assessment period (10.8 years on average) compared to their maximum of 4 years. A case-control study2 used similar categorization as previously described1 but used the lowest tertile (<60 DDDs) as reference. No dose-response associations for inhaled salbutamol users overall or among smokers were found. In summary, higher cumulative exposure has not been consistently protective in previous studies when not restricted on asthma/COPD.
There was evidence for differential association between annual exposure to long-acting β2AR agonists and risk of PD per pulmonary diagnosis type. Strongest aORs were observed in those with both asthma and COPD diagnoses and a lower risk of PD was observed only in the third quartile. Even though the confidence intervals in the highest quartile overlapped 1, point estimate was suggestive of decreased risk. This kind of finding might be due to more severe and complex pulmonary condition that we could not take into account in the analyses as this information is not recorded in registers. However, as was seen in our data, those with both asthma and COPD diagnoses were exposed to the highest amount of β2AR agonists compared to those with asthma or COPD alone. Thus, use of β2AR agonists can reflect the severity of the pulmonary disease. This group with both asthma and COPD diagnoses can resemble persons with Asthma–COPD Overlap Syndrome (ACOS). ACOS is a heterogeneous condition characterized by persistent airflow limitation22 and can occur particularly in smokers and older adults.23 Persons with ACOS can have more symptoms and exacerbations than asthma and COPD alone.24
Protective association could be confounded by smoking as protective effect of smoking against PD is well-known.14 The role of smoking on the development of PD in epidemiological studies on β2AR agonists has been speculated previously.2,3,7 According to a case-control study, long-term use of β2AR agonists was associated with a lower risk of PD compared to nonuse, but since markers of smoking were also associated with reduced risk of PD, authors interpreted the results to be indirectly mediated by smoking.7 Number of smokers could be assumed to be highest among the COPD-only group, and higher among those with both asthma and COPD than asthma alone, since COPD is more likely smoking-related disease25 than asthma.26 The number of persons with COPD only was low in our study. Consequently, we had low power to detect associations but the point estimates in this group were not supportive of protective dose-response association. In the COPD group smoking may no longer be a significant confounder and mediate the association since the entire group is formed most likely by smokers and β2AR agonists might not be associated with risk of PD as also reported in a previous study restricted on people with COPD.5 As for asthma, there was a suggestion of lower risk of PD in the highest exposure quartile, although there was otherwise no indication for dose-dependent association in the asthma-only group. Among asthmatics, smoking has been associated with increased asthma severity26 and worse asthma control,27 which could result in increased need of β2AR agonists, thus, the protective association in the highest exposure category could be indirectly mediated by smoking. The same phenomenon may explain the findings in the group with both asthma and COPD.
There are several strengths in our study. We were able to restrict the study population on asthma/COPD due to a large nationwide register data with a long follow-up period thus controlling for confounding by indication. In addition, we could have a 3-year lag period in exposure assessment. By applying a lag-period we could ascertain that drug exposure has happened before PD diagnosis and minimize reverse causality ie the likelihood of initiation or discontinuation of drugs due to prodromal symptoms of PD. We were able to account for multiple confounding factors even though we lacked information on smoking.
In Finland, diagnosis of asthma is based on patient history and clinical examination and objective evidence of reversible airway obstruction.28 Diagnosis of COPD is based on relevant exposure history, symptoms and on the presence of fixed airway obstruction in spirometry.29 One explanation for low prevalence of COPD in our study population can be an underdiagnosis of COPD in Finland.30
The register-based approach poses some limitations. Exposure for inhaled β2AR agonists defined from registers either as estimated duration of use from prescriptions filled or as DDDs are not necessarily exact as actual use and adherence is unknown. Especially in asthma, short-acting β2AR agonists can be used as as-needed rescue medications31 and therefore a new inhaler can be purchased due to expiration of the previous one or due to stockpiling. Persons with persistent respiratory symptoms might purchase more frequently and use higher DDDs. In dose-response analyses the lowest exposure category was used as reference instead of nonusers for less biased comparison. For example, disease severity can differ between users vs nonusers regardless of the initial restriction to asthma/COPD. We did not have information on disease severity, but we matched cases and controls by duration of asthma/COPD.
In conclusion, inhaled β2AR agonists were not associated with a risk of PD among persons with asthma/COPD. Findings from dose-response analyses suggest that higher levels of exposure to β2AR agonists are not consistently associated with reduced risk of PD among persons with asthma/COPD. The protective association in the highest category of annual exposure to long-acting β2AR agonists may be explained by unmeasured confounding such as disease severity or smoking.
Data Sharing Statement
The datasets generated and/or analysed during the current study are not publicly available due to restrictions by the register maintainers and Finnish legislation but are available from the corresponding author upon reasonable request and with permission of the register maintainers.
