Back to Journals » International Journal of Nanomedicine » Volume 19
Zinc Oxide Nanoparticles Exacerbate Epileptic Seizures by Modulating the TLR4-Autophagy Axis
Authors Ke P, Liu J, Chen C , Luo S, Gu H, Gu J, Liu Y, Ma Y, Meng Y, Hu L, Tian X, Xiao F
Received 23 October 2023
Accepted for publication 16 February 2024
Published 29 February 2024 Volume 2024:19 Pages 2025—2038
DOI https://doi.org/10.2147/IJN.S442623
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Pingyang Ke,1,* Jing Liu,1,2,* Chengzhi Chen,3,* Sen Luo,3,* Huiwen Gu,3,* Juan Gu,4 Yan Liu,1 Yuanlin Ma,1 Yuan Meng,1 Liqin Hu,1 Xin Tian,1 Fei Xiao1
1Department of Neurology, The First Affiliated Hospital of Chongqing Medical University, Chongqing Key Laboratory of Neurology, Chongqing, People’s Republic of China; 2Department of Neurology, Chongqing University Three Gorges Hospital, Chongqing, People’s Republic of China; 3Department of Occupational and Environmental Health, School of Public Health and Management, Chongqing Medical University, Chongqing, People’s Republic of China; 4Key Laboratory of Medical Electrophysiology of Ministry of Education and Medical Electrophysiological Key Laboratory of Sichuan Province, Institute of Cardiovascular Research, Southwest Medical University, Luzhou, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fei Xiao; Xin Tian, Email [email protected]; [email protected]
Background: Zinc oxide nanoparticles (ZnO NPs) has been widely used in various fields and has had an important impact on human public health. In addition, it inevitably damages human health, including neurological diseases. Therefore, this study explored the effect of ZnO NPs on epilepsy.
Methods: The effect of ZnO NPs on epilepsy was observed by behavioral analysis. TLR4 expression and autophagy related pathways were detected by RNA-seq and Western blot. In addition, the cell types of autophagy were detected by immunofluorescence. Further, the electrophysiological changes of ZnO NPs induced autophagy were detected by whole-cell patch-clamp. Finally, the recovery experiment was carried out by TLR4 inhibitor (TAK-242).
Results: We found that ZnO NPs enhanced epilepsy susceptibility and severity. Through RNA-seq analysis and Western blot, it was found that ZnO NPs affected the changes of TLR4 and autophagy related pathways. In addition, we found that ZnO NPs mainly affects autophagy of inhibitory neurons, resulting in excitation/inhibition imbalance. The autophagy and epileptic phenotypes were reversed with TAK-242. In general, ZnO NPs exacerbate epileptic seizures by modulating the TLR4-autophagy axis.
Conclusion: ZnO NPs enhanced the susceptibility and severity of epilepsy. Mechanistically, ZnO NPs affected autophagy by changing the expression of TLR4. In particular, the ZnO NPs mainly affected the synaptic function of inhibitory neuron, leading to excitation/inhibition imbalances.
Keywords: zinc oxide nanoparticles, epilepsy, excitation/inhibition balance, autophage
Introduction
Nanomaterials refer to materials with at least one dimension on the nanometer (10−9 m) scale in their 3D structure.1 Because of the special physical and chemical properties of nanomaterials, they have been used in many fields, such as chemical engineering, ceramics, microelectronics, metrology, electricity, optics and information communication.2 Studies have found that nanotechnology has great application potential in biology and medicine. As technology advances, an increasing number of nanoproducts are being developed.
The wide application of nanometer zinc oxide depends on the promotion and development of nanotechnology, and the annual production of nanometer zinc oxide reaches approximately 10–120 tons.3 Due to the widespread use of ZnO NPs, human exposure to these nanomaterials is inevitable Humans are directly exposed to ZnO NPs mainly through direct inhalation, skin contact, or digestive tract ingestion.4 Previous studies have reported that repeated exposure to ZnO NPs can cause damage to organs and tissues such as the liver, kidney, lung, and nerves.5 In the nervous system, ZnO NPs cross the blood-brain barrier and trigger pathological reactions such as oxidative stress, inflammation, apoptosis, and genotoxicity, leading to neurotoxicity.6–9 Therefore, a toxicity study of ZnO NPs is a prerequisite for nanometer material application and development and is necessary and urgent to solve the ZnO NPs problem in the human public health field.
