Back to Journals » Drug Design, Development and Therapy » Volume 12
Zoledronate inhibits fibroblasts’ proliferation and activation via targeting TGF-β signaling pathway
Authors Zhao Z, Shen W, Zhu H, Lin L, Jiang G, Zhu Y, Song H, Wu L
Received 21 March 2018
Accepted for publication 6 August 2018
Published 17 September 2018 Volume 2018:12 Pages 3021—3031
DOI https://doi.org/10.2147/DDDT.S168897
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Zichang Zhao,1,2,* Wei Shen,2,* Hanbin Zhu,3,* Lin Lin,4 Gening Jiang,1 Yongzhe Zhu,5 Hongyuan Song,2 Liang Wu1
1Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China; 2Department of Ophthalmology, Changhai Hospital, Second Military Medical University, Shanghai, China; 3Company 11 of Student Brigade, Second Military Medical University, Shanghai, China; 4Department of Regenerative Medicine, Tongji University School of Medicine, Shanghai, China; 5Department of Microbiology, Second Military Medical University, Shanghai, China
*These authors contributed equally to this work
Background: Previous preclinical and clinical studies have demonstrated that zoledronate might inhibit neointimal hyperplasia at least partly by inhibiting the proliferation, adhesion and migration of vascular smooth muscle cells (VSMCs). However, whether zoledronate influences fibroblasts’ proliferation and activation, which also play a key role in neointimal hyperplasia and vascular remodeling, remains largely unknown. In the present study, the effect of zoledronate on fibroblasts was investigated and the underlying molecular mechanisms were examined.
Methods: After treatment with zoledronate, changes in biological behaviors, including the morphology, proliferation, cell-cycle distribution and migration of fibroblasts (NIH3T3 cells), were observed. The expression of α-SMA, TGF-β1 and TGF-β2 and the level of Smad2/3 phosphorylation in cultured fibroblasts were examined by Western blot. In vivo expression of α-SMA and TGF-β1 was assessed by immunohistochemical staining.
Results: It was shown that the typical fibroblast cell morphology was altered after zoledronate exposure. Cultured fibroblasts treated with zoledronate displayed dose-dependent inhibition of cell proliferation due to cell-cycle arrest in the S phase. Cell migration activities were also dose dependently suppressed by zoledronate treatment. Expression of α-SMA in cultured fibroblasts was significantly reduced by zoledronate treatment. Further analysis showed decreased expression of TGF-β1 and α-SMA by periadventitial delivery of zoledronate in the rat carotid balloon-injury model. The expression of TGF-β1 and TGF-β2 and the phosphorylation of Smad2/3 in cultured fibroblasts were significantly inhibited by zoledronate treatment.
Conclusion: Our findings demonstrated that zoledronate can inhibit the proliferation, migration and activation of fibroblasts via the TGF-β signaling pathway and revealed a novel mechanism of zoledronate action against neointimal hyperplasia.
Keywords: zoledronate, fibroblasts, neointimal hyperplasia, TGF-β pathway
Introduction
Bisphosphonates, which are stable analogs of inorganic pyrophosphate, have been widely used in the treatment of bone metastasis and osteoporosis.1,2 They have been reported to inhibit the development of experimental neointimal hyperplasia due to reduced local inflammation by transient systemic inactivation of monocytes and macrophages.3 However, our previous study demonstrated that zoledronate, a third-generation bisphosphonate, could directly inhibit the proliferation, adhesion and migration of vascular smooth muscle cells (VSMCs).4 We further discovered that both systemic and periadventitial delivery of zoledronate resulted in a significant inhibition of neointimal hyperplasia in the rat carotid balloon-injury model.5
As the predominant resident population of the adventitia, fibroblasts are able to proliferate, migrate, and deposit abundant collagen fibrils and secrete inflammatory cytokines and chemokines when activated. The activated adventitial fibroblasts, also known as myofibroblasts, can be identified by increased expression of contractile proteins, such as α-smooth muscle actin (α-SMA). Abundant evidence indicates that these resident fibroblasts from the adventitia contribute to neointima formation and vascular remodeling.6,7
Since bisphosphonates have been reported to concentrate in adventitia and periadventitial delivery of zoledronate remarkably inhibits neointimal growth in the rat carotid balloon-injury model,5,8 we hypothesized that the effect of zoledronate on neointimal hyperplasia may result from direct inhibition of fibroblasts. Therefore, in this investigation, we analyzed the impact of zoledronate on fibroblasts and examined possible mechanisms.
