Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Yttrium-90 Radioembolization as the Major Treatment of Hepatocellular Carcinoma
Authors Yu CY, Huang PH , Tsang LLC , Hsu HW, Lim WX, Weng CC, Huang TL, Hsu CC, Chen CL, Ou HY , Cheng YF
Received 11 August 2022
Accepted for publication 4 January 2023
Published 11 January 2023 Volume 2023:10 Pages 17—26
DOI https://doi.org/10.2147/JHC.S385478
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Manal Hassan
Chun-Yen Yu,1,* Po-Hsun Huang,1,* Leo Leung-Chit Tsang,1 Hsien-Wen Hsu,1 Wei-Xiong Lim,1 Ching-Chun Weng,1 Tung-Liang Huang,1 Chien-Chin Hsu,2 Chao-Long Chen,3 Hsin-You Ou,1 Yu-Fan Cheng1
1Department of Diagnostic Radiology, Kaohsiung Chang Gung Memorial Hospital, and Chang Gung University College of Medicine, Kaohsiung, Taiwan; 2Department of Nuclear Medicine, Kaohsiung Chang Gung Memorial Hospital, and Chang Gung University College of Medicine, Kaohsiung, Taiwan; 3Liver Transplantation Center and Department of Surgery, Kaohsiung Chang Gung Memorial Hospital, and Chang Gung University College of Medicine, Kaohsiung, Taiwan
*These authors contributed equally to this work
Correspondence: Yu-Fan Cheng; Hsin-You Ou, Tel/Fax +88677317123-3027, Email [email protected]; [email protected]
Background: The purpose of this study was to assess the safety and efficacy of Yttrium-90 radioembolization using in unresectable hepatocellular carcinoma.
Methods: From 2017 to 2021, 32 patients with unresectable hepatocellular carcinoma, with mean tumor diameter about 7cm (21 males, 11 females; median age, 57.5 years of age), treated with Yttrium-90 radioembolization using resin microspheres were reviewed at pre-Yttrium-90 and post-Yttrium-90 follow-up. Tumor response was assessed according to the modified Response Evaluation Criteria in Solid Tumors. Outcomes including overall survival and progression-free survival were reported.
Results: Median follow-up was 18 months. At follow-up examinations at 3-, 6-, and 12-months follow-up, the overall survival rates were 94%, 87% and 59%, and the progression-free survival rates were 78%, 64% and 60%, respectively. Complete response, partial response, stable disease, and progressive disease were noted in 7 (21.9%), 14 (43.7%), 4 (12.5%), and 7 (21.9%) patients, respectively. The disease control rate was 78.1%, the objective response rate was 65.6%, and the successful downstage rate was 34.4% (11 of 32). Nine of thirty-two patients underwent resection or transplantation after Yttrium-90 radioembolization with 2-year overall survival being 100%. No serious adverse events occurred after Yttrium-90 treatment. Worse overall survival was related to the larger tumor, higher stage, Eastern Cooperative Oncology Group performance status, and Child-Pugh score. And worse progression-free survival was related to the higher tumor burden, and pre-Yttrium-90 serum α-fetoprotein level > 100.
Conclusion: Yttrium-90 Radioembolization can control hepatocellular carcinoma well even in advanced diseases. Patients successfully downstaging/bridging to resection or transplantation have excellent overall survival.