Epilepsy is a disease caused by abnormal synchronous discharge of neurons, which leads to brain function impairment.10 It is one of the most common and serious nervous system diseases with an incidence of epilepsy is 8.2‰. There are approximately 50 million individuals with epilepsy worldwide. Our country has 10 million individuals with epilepsy.11 The high incidence and associated disability and mortality of epilepsy seriously threaten the health of affected individuals and increase the burden not only on these individuals but also on society.12 Studies on the etiology of epilepsy have confirmed that the interaction of genetic and environmental factors leads to increased susceptibility to seizures.13–18 However, it is not clear whether ZnO NPs affect the occurrence and development of epilepsy.
Here, through behavioral, biochemical, and electrophysiological experiments, we illustrate that in epilepsy, ZnO NPs promote neuronal autophagy by activating TLR4 and autophagy-related pathways, leading to an excitation/inhibition imbalance. For the first time, we elucidate the effect of ZnO NPs on epileptic seizures and the possible underlying mechanism, providing new insights into the influence of ZnO NPs on neurological disorders.
Materials and Methods
Animal Samples
All C57BL/6J mice (aged 6–8 weeks and weighing 20–25 g) were purchased from the Laboratory Animal Center of Chongqing Medical University. C57BL/6J mice were kept in a specific pathogen-free (SPF) environment with a temperature of 20–26 °C, a relative humidity of 40%-70%, alternating light and dark lighting for 12h/12h, and adequate food and water. All animal experiments were approved by the Ethics Committee of Chongqing Medical University and the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University. The number of animal samples was statistically calculated. In addition, all animal experiments followed the principles of Laboratory Animal - Guidelines for ethical review of animal welfare (GB/T 35892–2018).
Preparation and Dose of ZnO NPs
ZnO NPs, <50 nm particle size, were purchased from Sigma Corporation (USA). ZnO NPs was prepared according to previous literature.19 The ZnO NP was suspended in 2% sibling mouse serum in MilliQ water. The dose was calculated according to the composition table of infant formula (GB 10765–2010), the standards for food and nutrition enhancers (GB14880-2012), and the national standards for food safety in China.20 In the food and nutrition enhancer standard, the baby food ZnO dose was 217 mg/kg. If an infant (10 kg) is fed a bag of milk powder (65 g) in the morning and evening, an infant will consume 2.821 mg/kg of zinc oxide per day. The converted mouse to human equivalent dose ratio was 9.1, so the mice were administered ZnO NPs by gavage at 26 mg/kg. The mice were divided into a vehicle group, a low ZnO NP group (13 mg/kg) and a high ZnO NP group (26 mg/kg). Intragastric administration was performed at regular intervals every morning for 30 days.
Epilepsy Model Construction
After 30 days of intragastric administration, PTZ (10 mg/kg, Sigma-Aldrich, MO, USA) was injected intraperitoneally for 8 consecutive times with an interval of 10 minutes.21 The seizure grade of mice was evaluated according to the Racine score.22 Epilepsy induction in mice was considered successful when seizures reached grade 4 or higher.
In addition, 1.0 nmol KA (1.0 nmol in 50 nl saline, Sigma-Aldrich, St. Louis, MO, USA) was injected into the hippocampus of mice after gavage. The injection position was as follows: dorsal, 2.0 mm; lateral, 1.5 mm; and depth, 1.5 mm. After the completion of the injection, SRSs were monitored by video for 30 days. SRSs (grade 4 and above) were evaluated according to the Racine score. The first SRS and the number of SRSs during the last 7 days of video monitoring were recorded.
RNA-Seq
Total RNA was extracted from the hippocampus of KA mice treated with ZnO NPs using TRIzol reagent (Thermo Fisher, California, USA). mRNA was enriched using magnetic beads with Oligo dT. After mRNA fragmentation, reverse transcription of cDNA was performed according to the SuperScript double-stranded cDNA short kit (Invitrogen, CA). Finally, sequencing analysis was performed on a NovaSeq 6000 sequencer (illumina).
The expression of transcripts was calculated according to the transcripts per million reads (TPM) method. Differential expression analysis was performed using DESeq2 or DEGseq. Goatools and KOBAS were used for Gene Ontology (GO) function of enrichment and KEGG pathway analysis, respectively, to find functional differences.