Materials and methods
Materials
Zoledronate was kindly provided by Novartis Pharma Stein AG (Stein, Switzerland). Antibodies to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), TGF-β1, TGF-β2 and α-SMA were purchased from Proteintech Group (Wuhan, China). Antibodies to Smad2, Smad3, p-Smad2, p-Smad3 and α-SMA (Alexa Fluor® 555) were obtained from Abcam (Shanghai, China). Recombinant human TGF-β1 was purchased from R&D Systems, Inc. (Minneapolis, MN, USA). FBS, DMEM, DAPI, Super Signal West Pico Chemiluminescent Substrate and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The 5-ethynyl-2-deoxyuridine (EdU) cell proliferation kit was purchased from Guangzhou RiboBio (Guangzhou, China). Propidium iodide reagent was obtained from Tianjin Sungene Biotech (Tianjin, China). Cell culture plates and transwell plates with 8-μm pore polycarbonate membranes were purchased from Corning Incorporated (Corning, NY, USA). Rat tail tendon collagen type I (5 mg/mL) in 0.006 N acetic acid was purchased from Shengyou Biotechnology Co., Ltd. (Hangzhou, China).
Cell culture
NIH3T3 mouse fibroblasts were purchased from KeyGen Biotech (Nanjing, China). The cells were cultured in complete DMEM supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. Cultures were maintained at 37°C in a humidified 95% air and 5% CO2 atmosphere. The cells were seeded into the indicated plates for different assays when the cells had attained 80% confluence.
Cell morphology analysis
Approximately 2×104 NIH3T3 cells were seeded in each well of a 24-well plate and incubated in DMEM containing 10% FBS with increasing concentrations (0–20 μM) of zoledronate for 48 hours. The morphologic changes in fibroblasts were analyzed using an inverted phase-contrast microscope (Olympus IX81; Olympus Corporation, Tokyo, Japan). Cells grown in DMEM with 10% FBS alone served as controls.
Cell proliferation assay
EdU assays were used to assess cell proliferation ability according to the methods we previously described.9 Briefly, 6×103 NIH3T3 cells were seeded into each well of 96-well plates and incubated for 8 hours before the experiment. The cells were incubated with different concentrations of zoledronate (0–20 μM) for 48 hours. The culture medium was then removed, and 100 μL of fresh DMEM containing EdU (100 μM) was added to the wells. After a 1.5-hour incubation, the EdU medium was discarded and the fibroblasts were fixed with 4% paraformaldehyde for 20 minutes. The cells were washed twice with glycine for 5 minutes per wash and then stained with Apollo fluorescent azide for 30 minutes and Hoechst stain for 15 minutes. Finally, images were captured using the Olympus IX81 microscope.
Cell-cycle distribution analysis
Approximately 4×104 NIH3T3 cells were seeded into each well of a six-well plate and incubated for 8 hours. The culture medium was replaced with DMEM without FBS to starve the cells for 12 hours. The cells were then incubated with different concentrations of zoledronate (0–20 μM) for 48 hours before the experiment. The cells were collected and fixed using 70% ethanol at 4°C for 10 hours. The ethanol was removed by centrifugation, and the cells were washed twice with PBS before staining. Then, the cells were resuspended in the propidium iodide solution and incubated for 20 minutes in the dark. We analyzed the samples using a flow cytometer (Cell Lab Quanta SC; Beckman Coulter Inc. Brea, CA, USA). The acquired data were further analyzed using ModFit software.
