Back to Journals » Drug Design, Development and Therapy » Volume 10
Zolav®: a new antibiotic for the treatment of acne
Received 15 February 2016
Accepted for publication 25 February 2016
Published 22 March 2016 Volume 2016:10 Pages 1235—1242
DOI https://doi.org/10.2147/DDDT.S106462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Alexa Dinant,1 Ramiz A Boulos2,3
1AXD Pty Ltd, Semaphore Park, 2School of Chemical and Physical Sciences, Flinders University, Bedford Park, 3Boulos & Cooper Pharmaceuticals Pty Ltd, Port Adelaide, SA, Australia
Background: Acne is a prominent skin condition affecting >80% of teenagers and young adults and ~650 million people globally. Isotretinoin, a vitamin A derivative, is currently the standard of care for treatment. However, it has a well-established teratogenic activity, a reason for the development of novel and low-risk treatment options for acne.
Objective: To investigate the effectiveness of Zolav®, a novel antibiotic as a treatment for acne vulgaris.
Materials and methods: Minimum inhibitory concentration of Zolav® against Propionibacterium acnes was determined by following a standard protocol using Mueller-Hinton broth and serial dilutions in a 96-well plate. Cytotoxicity effects on human umbilical vein endothelial cells and lung cells in the presence of Zolav® were investigated by determining the growth inhibition (GI50) concentration, total growth inhibition concentration, and the lethal concentration of 50% (LC50). The tryptophan auxotrophic mutant of Escherichia coli strain, WP2 uvrA (ATCC 49979), was used for the AMES assay with the addition of Zolav® tested for its ability to reverse the mutation and induce bacterial growth. The in vivo effectiveness of Zolav® was tested in a P. acnes mouse intradermal model where the skin at the infection site was removed, homogenized, and subjected to colony-forming unit (CFU) counts.
Results: Susceptibility testing of Zolav® against P. acnes showed a minimum inhibitory concentration of 2 µg/mL against three strains with no cytotoxicity and no mutagenicity observed at the highest concentrations tested, 30 µM and 1,500 µg/plate, respectively. The use of Zolav® at a concentration of 50 µg/mL (q8h) elicited a two-log difference in CFU/g between the treatment group and the control.
Conclusion: This study demonstrates the potential of Zolav® as a novel treatment for acne vulgaris.
Keywords: acne, MscL, Zolav®, benzoyl peroxide, isotretinoin, antibiotic resistance
Corrigendum for this paper has been published
Introduction
Acne is a prominent skin condition manifesting itself mostly in adolescents. It affects ~80% of teenagers and young adults and ~650 million people globally,1 making it one of the top ten most common diseases worldwide. It is caused by the bacterium Propionibacterium acnes that resides within the pilosebaceous follicles, adjacent to the sebaceous glands. Although it is still unknown what triggers it, the overproduction of sebum by these glands, or the blockage of follicles, causes the overgrowth of P. acnes, which causes inflammation in the skin.2 It is often accompanied with a feeling of discomfort and affects the physical appearance and self-esteem of affected individuals.2
Current treatment options for acne include the use of topical and oral treatments, including hormonal agents.3 Benzoyl peroxide is a bactericidal topical agent used for the treatment of mild to moderate acne, but it is ineffective in severe acne.3 Topical antibiotics such as erythromycin and clindamycin are used in more serious acne cases; however, the decreased sensitivity of P. acnes to these antibiotics has limited their use as monotherapies.3 Oral antibiotics have also been used; however, resistance emergence aside,4,5 adverse effects such as photosensitivity associated with the use of doxycycline and skin and teeth pigmentation associated with the use of minocycline have been reported.3 The standard of care for the treatment of acne is the use of isotretinoin, a vitamin A derivative, and although effective, isotretinoin has a number of adverse effects affecting the mucocutaneous, musculoskeletal, and ophthalmic systems as well as the central nervous system.3 Side effects can include changes in mood, suicide ideation, and headaches.3 However, the biggest side effect of isotretinoin comes from its teratogenic effect on the fetus during pregnancy,2,3 and it is therefore a legal requirement to consent to effect nonpregnancy during treatment and at least 4 weeks after treatment. Often, a number of treatment options are combined4 to deliver the best outcome, although this comes at the expense of the treatment cost. In light of the above discussion, there is a need for new and low-risk treatment options for acne vulgaris.