Western Blot
Hippocampal tissue was lysed and the supernatant was centrifuged to obtain hippocampal protein. Proteins were separated by electrophoresis on SDS-PAGE gels (10%). Proteins were then transferred to 0.45 μm polyvinylidene fluoride (PVDF) membranes by electrophoresis. The membranes were then blocked and incubated with primary antibodies overnight. The next day, imaging was performed after incubation with secondary antibodies. Antibodies against the following proteins were used for Western blot experiments: TLR4 (Abcam, Cat No: ab13556), LC3A/B (Cell Signaling Technology, 4108), P62 (Proteintech, Cat No.: 18,420-1-AP), and beclin1 (Proteintech, Cat No.: 11,306-1-AP).
TLR4 Inhibitor Intervention
The TL4 inhibitor (TAK-242) was purchased from MedChemExpress. Specific interventions were performed according to the previous literature.23 In brief, TAK-242 was dissolved in PBS containing 0.9% DMSO. One hour after the end of each gavage, TAK-242 was intraperitoneally injected into mice (3 mg/kg).
Immunofluorescence
After anesthetizing the mice, the brain tissue was removed with PBS cardiac perfusion and then fixed with 4% paraformaldehyde (PFA). Mouse brain tissues were fixed overnight in 4% PFA and dehydrated in 30% sucrose for 48 h. The brain tissue was embedded in optimal cutting temperature compound (OCT) compound. Brain slices were obtained using a freezing microtome. Transparent slices were incubated with 0.3% Triton X-100 (Beyotime, Shanghai) at 37 °C for 30 min. The brain slices were blocked using goat serum (Boster, Wuhan) for 1 h at 37 °C. Brain slices were incubated overnight with primary antibodies at 4 °C. The next day, brain slices were incubated with secondary antibodies for 1 h at 37 °C. The following primary and secondary antibodies were used: anti-LC3A/B (Cell Signaling Technology, Cat. No. 4108), anti-NeuN (Millipore, NBP192693PE), anti-GFAP (Abcam, ab4648), anti-Iba1 (Abcam, ab5076), anti-Vglut1 (Synaptic Systems, Cat. No. 135 304), anti-VGAT (Synaptic System, Cat. No. 131 011), DyLight 488 goat anti-rabbit IgG (Abbkine, A23220), DyLight 594 goat anti-rabbit IgG (Abbkine, A23420), and goat anti-guinea pig IgG (Abcam, ab150186).
Whole-Cell Patch-Clamp Recording
After mice were anesthetized, brain tissue was removed and cut into 300 μm sections using cryosectioning solution. Then, at 32 °C, artificial cerebrospinal fluid was used for recovery for 30 min. For sEPSC recording, the glass electrode internal fluid contained the following components (in mM): 30 NMG (pH 7.4, 280–290 mOsm), 10 HEPES, CsCl, 100 1 EGTA, 1 MgCl2, 0.5 Na3GTP, 5 MgATP, 12 phosphocreatine, and 100 μM picrotoxin. For sIPSC recordings, the glass electrode internal fluid contained the following components (in mM): 12 mM phosphocreatine (pH 7.25; 280–300 mOsm), 10 HEPES, 100 CsCl, 30 NMG, 1 MgCl2, 5 MgATP, 1 EGTA, 0.5 Na3GTP, 20 μM DNQX and 50 μM D-AP5.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8. A P value of less than 0.05 was considered to indicate statistical significance. The chi-square test was used to analyze the PTZ kindling rate. For other statistical analyses, unpaired t test was used if samples conformed to normal distribution, and an unpaired t test with Welch’s correction was used otherwise. If the sample value was more than 3 times the standard deviation, it was considered as an outlier and was eliminated.
Results
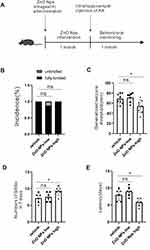
ZnO NPs Increase the Epileptic Seizure Susceptibility and Severity
To investigate the effect of ZnO NPs on susceptibility to epilepsy, pentetrazol (PTZ) and kainic acid (KA) models were established in mice. There are showed on the KA mouse model-related behavioral monitoring time axis (Figure 1A). In the PTZ model, the low and high ZnO NPs groups did not show significant changes in the PTZ kindling rate (Figure 1B). In addition, we found that a high concentration of ZnO NPs reduced the seizure latency of mice in the PTZ group compared with those in the vehicle group, but a low concentration of ZnO NPs did not cause significant changes (Figure 1C). Spontaneous recurrent seizures (SRSs) are an important feature of epilepsy. Compared with the control group, the ZnO NPs-high group exhibited increased the SRSs frequency and decreased latency in KA mice (Figure 1D and E). In general, high-dose ZnO NPs treatment increased epileptic seizure susceptibility and severity. Since only high doses of ZnO NPs caused epileptic behavioral changes, we next studied only high-dose ZnO NPs.