Keywords: Yttrium-90 radioembolization, selective internal radiation therapy, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, and it represents the third leading cause of cancer death worldwide, and also the leading cause of cancer-related mortality in Taiwan.1,2 Despite advanced screening recommendations, more than one-third of patients present an advanced disease at diagnosis.3,4 While surgical treatments including resection and liver transplantation provide the greatest cure potential and survival, for patients with unresectable tumor or beyond the selection criteria, locoregional therapies (LRT) such as radiofrequency ablation and transarterial chemoembolization (TACE) have various potential roles, including palliation, tumor down-staging, and bridging therapy prior to resection or liver transplantation (LT). Despite the American Association for the Study of Liver Disease (AASLD) does not recommend one form of LRT over another, there is growing evidence of positive clinical outcomes with Yttrium-90 (Y90) radioembolization in the treatment of HCC. It has been reported that Y90 radioembolization can provide significan tly better time to progression (TTP) than conventional transarterial chemoembolization (cTACE) with favorable survival in Barcelona clinic liver cancer stage A-C.5–7 Also, due to prolonged TTP, induction of contralateral hepatic hypertrophy, and lower risk of liver ischemia in the patient with portal vein thrombosis (PVT), Y90 is an effective treatment of HCC for bridging/downstaging for surgery.8,9 In our study, we report our institutional experience of Y90 radioembolization using in unresectable HCC, as the primary or major treatment combined with other local regional and systemic treatment.
Materials and Methods
Patients
This study had institutional review board approval, and all patients provided written informed consent for the treatment. Between February 2017 and October 2021, 32 patients with unresectable HCC received Y90 radioembolization as part of therapy. Inclusion criteria included relatively preserved liver function (Child-Pugh (CP) A-B), platelet count >100,000 c/μL and international normalized ratio (INR) <1.5. Main portal vein thrombosis has been considered the relative contraindication. A retrospective chart review was performed, and imaging and survival outcomes were also evaluated.
Evaluation and Staging
Triphasic contrast-enhanced computed tomography (CT) or contrast-enhanced magnetic resonance imaging (MRI) was performed before Y90 radioembolization.
HCC was diagnosed according to guidelines,3 liver function was assessed by CP class, and tumor staging was according to American Joint Committee on Cancer (AJCC) 8th Edition.10
Y90 Radioembolization
All patients underwent planning simulation using technetium-99m-macroaggregated albumin (Tc-99m-MAA) scintigraphy, which simulates the distribution of Y90 microspheres, to derive the estimated tumor-to-normal liver (T/N) ratio, and simulate the hepatic field of Y-90 radioembolization for the purpose of personalized planning and dosimetry. Angiographic evaluation was performed to identify the vascular anatomy, tumor feeding vessels and to detect aberrant vessels, arterioportal and arteriovenous shunting, and extrahepatic collateral supply which may feed extrahepatic organs, especially the gastrointestinal tract. Selective injection of each tumor’s feeding vessel branches in segment or subsegment was performed if possible.
Tc-99m-MAA was then injected with the delivery catheter in the planning position for Y-90 microsphere infusion, followed by single photon emission computed tomography/computed tomography (SPECT/CT) to assess pulmonary shunt and any unexpected flow to extrahepatic organs. Within one week of the planning simulation, all patients underwent Y90 radioembolization (SIR-Spheres Y90 resin microspheres, Sirtex, Singapore) according to the standardized procedure.11
Dosimetry
Activities were calculated according to the partition model with the aim of delivering of the highest dose to the tumor while limiting the mean absorbed dose to non-tumoral liver not more than 70 Gy in the non-cirrhotic liver, or 40 Gy in the cirrhotic liver. As for lung shunt management, the cut-off values for lung exposure were 30 Gy and 20% for lung dose and lung shunt fraction, respectively.12 The partition model used pre-treatment Tc-99m-MAA SPECT/CT image with semi-automated regions of interest were drawn around the targeted tumors and non-tumoral liver. About one day after the Y90 radioembolization, Y90 bremsstrahlung SPECT/CT was performed to demonstrate the desired distribution of Y90 microspheres.