Ethics Approval and Informed Consent
Register maintainers have approved the FINPARK study plan. Data were pseudonymized before submission to the research team and study participants were not contacted. Therefore, according to Finnish legislation (including Personal Data Act 23/1999, Act on the Openness of Government Activities 621/1999 and Act on the Secondary Use of Health and Social Data 552/2019, and previous Act on the National Healthcare registers [no official English translation as this is not available] 556/1989), the study has been granted an exemption from requiring ethics approval or informed consent.
Acknowledgments
This paper was presented at the 18th Congress of the European Geriatric Medicine Society as online poster with interim findings. The poster’s abstract was published in Abstracts of the 18th Congress of the European Geriatric Medicine Society. Eur Geriatr Med 13 (Suppl 1), 1–439 (2022). https://doi.org/10.1007/s41999-022-00711-8. An oral presentation of this paper with interim findings was given at the 38th International Conference on Pharmacoepidemiology & Therapeutic Risk Management and at the 14th annual Nordic Pharmacoepidemiological Network meeting in 2022.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Michael J Fox Foundation for Parkinson’s research (grant MJFF-008834 to AMT, which paid salary for AP).
Disclosure
AP reports grant from the Finnish Parkinson Foundation and The Michael J. Fox Foundation for Parkinson’s Research. HK reports grants from Kuopio Area Respiratory Foundation and The Research Foundation of the Pulmonary Diseases and payment for lectures from Boehringer Ingelheim Ltd and MSD Ltd and Stock ownership of Orion Ltd, all outside the submitted work. JT reports grant from Janssen-Cilag paid to his employing institution, Karolinska Institutet. JT is also a consultant to HLS Therapeutics and WebMed Global and has received lecture fees from Janssen-Cilag and Otsuka; all outside of the submitted work. AMT reports grant from the Michael J Fox Foundation for Parkinson’s research (grant MJFF-008834 which paid salary for AP) related to this manuscript. AMT also reports research grant from Amgen, paid through the institution she is employed at, outside of the submitted manuscript. SH has got lecture fees from Eisai Ltd. MT and MK have no conflicts of interest to declare for this work.
References
1. Mittal S, Bjørnevik K, Im DS, et al. β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease. Science. 2017;357(6354):891–898. doi:10.1126/science.aaf3934
2. Searles Nielsen S, Gross A, Camacho-Soto A, Willis AW, Racette BA. β2-adrenoreceptor medications and risk of Parkinson disease. Ann Neurol. 2018;84(5):683–693. doi:10.1002/ana.25341
3. Hopfner F, Höglinger GU, Kuhlenbäumer G, et al. β-adrenoreceptors and the risk of Parkinson’s disease. Lancet Neurol. 2020;19(3):247–254.
4. Gronich N, Abernethy DR, Auriel E, Lavi I, Rennert G, Saliba W. β2-adrenoceptor agonists and antagonists and risk of Parkinson’s disease. Mov Disord off J Mov Disord Soc. 2018;33(9):1465–1471. doi:10.1002/mds.108
5. Chen W, Sadatsafavi M, Tavakoli H, Samii A, Etminan M. Effects of β2-adrenergic agonists on risk of Parkinson’s disease in COPD: a population-based study. Pharmacotherapy. 2020;40(5):408–415. doi:10.1002/phar.2383
6. Marras C, Pequeno P, Austin PC, et al. Beta agonists and progression of Parkinson’s disease in older adults: a retrospective cohort study. Mov Disord off J Mov Disord Soc. 2020;35(7):1275–1277. doi:10.1002/mds.28085
7. Hopfner F, Wod M, Höglinger GU, et al. Use of β2-adrenoreceptor agonist and antagonist drugs and risk of Parkinson disease. Neurology. 2019;93(2):e135–e142. doi:10.1212/WNL.0000000000007694
8. Giorgianni F, Ernst P, Dell’Aniello S, Suissa S, Renoux C. β2-agonists and the incidence of Parkinson disease. Am J Epidemiol. 2020;189(8):801–810. doi:10.1093/aje/kwaa012
9. de Germay S, Conte C, Rascol O, Montastruc JL, Lapeyre-Mestre M. β-adrenoceptor drugs and Parkinson’s Disease: a nationwide nested case-control study. CNS Drugs. 2020;34(7):763–772. doi:10.1007/s40263-020-00736-2