ZnO NPs Induce Autophagic Changes
To further explore the relevant mechanisms by which ZnO NPs affect epilepsy, we performed RNA-sequencing (RNA-seq) omics analysis of KA mice after high-dose ZnO NPs treatment. RNA-seq omics results showed that 872 genes were upregulated and 117 genes were downregulated by ZnO NPs exposure compared with vehicle (Figure 2A and B). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of the altered genes showed that ZnO NPs mainly affected phagocytosis (Figure 2C). In the phagocytosis related pathway, TLR4 was significantly increased (Figure 2D). The expression of TLR4 is closely related to the occurrence and development of epilepsy, especially through autophagy.
ZnO NPs Affect Epilepsy Through TLR4
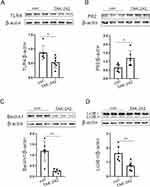
Next, we verified whether ZnO NPs affect epilepsy through TLR4 and the autophagy pathway. We performed Western blotting on protein samples from KA model mice treated with high concentrations of ZnO NPs. Western blot results showed that ZnO NPs-high promoted TLR4 expression compared with the vehicle group (Figure 3A). Moreover, ZnO NPs-high changed the expression of autophagy-related proteins (Figure 3B–D). Furthermore, KA mice treated with high-dose ZnO NPs were treated with TAK-242, a TLR4 inhibitor. TAK-242 reversed the changes of TLR4 and autophagy-related proteins induced by high-dose ZnO NPs, including decreased TLR4, increased P62, decreased Beclin1, and decreased LC3 (Figure 4A–D). Therefore, ZnO NPs affect epilepsy by affecting TLR4 and autophagy.
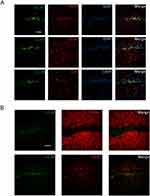
ZnO NPs Influence Inhibitory Neurons Autophagy
Autophagy is closely related to the occurrence and development of epilepsy. Epilepsy is a kind of disease characterized by abnormal synchronization discharge. To further explore what types of cells autophagy occur in the hippocampus, we performed immunofluorescence staining. Immunofluorescence results showed that autophagy mainly occurred in neurons, but not astrocytes or microglia (Figure 5A). Due to the abnormal synchronous firing of neurons caused by excitation/inhibition imbalance, we next costained LC3B with vGlut1 (a marker of excitatory neurons) and VGAT (a marker of inhibitory neurons), and the results showed that LC3B was mainly localized in inhibitory neurons (Figure 5B). In conclusion, ZnO NPs increased autophagy in inhibitory neurons.
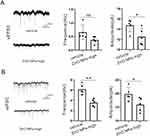
ZnO NPs Affect the Excitability of Neurons via TLR4
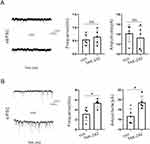
To further examine the effect of ZnO NPs on neuronal excitability, we examined the effect of ZnO NPs on excitatory and inhibitory neurons by whole-cell patch clamp recording. ZnO NPs reduced the amplitude but did not affect the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) (Figure 6A). Moreover, ZnO NPs reduced the frequency and amplitude of spontaneous inhibitory postsynaptic currents (sIPSCs) (Figure 6B). Then we performed the rescue experiment. Compared with the control group (which received high-dose ZnO NP treatment), the TAK-242 group (which received high-dose ZnO NPs + TAK-242) showed that their frequency and amplitude of sEPSCs were not affected (Figure.7A), but a reduced frequency and amplitude of sIPSCs (Figure 7B). Overall, ZnO NPs affect inhibitory neuronal synaptic transmission mainly through TLR4, which may be a potential cause of the excitation/inhibition imbalance.