We have recently reported the in silico modeling that led to the discovery of a new class of antibiotics that target the novel mechanosensitive ion channel of large conductance (MscL).6 MscL channels have evolved the ability to translate the mechanical stress across the membrane into an electrochemical response by opening up and allowing the loss of solutes and small proteins from within the bacteria, thereby preventing cell lysis.7 MscL channels are highly conserved in bacterial species and absent in the human genome and are hence a highly sought after target for drug discovery. We have found that by interacting with these channels, this new class of antibiotics can inhibit the growth of bacteria.6 This new class has a number of competitive advantages, including a highly efficient and economical chemical synthesis,8,9 antioxidant properties,7 and, with relevance to a topical therapeutic agent for acne treatment, high chemical stability. Here, we present the effectiveness of Zolav®, a first-generation antibiotic belonging to that class in the treatment of acne, noting that resistance emergence of another antibiotic in the same class is very low.10 We present the in vitro efficacy of Zolav® against a number of P. acnes strains, and we show that Zolav® has low cytotoxicity against human umbilical vein endothelial cells (HUVEC) and lung cells and no mutagenic activity at therapeutic concentration. Finally, we present the effectiveness of topically administered Zolav® in the treatment of acne in a P. acnes mouse intradermal infection model.
Materials and methods
Chemicals and media
Zolav® was provided by Boulos & Cooper Pharmaceuticals Pty Ltd (Port Adelaide, SA, Australia) and stored until needed. Aroclor 1254-induced male Sprague Dawley rat liver S9 was purchased from Molecular Toxicology, Inc. (Boone, NC, USA) (Cat# 11-01L.2). β-nicotinamide adenine dinucleotide phosphate, 2-Aminoanthracene and tryptophan were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Glucose-6-phosphate, glucose, dimethyl sulfoxide (DMSO) and potassium dichromate were purchased from Merck Millipore (Billerica, MA, USA), and magnesium chloride, potassium phosphate dibasic, potassium chloride, sodium dihydrogen phosphate and sodium chloride were purchased from Wako Pure Chemical Industries, Ltd (Osaka, Japan). Bacto agar (DIFCO, Carolina Biological Supply Company, Burlington, NC, USA), bottom agar (containing 1.5% bacto agar, 2% glucose, and 2% Vogel-Bonner salt), nutrient broth (Oxoid Ltd, Hampshire, England), and top agar (0.6% bacto agar and 0.5% NaCl supplemented with 0.025 mM tryptophan) were stored according to the manufacturer’s instructions until needed.
Bacterial cultures
Escherichia coli, WP2 uvrA (ATCC 49979), P. acnes ATCC 6919, ATCC 29399, and ATCC 11827 were obtained from the American Type Culture Collection (Manassas, VA, USA).
Minimum inhibitory concentration testing
The antimicrobial potency of Zolav® was measured using the in vitro broth microdilution assay under assay conditions described by the Clinical and Laboratory Standards Institute. In this assay, the minimum inhibitory concentration (MIC) is defined as the lowest concentration of an agent that completely inhibits visible growth in vitro of the microorganism. The test substance was dissolved in 100% DMSO, suspended completely by sonication or vortexing, and diluted by twofold serial titrations in the same vehicle, for a total of eleven test concentrations. A 2 μL aliquot of each dilution was added to 198 μL of broth medium seeded with the organism suspension in wells of a 96-well plate (bacterial count: 2–8×105 CFU/mL final). The final volume was 200 μL in each well, and the final DMSO concentration was 1%. Following incubation, the test plates were visually examined and wells were scored for growth or complete growth inhibition to define the MIC. Each test substance was evaluated in duplicate, and the results presented are the duplicate test values. Vehicle control and clindamycin were used as blank and positive controls, respectively.