Follow-Up and Safety
Patients received regular follow up every 2 weeks for the first month, and monthly for 3 months, and then every 3 months. Liver function tests, serum α-fetoprotein (AFP), serum creatinine level, platelet count and INR were assessed. Triphasic contrast-enhanced CT imaging 1.5–3 months after the treatment was used for tumor response assessment according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST).13,14 Afterwards, CT imaging follow-up was performed at 6 month and then every 6 months. Post-Y90 toxicities and the adverse events were evaluated as per the National Cancer Institute Common Toxicity Criteria (CTCAE – Version 5.0).15
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 and R Statistical Software, Version 4.2.1. A P value of less than 0.05 is statistically significant. Baseline Characteristics of patients were calculated using frequency and descriptive analysis. Follow-up time was calculated until the date of death or the last follow-up. Paired t-test was performed for the comparison of pre- and post-Y90 radioembolization tumor number and sizes. Outcomes including overall survival (OS) and progression-free survival (PFS). Time-to-endpoint analyses were estimated using Kaplan–Meier. Univariate and multivariate analyses were performed using a Log rank test and Cox proportional-hazards model, respectively.
Results
Patient Characteristics
A total of 32 patients with unresectable HCC were reviewed retrospectively between February 2017 and October 2021. Baseline Characteristics are listed in Table 1. The median age was 57.5 (range, 31–79); most were male (65.6%, 21 of 32), afflicted hepatitis B (56.3%, 18 of 32), with multiple nodules (81.3%, 26 of 32), Eastern Cooperative Oncology Group (ECOG) performance status 0 (90.6%, 29 of 32) and CP A (93.8%, 30 of 32). There were 3 (9.4%), 11 (34.4%), 15 (46.9%), and 3(9.4%) patients who were AJCC stage T1, T2, T3, and T4, respectively. The 3 cases of AJCC stage T4 was staged due to major branch of the portal vein invasion. The median of number of nodules per patient was 4 [interquartile range (IQR), 3 to 9], with the median tumor size was 7.5cm (IQR, 4.1 to 9.5). PVT was present in 3 patients (9.4%) with Japan’s portal vein tumor thrombus (PVTT) portal vein invasion (VP) classification of portal vein tumor thrombus type II (2 of 3) and type IV (1 of 3, due to thrombus in partial main portal vein and contralateral branch), and tumor burden was more than 50% in 4 patients (12.5%). Cirrhosis was present in 28 (87.5%) patients. Median of the Model For End-Stage Liver Disease (MELD) score was 7 (IQR, 6 to 9). Fourteen (43.8%) patients had AFP level <100, whereas 18 (56.3%) patients exhibited AFP level >100. Before the Y90 radioembolization, 15 patients (45.9%) had previous HCC treatment including 7 (21.9%) cases of LRT, 3 (9.4%) cases of surgical resection, and 5 (15.6%) cases of surgical resection with LRT. The median dose of Y90 (GBq) was 1.4 (IQR, 1.0 to 1.8), tumor absorbed dose (Gy) was 143.24 (IQR, 118.16 to 164.69) and lung shunt fraction was 4.57 (IQR, 3.48 to 7.86). Treatment characteristics are listed in Table 2. The median dose of treatment activity was 1.4 GBq (IQR, 1 to 1.8). And the median tumor absorbed dose was 143.24 GBq (IQR, 118.16 to 164.69).
 |
Table 1 Patient Characteristics |
 |
Table 2 Treatment Characteristics |
Treatment Response
Median follow-up time was 18 months by reverse Kaplan-Meier. At follow-up examinations at 3-, 6-, and 12-months follow-up, the OS rates were 94%, 87% and 59%, and the PFS rates were 78%, 64% and 60%, respectively. The median OS and median PFS were not reached. Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were noted in 7 (21.9%), 14 (43.7%), 4 (12.5%), and 7 (21.9%) patients, respectively. The disease control rate, defined as the sum of CR, PR, and SD, was 78.1%, the objective response rate, defined as the sum of CR and PR, was 65.6%, and the successful downstage rate was 34.4% (11 of 32). Figure 1 shows graphical representation of the treatment response, reduction rate in tumor size, and tumor number and total size before and after Y90 radioembolization of each patient.