Discussion
The present study shows that the interaction between gene regulation and environmental factors affects the occurrence and development of epilepsy. Due to the unique physical and chemical properties of ZnO NPs, they are widely used in pharmaceutical, food, packaging, and other fields.24 The widespread use of ZnO NPs in daily life has simultaneously triggered a series of public health problems, bringing unexpected consequences to the environment and human health. ZnO NPs exposure causes liver, kidney and lung damage, skin irritation and abnormal blood coagulation.25 Likewise numerous studies have reported that ZnO NPs cause neurological damage. ZnO NPs enter the central nervous system after lingual instillation and activate glial cells to induce inflammation. After intranasal injection, ZnO NPs are transferred to the brain, where they become especially concentrated in the olfactory bulb, hippocampus, striatum and cerebral cortex, and caused oxidative stress, an inflammatory response and histopathological changes.26 Different sizes and dosages of ZnO NPs cause different degrees of Parkinson’s disease (PD)-like symptoms.27 The neurotoxicity and pathophysiological changes caused by ZnO NPs may be the basis for the occurrence and development of epilepsy. In the present study, we found that ZnO NPs enhanced seizure susceptibility and seizure severity.
At the same time, changes in autophagy have been shown to be one of the pathogeneses of epilepsy. There is a correlation between epilepsy and autophagy, and autophagy related proteins are significantly upregulated in different epilepsy models.28 Through an RNA-seq omics study, we found that ZnO NPs significantly activated autophagy-related pathways. After ZnO NPs entered the brain, Zn concentration increased in all brain regions. And transmission electron microscopy showed that the mitochondria and Golgi apparatus had obvious pathological changes, inducing oxidative damage and inflammation. In particular, the hippocampal region showed different degrees of cell arrangement disorder, cell degeneration and inflammatory cell infiltration.26 These neurotoxicity induced by ZnO NPs are a potential basis for induced autophagy. And it has also been reported that the effect of ZnO NPs on autophagy is crucial.29–31 Interestingly, we found that TLR4 expression was altered in the autophagy pathway. In previous studies, the activation of TLR4 has been reported to affect autophagy through different signaling pathways.32–34 Further studies showed that TLR4 regulated epilepsy through the activation of autophagy and apoptosis in juvenile rats with KA-induced epilepsy.35 This indicates that the TLR4-autophagy pathway is a key pathway affecting epilepsy. Our study showed that the inhibition of TLR4 reversed the effects of ZnO NPs on autophagy. Therefore, the existence of a ZnO NPs-TLR4-autophagy axis provides us with a new understanding of the effect of ZnO NPs on epilepsy, and this is consistent with the effect of ZnO NPs neurotoxicity.
Neurons have unique cellular structures: somata, axons, and dendrites. Autophagy maintains the turnover of RNA, protein, lipids, and organelles, thereby maintaining the homeostasis and survival of neurons.36,37 We found that abnormal autophagy induced by ZnO NPs mainly occurred in neurons, but not in glial cells. Abnormal changes in autophagy in neurons cause changes at the molecular level, cellular level and circuit.38–40 For example, abnormal autophagy alters the expression and function of ion channels, leading to changes in the excitability of neural networks. Further exploration revealed that ZnO NPs caused autophagic changes in inhibitory neurons. Patch-clamp experiments showed that ZnO NPs was also found to mainly affected sIPSCs. However, sEPSCs were slightly affected, which was not consistent with immunofluorescence staining. This finding may be due to tissue and cellular differences. The frequency of sEPSCs/sIPSCs represents the change in the amount of transmitters released by the neuron, while the amplitude represents the change in the amount of presynaptic transmitters released and the change in the number or responsiveness of postsynaptic membrane receptors. The changes in sEPSCs/sIPSCs further suggested that the autophagy induced by ZnO NPs resulted in synaptic structural changes and eventually led to neural network abnormalities. Epilepsy is a disease characterized by an excitation/inhibition imbalance. Aberrant autophagy of inhibitory neurons, which may alter their cellular state, leads to enhanced overall neural excitability. It has previously been reported that activation of autophagy leads to abnormal synaptic transmission in GABAergic neurons.41 Autophagy also regulates GABAA receptor expression.42 This suggests that autophagy can regulate inhibitory synaptic transmission. In addition, autophagy proteins cause different neuronal excitability in excitatory and inhibitory neurons.43 And TLR4 was expressed in both excitatory and inhibitory neurons.44 Therefore, we speculate that ZnO NPs enhances the expression or activity of TLR4 or autophagy protein in inhibitory neurons. However, the reason why ZnO NPs mainly caused inhibitory neuronal autophagic changes needs to be further explored. But the specific mechanism needs to be further explored.