Cytotoxicity testing
HUVEC cells (ATCC CRL-1730), A549 human lung carcinoma cells (ATCC CCL-185), and NCI-H460 cells human lung carcinoma (ATCC HTB-177) (1.5×104/mL in growth medium) were placed in 96-well plates in an atmosphere of 5% CO2 at 37°C for overnight incubation. Zolav® was screened at 30 μM, 3 μM, 0.3 μM, 0.03 μM, and 0.003 μM. Staurosporine was used as a control. Zolav® and/or vehicle was then added, and the plates were incubated for an additional 72 hours. After 72-hour incubation, luminescence was measured using a microplate reader. Growth inhibition (GI50) (concentration that produces 50% growth inhibition), total growth inhibition or cytostatic effect, and lethal concentration of 50% (LC50) (concentration that produces 50% growth, a cytotoxicity parameter) represent cell growth with 50% reduction of the vehicle-treated control (T72), equal to the cell growth of the beginning cell density (T0), and a loss of cells of 50% relative to the beginning cell density (T0), respectively. The cell lines followed ATCC’s regulations and the use of cell lines for in vivo studies were monitored under IACUC (Institutional Animal Care and Use Committee) and IBC (Institutional Biosafety Committee).
AMES test
The tryptophan auxotrophic mutant of E. coli strain, WP2 uvrA (ATCC 49979), was used. The test strain was obtained from the frozen working stock vial and thawed at room temperature. A 0.2 mL aliquot was inoculated into 25 mL nutrient broth medium and then incubated at 35°C–37°C with shaking (120 rpm) for 16–18 hours. Zolav® was dissolved in DMSO and diluted, tenfold, to obtain four stock concentrations of 15,000 μg/mL, 1,500 μg/mL, 150 μg/mL, and 15 μg/mL. Rat liver microsome enzyme homogenate (S9) mixture was prepared, containing 8 mM MgCl2, 33 mM KCl, 4 mM β-nicotinamide adenine dinucleotide phosphate, 5 mM glucose-6-phosphate, 100 mM NaH2PO4 (pH 7.4), and 4% (v/v) Aroclor 1254-induced male rat liver microsome enzyme homogenate (S9). Stock test compound solution, 0.1 mL, was combined with strain culture, 0.1 mL, and with 0.5 mL rat liver enzyme homogenate (S9) mixture or 0.5 mL phosphate buffer saline and then incubated at 35°C–37°C with shaking (120 rpm) for 20 minutes. Molten top agar (2 mL, containing 0.025 mM tryptophan) was added, and the mixture was poured on to the surface of a minimal glucose agar plate (30 mL bottom agar per petri dish) to obtain final test substance concentrations of 1,500 μg/plate, 150 μg/plate, 15 μg/plate, and 1.5 μg/plate. The plates were incubated at 37°C for 72 hours, and then the numbers of revertant colonies were counted. Treatments resulting in a ≥ threefold increase in revertant colonies relative to vehicle control are considered mutagenic. Treatments that reduce the colony count to ≤50% of vehicle control are considered cytotoxic. Assays were performed in triplicate.
Animals
Female BALB/c mice, ordered from Harlan Sprague Dawley Inc. (Indianapolis, IN, USA) weighing 17–19 g, were acclimated to housing conditions for 4 days prior to bacterial challenge. Only animals deemed healthy and fully immunocompetent were included in this study. Cages were prepared with three or five mice per cage. The animals were fed Teklad Global Rodent Diet (Harlan) and water ad libitum. Mice were housed in static cages with Teklad 1/8″ corn cob bedding inside bioBubble® Clean Rooms that provide high-efficiency particulate air (HEPA) filtered air into the bubble environment at 100 complete air changes per hour. All treatments and infectious challenges were carried out in the bioBubble environment. The environment was controlled to a temperature of ~25°C and a humidity of 50%. Treatment groups were identified by cage card. All procedures carried out in this experiment were conducted in compliance with all the laws, regulations, and guidelines of the National Institutes of Health and with the approval of the TransPharm Animal Care and Use Committee.
Skin preparation
On Day 1, each mouse was anesthetized in an isoflurane induction chamber and the lesion site was cleared of hair. An area of ~2.0 cm ×2.0 cm of skin on the dorsal area of each mouse was cleared through use of the depilatory agent Nair®.