Cell migration assay
The effect of zoledronate on fibroblast migration was measured as described previously using transwell chambers.4 Briefly, NIH3T3 cells were starved overnight. The cells were then digested and resuspended in 0.5% FBS medium containing different concentrations of zoledronate. Approximately 104 cells were seeded into the upper chamber of transwell units, which were placed in 24-well plates, with each well filled with 750 μL cell culture medium with 1% FBS. After incubation for 12 hours, the transwell units were fixed with 4% paraformaldehyde for 20 minutes. Non-migrating cells on the upper surface were gently scraped away, and cells that had migrated to the lower surface of the membrane were stained with 0.1% crystal violet dye for 15 minutes at room temperature. Finally, images of the migrated cells were captured using the Olympus IX81 microscope.
Immunofluorescence assay
NIH3T3 cells were seeded on cover glasses and incubated in the culture medium containing increasing concentrations (0–20 μM) of zoledronate for 48 hours. The cells were washed with PBS once and fixed with 4% paraformaldehyde for 20 minutes at room temperature. Cells were then treated with 0.1% Triton X-100 for 20 minutes and blocked with 5% BSA for 1 hour. The cells were incubated with α-SMA antibody for 1 hour and DAPI for 20 minutes. Finally, images were obtained using the Olympus IX81 microscope.
Western blot
Total protein was extracted using a modified buffer with 0.5% sodium dodecyl sulfate (SDS) containing a protease inhibitor cocktail (Sigma-Aldrich Co., St Louis, MO, USA). Approximately 20 μg of protein were electrophoresed using 10% SDS/polyacrylamide gel electrophoresis (PAGE) gels. The separated proteins were transferred to 0.22 μm polyvinylidene fluoride membranes at 4°C. Nonfat milk (5%) was used to block nonspecific sites for 2 hours at room temperature. The membranes were then incubated with antibodies against GAPDH, α-SMA, TGF-β1, TGF-β2, Smad2, Smad3, p-Smad2 and p-Smad3 at 4°C overnight, followed by HRP-conjugated secondary antibodies for 2 hours. The membranes were washed three times in TBS-T, and bands were identified using enhanced chemiluminescence kits and visualized using the GeneGnome HR Image Capture System (Syngene, Frederick, MD, USA).
Animals
Sprague Dawley rats (male, 300–350 g) were purchased from the Shanghai Laboratory Animal Centre of the Chinese Academy of Sciences. Animals were fed in standard pathogen-free conditions. All surgeries were performed after the rats were anesthetized intraperitoneally with 10% chloral hydrate (3 mL/kg). All animal experiments were performed in agreement with the guidelines of Tongji University School of Medicine on the ethical use of animals and were approved by the Biomedical Research Ethics Committee of Tongji University.
Rat balloon-injury model
Balloon injury of the right common carotid arterial endothelium was performed as described previously.5 Briefly, rats were anesthetized intraperitoneally with 10% chloral hydrate (3 mL/kg). A midline incision was made in the neck, and the right common carotid artery was exposed. A small cut was then made in the external carotid artery, and an embolectomy catheter (Fogarty 2F; Edwards Lifesciences, Irvine, CA, USA) was passed into the common carotid artery. The balloon was inflated with water until slight resistance was met to expand the common carotid artery and then slowly twisted back to the carotid bifurcation. This procedure was done three times to achieve complete denudation of the endothelium, including a mild-to-moderate injury of the inner layers of the media smooth muscle cells. Finally, we removed the balloon and ligated the external carotid artery.
Therapy
A stock solution (10 mM) of zoledronate was prepared in calcium-free PBS as follows. A 1 mL solution of collagen (4.9 mg/mL) and zoledronate (100 μM) was mixed with 980 μL of 5 mg/mL collagen, 10 μL of 10 mM zoledronate and 6 μL of 1N NaOH. The mixture was kept on ice until ready for use.
Twelve rats were divided into a non-treated group and a local zoledronate-treated group. All rats underwent balloon injury on the right carotid artery. The left uninjured carotid arteries from the non-treated group were considered as normal artery samples. For the local zoledronate-treated group, 200 μL of a mixture of rat tail collagen (4.9 mg/mL) and zoledronate (100 μM) was coated around the adventitia of the injured carotid artery.