Temporal lobe epilepsy is the most common focal epilepsy, and KA model is the classic temporal lobe epilepsy model. So we choose KA model as the main research object. However, the limitation of this study is that it is not known whether Zno NPs has an effect on other types of epilepsy. In addition, our study lacked support from epileptic patients for its conclusions. All these need further study.
In summary, we describe for the first time the effect of ZnO NPs on epilepsy and show that ZnO NPs alter neural excitability by activating TLR4 and autophagy pathways. This finding provides further a understanding of the effects of ZnO NPs on the nervous system. Our study shows that ZnO NPs can promote epileptic seizure. Therefore, the development of drugs that inhibit TLR4 and autophagy pathways is beneficial for the prevention of epilepsy in people exposed to ZnO NPs for a long time.
Conclusion
We found that ZnO NPs enhanced the susceptibility and severity of epilepsy. Mechanistically, ZnO NPs affected autophagy by changing the expression of TLR4. In particular, the ZnO NPs mainly affected the synaptic function of inhibitory neuron, leading to excitation/inhibition imbalances.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
The animal study protocol was approved by the Ethics Committee of Chongqing Medical University and the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (2020-821). And all animal experiments followed the principles of Laboratory Animal - Guidelines for ethical review of animal welfare (GB/T 35892-2018).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study is supported by National Natural Science Foundation of China (82001378), Young and Middle-aged Medical Excellence Team Program of Chongqing, Chongqing Chief Expert Studio program, Science and Technology Research Program of Chongqing Education Commission (KJQN202200435), Chongqing Talents: Exceptional Young Talents Project (CQYC202005014), China Postdoctoral Science Foundation(2023M730443), Natural Science Foundation of Chongqing (CSTB2023NSCQ-JQX0035, CSTB2022NSCQ-LZX0038, cstc2021ycjh-bgzxm0035), the Joint Project of Chongqing Health Commission and Science and Technology Bureau (2023QNXM009), the Natural Science Foundation of Chongqing (CSTB2023NSCQ-MSX0720).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Diez-Pascual AM, Rahdar A. Functional nanomaterials in biomedicine: current uses and potential applications. ChemMedChem. 2022;17(16):e202200142. doi:10.1002/cmdc.202200142
2. Salata O. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2(1):3. doi:10.1186/1477-3155-2-3
3. Swain PS, Rao SBN, Rajendran D, Dominic G, Selvaraju S. Nano zinc, an alternative to conventional zinc as animal feed supplement: a review. Anim Nutr. 2016;2(3):134–141. doi:10.1016/j.aninu.2016.06.003
4. Iavicoli I, Leso V, Beezhold DH, Shvedova AA. Nanotechnology in agriculture: opportunities, toxicological implications, and occupational risks. Toxicol Appl Pharmacol. 2017;329:96–111. doi:10.1016/j.taap.2017.05.025
5. Esmaeillou M, Moharamnejad M, Hsankhani R, Tehrani AA, Maadi H. Toxicity of ZnO nanoparticles in healthy adult mice. Environ Toxicol Pharmacol. 2013;35(1):67–71. doi:10.1016/j.etap.2012.11.003
6. Tian L, Lin B, Wu L, et al. Neurotoxicity induced by zinc oxide nanoparticles: age-related differences and interaction. Sci Rep. 2015;5:16117. doi:10.1038/srep16117
7. Liang H, Chen A, Lai X, et al. Neuroinflammation is induced by tongue-instilled ZnO nanoparticles via the Ca(2+)-dependent NF-κB and MAPK pathways. Part Fibre Toxicol. 2018;15(1):39. doi:10.1186/s12989-018-0274-0
8. Amara S, Ben-Slama I, Mrad I, et al. Acute exposure to zinc oxide nanoparticles does not affect the cognitive capacity and neurotransmitters levels in adult rats. Nanotoxicology. 2014;8(Suppl 1):208–215. doi:10.3109/17435390.2013.879342
9. Kao YY, Cheng TJ, Yang DM, Wang CT, Chiung YM, Liu PS. Demonstration of an olfactory bulb-brain translocation pathway for ZnO nanoparticles in rodent cells in vitro and in vivo. J Mol Neurosci. 2012;48(2):464–471. doi:10.1007/s12031-012-9756-y
10. Cendes F. Epilepsy care in China and its relevance for other countries. Lancet Neurol. 2021;20(5):333–334. doi:10.1016/s1474-4422(21)00096-x