Challenge
Cultures were grown for 96 hours at 37°C in an anaerobic atmosphere on tryptic soy agar plates supplemented with 5% sheep blood cells. The culture was aseptically swabbed and transferred to tubes of Brain–Heart Infusion broth and allowed to grow for 72 hours. The cultures were diluted to provide challenge inoculum of ~7.0 log 10 CFU per 50 μL in phosphate buffer saline. On Day 0, each mouse was anesthetized using isoflurane. Each animal on the study was administered 50 μL of the bacterial suspension via intradermal injection in the dorsal area that was previously denuded of hair. The final CFU count from the challenge suspension determined that 5.6 log 10 CFU per mouse were delivered.
Formulation and dosing
Zolav® in the aqueous form was provided by Boulos & Cooper Pharmaceuticals Pty Ltd. Treatments were administered via topical application in a dose volume of 50 μL and spread evenly over the infection site. Treatments were administered at 2 hours, 10 hours, 18 hours, 26 hours, 34 hours, 42 hours, 50 hours, 58 hours, 66 hours, 74 hours, 82 hours, and 90 hours post-challenge. At the conclusion of the study, mice were humanely euthanized and the skin was aseptically removed from the infection site. After 2 hours postinfection, mice were sacrificed for determining the CFU/g baseline burden. Skin samples from mice were placed in homogenation vials with 2.0 mL tryptic soy broth, weighed, and homogenized using a mini-bead beater. The homogenate was serially diluted and plated anaerobically on tryptic soy agar plates for enumeration of CFUs per gram of skin tissue.
Results and discussion
MIC testing revealed that Zolav® at a concentration of 2 μg/mL inhibited the growth of three different strains of P. acnes (Table 1). This is a clinically significant result given that any successful treatment option for acne must show activity against the different strains of P. acnes, in light of different strains inducing pathogenicity. For comparison, clindamycin has shown an MIC of 0.063 μg/mL against the three strains, while gentamycin, ofloxacin, and vancomycin have shown MICs of 30 μg/mL, 1 μg/mL, and 1 μg/mL, respectively, against P. acnes ATCC 6919.
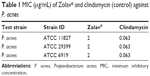  | Table 1 MIC (μg/mL) of Zolav® and clindamycin (control) against P. acnes |
Acne can affect the back, neck, and the T-zone area of affected individuals, and therefore, any topical treatment for acne should have low toxicity primarily to the skin and secondarily to the lungs, if the drug particles are inhaled. HUVEC cells were used here as a model cell line to gauge the cytotoxicity of skin cells, with the results showing no cell death observed in HUVEC and lung cells at the highest concentration tested at 30 μM, at least 7× the MIC of Zolav® (Table 2). For comparison, the natural antibiotic staurosporine, a promiscuous protein kinase inhibitor, has shown cytotoxicity at concentrations significantly lower than those of Zolav®.
The tryptophan auxotrophic mutant of the E. coli strain (WP2 uvrA, ATCC 49979) is incapable of producing tryptophan due to a mutation in the tryptophan locus. Reverse mutations of the strain to tryptophan (Trp+) prototrophy are detected as growth on minimal medium that is deficient for tryptophan. Induction of reversion (positive mutagenicity) is indicated by a threefold increase in the reversion frequency of treated groups versus control groups. The results have shown that no mutagenic activity was observed at the highest concentrations tested of Zolav®, up to 1,500 μg/plate, or the equivalent of 50 μg/mL (Table 3) in the presence of S9, the homogenate from the rat liver. S9 contains the cytosol and microsomes and is used in AMES tests since some compounds may only display a mutagenic activity upon activation via enzymes present in the homogenate. As a positive control, 30 μg/plate of 2-anthramine or the equivalent of 1 μg/mL elicited a significant mutagenic activity with >20 times (mean 754±4 revertant colonies) the number of revertant colonies of E. coli, as the negative control.