Immunohistochemical staining
After 21 days of arterial injury, the rats were euthanatized after anesthesia with chloral hydrate. The right (injured) and left (uninjured) segments of the common carotid arteries were removed and fixed in 10% buffered formalin. At 24 hours post fixation, the arterial segments were dehydrated, embedded in paraffin and cut into longitudinal sections. The sections were stained with H&E for morphology and TGF-β1 and α-SMA for immunohistochemistry. Histologic changes were evaluated using an Olympus IX81 microscope.
Statistical analyses
Data are shown as mean±SD values. Statistical significance was determined by one-way ANOVA and Bonferroni’s post hoc test. P<0.05 was considered as statistically significant. All statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
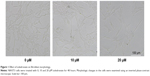
Effect of zoledronate on fibroblast morphology
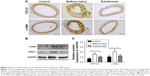
The morphology of untreated control fibroblasts and fibroblasts exposed to zoledronate at 10 μM or 20 μM was observed under a phase-contrast microscope. Untreated cells were more flattened and spread out with variable cytoplasmic shapes. In contrast, zoledronate-treated cells retracted from the substratum and lost contact with neighboring cells and were more spindle shaped with scant cytoplasm extending into two or more long slender cytoplasmic processes (Figure 1).
Effect of zoledronate on fibroblast proliferation
The effect of increasing concentrations (0–20 μM) of zoledronate on the proliferation of fibroblasts was assessed using EdU cell proliferation assays. There were fewer EdU-positive cells in both zoledronate-treated groups compared with those in the untreated group (Figure 2A). Quantitative analysis showed that 10 and 20 μM of zoledronate decreased EdU-positive fibroblasts by 89.7% and 95.0%, respectively, compared with the control group (Figure 2B).
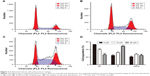
Effect of zoledronate on fibroblast cell-cycle distribution
To further examine whether the decrease in proliferation of fibroblasts reflected cell-cycle arrest, cell-cycle progression was analyzed by propidium iodide staining and flow cytometry. The control fibroblasts revealed a cell-cycle distribution typical of fast proliferating cells (Figure 3A and D), with an average of 53.6% of cells exhibiting a 2n DNA content (G1 phase), 21.3% of cells featuring a 4n DNA content (G2/M phase) and 25.1% of cells revealing a DNA content between 2 and 4n (S phase). After treatment with 10 or 20 μM zoledronate for 48 hours, the G1 phase occupied only 40.06% and 26.50% of the cell cycle, respectively, while cells in the S phase increased to 32.16% and 48.76%, respectively (Figure 3B–D). The results indicated that zoledronate downmodulated fibroblast proliferation via cell-cycle arrest at the S phase.
Effect of zoledronate on fibroblast migration
The effect of zoledronate on the migration of fibroblasts was measured using transwell migration assays. Treatment with increasing concentrations of zoledronate resulted in a dose-dependent inhibition of fibroblast migration (Figure 4A–C). Quantitative data showed that 10 and 20 μM zoledronate suppressed the migration of fibroblasts by ~47% and ~67%, respectively, compared with the untreated group (Figure 4D).
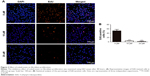
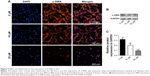
Effect of zoledronate on fibroblast expression of α-SMA in vitro
Activated fibroblasts accelerate the neointimal hyperplasia process, and increased expression of α-SMA is a major marker of these cells. The data from immunofluorescence assays showed that zoledronate inhibited fibroblast expression of α-SMA in a dose-dependent manner (Figure 5A), which was confirmed using Western blot (Figure 5B and C). These in vitro data indicated that zoledronate could inhibit the activation of fibroblasts.
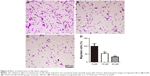
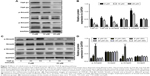
Effect of zoledronate on fibroblast expression of TGF-β1 and α-SMA in vivo
The data from the current investigation were consistent with our previous report that zoledronate could improve the histopathological changes and decrease neointima formation in injured arteries.5 In the non-treated group, the expression of TGF-β1 and α-SMA was significantly higher in injured arteries than in normal artery samples, and periadventitial delivery of zoledronate inhibited the expression of TGF-β1 and α-SMA (Figure 6A). Furthermore, we used Western blot to detect the expression of TGF-β1 and α-SMA. The data were consistent with those of immunohistochemical staining and showed that zoledronate inhibited the expression of TGF-β1 and α-SMA (Figure 6B and C). Thus, zoledronate was associated with a decreased expression of TGF-β1 and α-SMA in injured arteries, consistent with histopathological changes in neointima.