11. Ding D, Zhou D, Sander JW, Wang W, Li S, Hong Z. Epilepsy in China: major progress in the past two decades. Lancet Neurol Apr. 2021;20(4):316–326. doi:10.1016/s1474-4422(21)00023-5
12. Feigin VL, Nichols E, Alam T. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):459–480. doi:10.1016/s1474-4422(18)30499-x
13. Arrieta O, Palencia G, García-Arenas G, Morales-Espinosa D, Hernández-Pedro N, Sotelo J. Prolonged exposure to lead lowers the threshold of pentylenetetrazole-induced seizures in rats. Epilepsia. 2005;46(10):1599–1602. doi:10.1111/j.1528-1167.2005.00267.x
14. Cakmak S, Dales RE, Vidal CB. Air pollution and hospitalization for epilepsy in Chile. Environ Int. 2010;36(6):501–505. doi:10.1016/j.envint.2010.03.008
15. Xu C, Fan YN, Kan HD, et al. The novel relationship between urban air pollution and epilepsy: a time series study. PLoS One. 2016;11(8):e0161992. doi:10.1371/journal.pone.0161992
16. Hjortebjerg D, Nybo Andersen AM, Ketzel M, Raaschou-Nielsen O, Sørensen M. Exposure to traffic noise and air pollution and risk for febrile seizure: a cohort study. Scand J Work Environ Health. 2018;44(5):539–546. doi:10.5271/sjweh.3724
17. Ma J, Guo A, Wang S, et al. From the lung to the knee joint: toxicity evaluation of carbon black nanoparticles on macrophages and chondrocytes. J Hazard Mater. 2018;353:329–339. doi:10.1016/j.jhazmat.2018.04.025
18. He M, Jiang X, Zou Z, et al. Exposure to carbon black nanoparticles increases seizure susceptibility in male mice. Nanotoxicology. 2020;14(5):595–611. doi:10.1080/17435390.2020.1728412
19. Zhang S, Cheng S, Jiang X, et al. Gut-brain communication in hyperfunction of 5-hydroxytryptamine induced by oral zinc oxide nanoparticles exposure in young mice. Food Chem Toxicol. 2020;135:110906. doi:10.1016/j.fct.2019.110906
20. Wang Y, Yuan L, Yao C, et al. A combined toxicity study of zinc oxide nanoparticles and vitamin C in food additives. Nanoscale. 2014;6(24):15333–15342. doi:10.1039/c4nr05480f
21. Qin R, Cao S, Lyu T, Qi C, Zhang W, Wang Y. CDYL deficiency disrupts neuronal migration and increases susceptibility to epilepsy. Cell Rep. 2017;18(2):380–390. doi:10.1016/j.celrep.2016.12.043
22. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electro Clin Neurophy. 1972;32(3):281–294. doi:10.1016/0013-4694(72)90177-0
23. Ono Y, Maejima Y, Saito M, et al. TAK-242, a specific inhibitor of toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice. Sci Rep. 2020;10(1):694. doi:10.1038/s41598-020-57714-3
24. Meng J, Yang J, Pan T, Qu X, Cui S. ZnO nanoparticles promote the malignant transformation of colorectal epithelial cells in APC(min/+) mice. Environ Int. 2022;158:106923. doi:10.1016/j.envint.2021.106923
25. Fujihara J, Nishimoto N. Review of zinc oxide nanoparticles: toxicokinetics, tissue distribution for various exposure routes, toxicological effects, toxicity mechanism in mammals, and an approach for toxicity reduction. Biol Trace Elem Res. 2023;202(1):9–23. doi:10.1007/s12011-023-03644-w
26. Liu H, Yang H, Fang Y, et al. Neurotoxicity and biomarkers of zinc oxide nanoparticles in main functional brain regions and dopaminergic neurons. Sci Total Environ. 2020;705:135809. doi:10.1016/j.scitotenv.2019.135809
27. Jin M, Li N, Sheng W, et al. Toxicity of different zinc oxide nanomaterials and dose-dependent onset and development of Parkinson’s disease-like symptoms induced by zinc oxide nanorods. Environ Int. 2021;146:106179. doi:10.1016/j.envint.2020.106179
28. Li Q, Han Y, Du J, et al. Alterations of apoptosis and autophagy in developing brain of rats with epilepsy: changes in LC3, P62, Beclin-1 and Bcl-2 levels. Neurosci Res. 2018;130:47–55. doi:10.1016/j.neures.2017.08.004
29. Yang D, Zhang M, Gan Y, et al. Involvement of oxidative stress in ZnO NPs-induced apoptosis and autophagy of mouse GC-1 spg cells. Ecotoxicol Environ Saf. 2020;202:110960. doi:10.1016/j.ecoenv.2020.110960
30. Mawed SA, Marini C, Alagawany M, et al. Zinc Oxide Nanoparticles (ZnO-NPs) Suppress Fertility by Activating Autophagy, Apoptosis, and Oxidative Stress in the Developing Oocytes of Female Zebrafish. Antioxidants (Basel). 2022;11(8):1.