Similarly, no mutagenic activity of Zolav® was observed at therapeutic concentrations in the absence of S9 (Table S1). The low cytotoxicity and lack of mutagenicity observed here are similar to those of other first-generation antibiotics belonging to this class.6,7,9
The in vivo effectiveness of Zolav® was tested in a P. acnes mouse intradermal model where the skin at the infection site was removed, homogenized, and subjected to CFU counts. At 2 hours postinfection, mice from the vehicle group were harvested showing a mean baseline bacterial burden of 5.78 log 10 (n=3). The remaining mice were harvested at 96 hours post-challenge. The skin CFU burden in mice in the negative control group has shown a mean bacterial burden of 7.17 log 10 at 96 hours (n=5) (Figure 1; Table S2). The group treated with the Zolav® showed a bacterial burden at 96 hours of 5.07 log 10 (P=0.0252) (n=5), a significant difference (2.10 log 10) to the negative control group and a 0.71 log reduction compared to the mean baseline bacterial burden. None of the mice in the study displayed any acute adverse events associated with the treatments, and none of the mice succumbed to the infection or have shown signs of morbidity, which could be attributed to penetration of the infection into the circulatory system or deep tissue. Consistent with the significant decrease of P. acnes CFU burden in the group treated with Zolav® compared to the untreated group, photographs of mice from the two groups taken after 26 hours and after 50 hours demonstrate a reduction of inflammation, as indicated by the reduced redness, as well as a reduction in lesions in the treatment group (Figure S1). It is worth mentioning that no optimization of the treatment conditions, including doses, frequency of administration, and length of treatment, was attempted in this study. In future work, we will investigate the effect of shorter treatment times, lower doses, and twice a day administration or even once a day administration on the overall outcome of treatment, prior to toxicity assessment and first-in-man clinical trials.
Conclusion
In summary, we have established the in vitro and in vivo efficacy of Zolav® against P. acnes, and we have shown the low cytotoxic nature of the antibiotic and its lack of mutagenic activity. We show that a final concentration of 50 μg/mL is effective in reducing the P. acnes burden in a mouse intradermal infection model. These results show the potential of Zolav® as a novel, affordable, effective, and low-risk treatment option for acne.
Acknowledgments
The authors gratefully acknowledge funding from Boulos & Cooper Pharmaceuticals Pty Ltd for supporting the project. Zolav® is a trademark fully registered in Australia.
Disclosure
RAB is the Chief Executive Officer of Boulos & Cooper Pharmaceuticals Pty Ltd, which owns the intellectual property, and AD is consulting for Boulos & Cooper Pharmaceuticals Pty Ltd. The authors report no other conflicts of interest in this work.
References
Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. | ||
Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2011;379:361–372. | ||
Strauss JS, Krowchuk DP, Leyden JJ, et al; American Academy of Dermatology/American Academy of Dermatology Association. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56:651–663. | ||
Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. Epub 2015 Oct 30. | ||
Leyden JJ, McGinley KJ, Cavalieri S, Webster GF, Mills OH, Kligman AM. Propionibacterium acnes resistance to antibiotics in acne patients. J Am Acad Dermatol. 1983;8:41–45. | ||
Iscla I, Wray R, Blount P, et al. A new antibiotic with potent activity targets MscL. J Antibiot. 2015;68:453–462. | ||
James E, Viola H, Hool L, Eggers PK, Raston CL, Boulos RA. A novel antimicrobial agent reduces oxidative stress in cells. RSC Adv. 2013;3:7277–7281. | ||
Lengkeek NA, Boulos RA, McKinley AJ, Riley TV, Martinac B, Stewart SG. The synthesis of fluorescent DNA intercalator precursors through efficient multiple heck reactions. Aust J Chem. 2011;64:316–323. | ||
Boulos RA, Man NY, Lengkeek NA, et al. Inspiration from old dyes: tris(stilbene) compounds as potent gram-positive antibacterial agents. Chemistry. 2013;19:17980–17988. | ||
Rao S, Prestidge CA, Miesel L, Sweeney D, Shinabarger DL, Boulos RA. Preclinical development of Ramizol®, an antibiotic belonging to a new class, for the treatment of Clostridium difficile colitis. J Antibiot. In press 2016. |
Supplementary materials
  | Table S2 Summary of Propionibacterium acnes intradermal infection model in mice in CFU/g (log10) |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.