Effect of zoledronate on TGF-β expression and the TGF-β signaling pathway
The TGF-β pathway is one of the major signaling pathways that regulate cell growth, differentiation and morphogenesis, which is usually mediated by the Smad pathway.10 In order to examine the impact of zoledronate on TGF-β expression levels and phosphorylation of Smads in fibroblasts, we analyzed the protein expression levels of TGF-β1, TGF-β2 and p-Smad2/3 protein as well as total levels of Smad2/3 in fibroblasts by Western blot. As shown in Figure 7A and B, protein levels of TGF-β1 and TGF-β2 were downregulated dose dependently in fibroblasts, and phosphorylated forms of Smad2/3 were significantly decreased by zoledronate. Further data showed that zoledronate inhibited the phosphorylation of Smad2/3 induced by TGF-β1 (Figure 7C and D). These data suggested that zoledronate could suppress fibroblast proliferation and activation by inhibiting TGF-β expression levels and the TGF-β/Smad pathway.
Discussion
In the present study, we provide evidence that zoledronate can inhibit the proliferation, migration and activation of fibroblasts via the intervention of the TGF-β signaling pathway. We found that zoledronate induced dramatic morphologic changes in fibroblasts. After 48 hours of zoledronate treatment, cell bodies retracted from the substratum and lost contact with adjacent cells, which appeared to become more spindle shaped. These results confirmed previous published studies showing that zoledronate caused morphologic changes in VSMCs and some cancer cells.4,13–15 These morphological changes might be explained by the fact that zoledronate prevents protein prenylation of a wide variety of small G-proteins, including Ras, Rac, Rab and Rho, which are associated with cytoskeleton organization.11,16
In our study, zoledronate induced significant antiproliferative effects in the range of 10–20 μM. Similar concentrations (typically 10–50 μM) of zoledronate have been utilized elsewhere to produce antiproliferative effects against VSMCs and some cancer cells.4,12,13 However, the peak serum concentration of zoledronate following administration of a 4 mg dose ranges only from 1 to 3 μM.17 The primary concern is that the doses required to inhibit cell growth may not be achieved in vivo using current treatment regimens. This concern has led us to the concept of local drug delivery where higher tissue concentrations of a drug could be achieved while minimizing the likelihood of systemic side effects. Our previous work demonstrated that periadventitial delivery of zoledronate could remarkably improve histopathological changes and decrease neointima formation in injured arteries.5
To further examine whether the decrease in cell proliferation reflected cell-cycle arrest, cell-cycle progression was analyzed by flow cytometry. The results revealed an accumulation of cells in the S phase after zoledronate treatment, which indicated that zoledronate inhibited fibroblast proliferation, at least in part, by arresting the cell cycle at the S phase. This effect has also been observed in previous studies on several tumor cells and oral keratinocytes after zoledronate exposure.18–20 Thus, cell-cycle arrest at the S phase may be a common cellular response induced by zoledronate. However, the detailed mechanism underlying regulation of the cell cycle at the S phase remains to be explored.
It is well known that α-SMA is the marker of activated myofibroblasts, which can proliferate, migrate, deposit extracellular matrix and secrete inflammatory cytokines. To evaluate the effect of zoledronate on fibroblast activation and differentiation, we assessed the expression of α-SMA by immunofluorescence staining and Western blot. The results showed that zoledronate suppressed α-SMA protein levels dose dependently. Furthermore, α-SMA expression in balloon-injured arteries after 21 days decreased significantly with the periadventitial delivery of zoledronate in rats. Therefore, our in vitro and in vivo results indicate that zoledronate may reduce neointima formation by inhibiting the activation and differentiation of fibroblasts.