31. Zhang H, Chen F, Li Y, et al. More serious autophagy can be induced by ZnO nanoparticles than single-walled carbon nanotubes in rat tracheal epithelial cells. Environ Toxicol: Int J. 2021;36(2):238–248. doi:10.1002/tox.23029
32. Kim TS, Jin YB, Kim YS, et al. SIRT3 promotes antimycobacterial defenses by coordinating mitochondrial and autophagic functions. Autophagy. 2019;15(8):1356–1375. doi:10.1080/15548627.2019.1582743
33. Kandadi MR, Frankel AE, Ren J. Toll-like receptor 4 knockout protects against anthrax lethal toxin-induced cardiac contractile dysfunction: role of autophagy. Br J Pharmacol. 2012;167(3):612–626. doi:10.1111/j.1476-5381.2012.02040.x
34. Wang S, Song X, Zhang K, et al. Overexpression of toll-like receptor 4 affects autophagy, oxidative stress, and inflammatory responses in monocytes of transgenic sheep. Front Cell Dev Biol. 2020;8:248. doi:10.3389/fcell.2020.00248
35. Wang L, Song LF, Chen XY, et al. MiR-181b inhibits P38/JNK signaling pathway to attenuate autophagy and apoptosis in juvenile rats with kainic acid-induced epilepsy via targeting TLR4. CNS Neurosci Ther. 2019;25(1):112–122. doi:10.1111/cns.12991
36. Lee JA. Neuronal autophagy: a housekeeper or a fighter in neuronal cell survival? Exp Neurobiol. 2012;21(1):1–8. doi:10.5607/en.2012.21.1.1
37. Crawley O, Grill B. Autophagy in axonal and presynaptic development. Curr Opin Neurobiol. 2021;69:139–148. doi:10.1016/j.conb.2021.03.011
38. Bejarano E, Rodríguez-Navarro JA. Autophagy and amino acid metabolism in the brain: implications for epilepsy. Amino Acids. 2015;47(10):2113–2126. doi:10.1007/s00726-014-1822-z
39. McMahon J, Huang X, Yang J, et al. Impaired autophagy in neurons after disinhibition of mammalian target of rapamycin and its contribution to epileptogenesis. J Neurosci. 2012;32(45):15704–15714. doi:10.1523/jneurosci.2392-12.2012
40. Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29(25):8259–8269. doi:10.1523/jneurosci.4179-08.2009
41. Liu J, Ke P, Guo H, et al. Activation of TLR7-mediated autophagy increases epileptic susceptibility via reduced KIF5A-dependent GABA(A) receptor transport in a murine model. Exp Mol Med. 2023;55(6):1159–1173. doi:10.1038/s12276-023-01000-5
42. Sumitomo A, Yukitake H, Hirai K, et al. Ulk2 controls cortical excitatory-inhibitory balance via autophagic regulation of p62 and GABAA receptor trafficking in pyramidal neurons. Hum Mol Genet. 2018;27(18):3165–3176. doi:10.1093/hmg/ddy219
43. Overhoff M, Tellkamp F, Hess S, et al. Autophagy regulates neuronal excitability by controlling cAMP/protein kinase A signaling at the synapse. EMBO j. 2022;41(22):e110963. doi:10.15252/embj.2022110963
44. Giansante G, Marte A, Romei A, et al. Presynaptic L-type ca(2+) channels increase glutamate release probability and excitatory strength in the hippocampus during chronic neuroinflammation. J Neurosci. 2020;40(36):6825–6841. doi:10.1523/jneurosci.2981-19.2020
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.