10. Belvisi D, Pellicciari R, Fabbrini A, et al. Risk factors of Parkinson disease: simultaneous assessment, interactions, and etiologic subtypes. Neurology. 2020;95(18):e2500–e2508. doi:10.1212/WNL.0000000000010813
11. Courtois É, Nguyen TTH, Fournier A, et al. Identifying protective drugs for Parkinson’s disease in health-care databases using machine learning. Mov Disord off J Mov Disord Soc. 2022;37:2376–2385. doi:10.1002/mds.29205
12. Magistrelli L, Comi C. Beta2-adrenoceptor agonists in Parkinson’s disease and other synucleinopathies. J Neuroimmune Pharmacol off J Soc NeuroImmune Pharmacol. 2020;15(1):74–81. doi:10.1007/s11481-018-09831-0
13. Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–192. doi:10.1038/nri2254
14. Hernán MA, Takkouche B, Caamaño-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52(3):276–284. doi:10.1002/ana.10277
15. Yawn BP, Mintz ML, Doherty DE. GOLD in practice: chronic obstructive pulmonary disease treatment and management in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2021;16:289–299. doi:10.2147/COPD.S222664
16. Hentilä E, Tiihonen M, Taipale H, Hartikainen S, Tolppanen AM. Incidence of antidepressant use among community dwellers with and without Parkinson’s disease - a nationwide cohort study. BMC Geriatr. 2021;21(1):202–206. doi:10.1186/s12877-021-02145-6
17. Joutsa J, Gardberg M, Röyttä M, Kaasinen V. Diagnostic accuracy of parkinsonism syndromes by general neurologists. Parkinsonism Relat Disord. 2014;20(8):840–844. doi:10.1016/j.parkreldis.2014.04.019
18. Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86(6):566–576. doi:10.1212/WNL.0000000000002350
19. Paakinaho A, Karttunen N, Koponen M, et al. Incidence of muscle relaxant use in relation to diagnosis of Parkinson’s disease. Int J Clin Pharm. 2020;42(2):336–340. doi:10.1007/s11096-020-01002-7
20. WHOCC. Definition and general considerations; 2022. Available from: https://www.whocc.no/ddd/definition_and_general_considera/.
21. Cheng CM, Wu YH, Tsai SJ, et al. Risk of developing Parkinson’s disease among patients with asthma: a nationwide longitudinal study. Allergy. 2015;70(12):1605–1612. doi:10.1111/all.12758
22. GINA & GOLD. Diagnosis of diseases of chronic airflow limitation: asthma, COPD and Asthma-COPD Overlap Syndrome (ACOS) 2015; 2015. Available from: https://goldcopd.org/asthma-copd-asthma-copd-overlap-syndrome/.
23. Zeki AA, Schivo M, Chan A, Albertson TE, Louie S. The asthma-COPD overlap syndrome: a common clinical problem in the elderly. J Allergy. 2011;2011:1–10. doi:10.1155/2011/861926
24. Nielsen M, Bårnes CB, Ulrik CS. Clinical characteristics of the asthma-COPD overlap syndrome--a systematic review. Int J Chron Obstruct Pulmon Dis. 2015;10:1443–1454. doi:10.2147/COPD.S85363
25. Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385(9971):899–909. doi:10.1016/S0140-6736(14)60446-3
26. Siroux V, Pin I, Oryszczyn MP, Le Moual N, Kauffmann F. Relationships of active smoking to asthma and asthma severity in the EGEA study. Eur Respir J. 2000;15(3):470–477. doi:10.1034/j.1399-3003.2000.15.08.x
27. Polosa R, Thomson NC. Smoking and asthma: dangerous liaisons. Eur Respir J. 2013;41(3):716–726. doi:10.1183/09031936.00073312
28. Koskela HO, Hyva¨rinen L, Brannan JD, Chan HK, Anderson SD. Responsiveness to three bronchial provocation tests in patients with asthma. Chest. 2003;124(6):2171–2177. doi:10.1378/chest.124.6.2171
29. Kankaanranta H, Harju T, Kilpeläinen M, et al. Diagnosis and pharmacotherapy of stable chronic obstructive pulmonary disease: the finnish guidelines. Basic Clin Pharmacol Toxicol. 2015;116(4):291–307. doi:10.1111/bcpt.12366
30. Kainu A, Rouhos A, Sovijärvi A, Lindqvist A, Sarna S, Lundbäck B. COPD in Helsinki, Finland: socioeconomic status based on occupation has an important impact on prevalence. Scand J Public Health. 2013;41(6):570–578. doi:10.1177/1403494813484554
31. Cloutier MM, Dixon AE, Krishnan JA, Lemanske RF Jr, Pace W, Schatz M. Managing asthma in adolescents and adults: 2020 asthma guideline update from the national asthma education and prevention program. JAMA. 2020;324(22):2301–2317. doi:10.1001/jama.2020.21974
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.