Transwell assay results showed that zoledronate inhibited the migration of fibroblasts in a dose-dependent manner. Previous studies have similarly shown the inhibitory effects of zoledronate on the migration of various cell types.4,21–23 As mentioned earlier, nitrogen-containing bisphosphonates can prevent protein prenylation of a wide variety of small G-proteins, including Ras, Rac, Rab and Rho, which are associated with cell migration. Our previous study and other studies showed that zoledronate inhibited cell migration by decreasing phosphorylation of focal adhesion kinase,4 suppressing prenylation-dependent RhoA/ROCK signaling pathway21 and downregulating expressions of vascular endothelial growth factor and matrix metalloproteinases.22 In agreement with our results, zoledronate was also indicated to inhibit TGF-β1-induced cell migration through the Smad pathway in human gingival fibroblasts.23
TGF-β is regarded as a key fibrogenic cytokine and can drive fibroblasts to myofibroblast differentiation.24,25 The TGF-β pathway is one of the major signaling pathways that regulates cell growth, differentiation, migration and extracellular matrix production,26,27 which is usually mediated through its transmembrane receptor and subsequent activation of cytoplasmic Smad proteins.28 Meanwhile, the expression of α-SMA is regulated by the TGF-β pathway.29,30 In order to examine the impact of zoledronate on TGF-β expression levels and TGF-β downstream signaling molecules in fibroblasts, we analyzed the protein expression levels of TGF-β1 and TGF-β2 and the level of Smad2/3 phosphorylation in fibroblasts by Western blot. The results showed that protein levels of TGF-β1 and TGF-β2 were downregulated in fibroblasts dose dependently, and the phosphorylation of Smad2/3 was significantly inhibited by zoledronate. These data suggest that zoledronate may suppress fibroblast proliferation, migration and activation by inhibiting TGF-β expression levels and the TGF-β/Smad signaling pathway.
In this work, several aspects of the molecular mechanism of zoledronate action on fibroblasts have been explored. However, in addition to the TGF-β/Smad signaling pathway, other signaling pathways including the MAPK, PI3K and JNK pathways are also involved in fibroblast activation and differentiation.31 Therefore, it will be necessary to evaluate the influence of zoledronate on these pathways as well.
Conclusion
Our findings demonstrate that zoledronate can change the morphology of fibroblasts and inhibit their proliferation, migration and activation via inhibition of TGF-β expression levels and intervention in the TGF-β signaling pathway. These results reveal a novel mechanism of bisphosphonates against neointimal hyperplasia and shed new light on the therapeutic role of zoledronate in the treatment of neointimal hyperplasia.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (81500076, 81700839), the Research Project of Shanghai Municipal Commission of Health and Family Planning (20144Y0200, QNYS15-03) and the Research Foundation for Youth of Second Military Medical University (2016QN13). We thank Luis Angel Hernandez-Arenas for reviewing the manuscript and for providing detailed comments.
Disclosure
The authors report no conflicts of interest in this work.
References
Eriksen EF, Díez-Pérez A, Boonen S. Update on long-term treatment with bisphosphonates for postmenopausal osteoporosis: a systematic review. Bone. 2014;58:126–135. | ||
Holen I, Coleman RE. Bisphosphonates as treatment of bone metastases. Curr Pharm Des. 2010;16(11):1262–1271. | ||
Danenberg HD, Golomb G, Groothuis A, et al. Liposomal alendronate inhibits systemic innate immunity and reduces in-stent neointimal hyperplasia in rabbits. Circulation. 2003;108(22):2798–2804. | ||
Wu L, Zhu L, Shi WH, Zhang J, Ma D, Yu B. Zoledronate inhibits the proliferation, adhesion and migration of vascular smooth muscle cells. Eur J Pharmacol. 2009;602(1):124–131. | ||
Wu L, Zhu L, Shi WH, Yu B, Cai D. Zoledronate inhibits intimal hyperplasia in balloon-injured rat carotid artery. Eur J Vasc Endovasc Surg. 2011;41(2):288–293. | ||
Sartore S, Chiavegato A, Faggin E, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89(12):1111–1121. | ||
Dutzmann J, Koch A, Weisheit S, et al. Sonic hedgehog-dependent activation of adventitial fibroblasts promotes neointima formation. Cardiovasc Res. 2017;113(13):1653–1663. | ||
Ylitalo R, Kalliovalkama J, Wu X, et al. Accumulation of bisphosphonates in human artery and their effects on human and rat arterial function in vitro. Pharmacol Toxicol. 1998;83(3):125–131. | ||
Song H, Wang W, Zhao P, Qi Z, Zhao S. Cuprous oxide nanoparticles inhibit angiogenesis via down regulation of VEGFR2 expression. Nanoscale. 2014;6(6):3206–3216. | ||
Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. | ||
Csépányi-Kömi R, Lévay M, Ligeti E. Small G proteins and their regulators in cellular signalling. Mol Cell Endocrinol. 2012;353(1–2):10–20. | ||
Albadawi H, Haurani MJ, Oklu R, et al. Differential effect of zoledronic acid on human vascular smooth muscle cells. J Surg Res. 2013;182(2):339–346. | ||
Tassone P, Tagliaferri P, Viscomi C, et al. Zoledronic acid induces antiproliferative and apoptotic effects in human pancreatic cancer cells in vitro. Br J Cancer. 2003;88(12):1971–1978. | ||
Denoyelle C, Hong L, Vannier JP, Soria J, Soria C. New insights into the actions of bisphosphonate zoledronic acid in breast cancer cells by dual RhoA-dependent and -independent effects. Br J Cancer. 2003;88(10):1631–1640. | ||
Cheng HL, Lin CW, Yang JS, et al. Zoledronate blocks geranylgeranylation not farnesylation to suppress human osteosarcoma U2OS cells metastasis by EMT via Rho A activation and FAK-inhibited JNK and p38 pathways. Oncotarget. 2016;7(9):9742–9758. | ||
Okamoto S, Jiang Y, Kawamura K, et al. Zoledronic acid induces apoptosis and S-phase arrest in mesothelioma through inhibiting Rab family proteins and topoisomerase II actions. Cell Death Dis. 2014;5:e1517. | ||
Skerjanec A, Berenson J, Hsu C, et al. The pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with varying degrees of renal function. J Clin Pharmacol. 2003;43(2):154–162. | ||
Iguchi T, Miyakawa Y, Saito K, et al. Zoledronate-induced S phase arrest and apoptosis accompanied by DNA damage and activation of the ATM/Chk1/cdc25 pathway in human osteosarcoma cells. Int J Oncol. 2007;31(2):285–291. | ||
Romani AA, Desenzani S, Morganti MM, et al. Zoledronic acid determines S-phase arrest but fails to induce apoptosis in cholangiocarcinoma cells. Biochem Pharmacol. 2009;78(2):133–141. | ||
Ohnuki H, Izumi K, Terada M, et al. Zoledronic acid induces S-phase arrest via a DNA damage response in normal human oral keratinocytes. Arch Oral Biol. 2012;57(7):906–917. | ||
Hasmim M, Bieler G, Rüegg C. Zoledronate inhibits endothelial cell adhesion, migration and survival through the suppression of multiple, prenylation-dependent signaling pathways. J Thromb Haemost. 2007;5(1):166–173. | ||
Li XY, Lin YC, Huang WL, et al. Zoledronic acid inhibits proliferation and impairs migration and invasion through downregulating VEGF and MMPs expression in human nasopharyngeal carcinoma cells. Med Oncol. 2012;29(2):714–720. | ||
Ge C, Li Q, Li X, et al. Zoledronic Acid Inhibits TGF-β1-Induced the Proliferation, Migration and EMT Through Smad Pathway in Human Gingival Fibroblasts. J Biomater Tissue Eng. 2017;7(4):319–326. | ||
Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331(19):1286–1292. | ||
Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. Biochim Biophys Acta. 2013;1832(7):1049–1060. | ||
Duncan MR, Frazier KS, Abramson S, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. Faseb J. 1999;13(13):1774–1786. | ||
Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136(22):3699–3714. | ||
Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114(Pt 24):4359–4369. | ||
Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–111. | ||
Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol. 2003;29(3 Pt 1):397–404. | ||
Maclean J, Pasumarthi KB. Signaling mechanisms regulating fibroblast activation, phenoconversion and fibrosis in the heart. Indian J Biochem Biophys. 2014;51(6):476–482. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.