Pre- and post-Y90 radioembolization tumor number and sizes comparison are listed in Table 3, which shows significant reduction in tumor number and sizes. The difference in pre- and post-Y90 radioembolization is also listed with mean reduction rate in tumor size being 48.58%.
 |
Table 3 Pre- and Post-Y90 Radioembolization Tumor Number and Sizes Comparison |
After the Y90 radioembolization, 9 of 32 (28.1%) patients received subsequent surgery including LT and surgical resection with 2-year OS, 1-year and 2-year disease-free survival being 100%, 96.9% and 93.8%, respectively (median follow-up time 20.6 months). The median time from Y90 radioembolization to surgery was 114 days.
Factors contributing to OS and PFS are listed in Table 4. Maximum tumor diameter larger than 10cm and higher AJCC stage correlated with worse OS (Figure 2). Meanwhile, higher tumor burden (>50%), and pre-Y90 AFP more than 100 ng/mL, correlated with worse PFS. Nine of thirty-two (28.1%) patients underwent resection (7 patients) or transplantation (2 patients) after Y90 radioembolization and were associated with better OS (Figure 3.).
 |
Table 4 Factors Contributing to OS and PFS (Univariate Analysis) |
 |
Figure 2 Overall Survival of patients with maximum tumor diameter <10cm or ≥10cm. |
 |
Figure 3 Overall Survival of patients with or without subsequent operation. |
Treatment-Related Toxicities
There was 1 patient (3.1%) developed grade 3 radiation pneumonitis. Otherwise, no radiation induced liver disease or other grade 3–4 adverse effects were observed after the treatment. There was no significant difference in the pre-Y90 and post-Y90 liver function tests, AFP and serum creatinine level.
Discussion
Y90 radioembolization, a kind of selective internal radiation therapy (SIRT) with Y90-loaded microspheres injected through the feeding artery, has emerged over the last decades with growing evidence of the safety and efficacy as a LRT for HCC, intrahepatic cholangiocarcinoma, and liver metastases of malignancies including neuroendocrine tumors and colorectal cancer.6,16–18 In HCC treatment, it has been well adopted across different stages, with the goals of the treatment ranging from symptom palliation to complete tumor control, and even ablative therapy for early-stage HCC and bridging/downstaging for curative treatments.19,20
In this study, we reviewed our institutional experience with Y-90 radioembolization using in 32 patients with unresectable HCC. Although most of the patients had advanced stage with T3 and T4 (56.3%, 18 of 32), our study showed promising result with 2-year OS rates 59%, and 2-year PFS rates 60%. The response rate was comparable with our previous study of TACE treatment for HCC which consisted of more early-stage patients,21 with the disease control rate and the objective response rate being 78.1% and 65.6%, respectively. The successful downstaging was achieved in 11 of 32 patients with downstaging rate being 34.4%. As for the predictor of OS and PFS, we found that patients with larger tumor diameter had significantly worse OS. And patients with higher tumor burden, and pre-Y90 AFP more than 100 ng/mL correlated with worse PFS. The above findings might be explained easily as advanced disease, poor patient condition, as well as high pretreatment AFP level may lead to unfavorable outcomes.22
There are two calculation methods commonly used for of Y-90 activity of resin microsphere, including the body surface area (BSA) method and the partition model. The partition model is more precise and personalized, but also more complex than BSA method. In partition model, lungs, tumor and non-tumorous liver are separated to different compartments for radiation dose calculation, and the dose will be adjusted according to the measured T/N ratio and lung shunt fraction.23 In regard to the efficacy dose cut-off of mean tumor absorbed dose in resin Y90, a cut-off of 100–120 Gy is recommended for HCC to yield response. In the meantime, the mean absorbed dose to non-tumoral liver should be limited to below 40 Gy in the cirrhotic liver.12 Although most of our patients had cirrhotic liver (28 of 32), we managed to have most of the patients with mean tumor absorbed dose more than 120 Gy (24 of 32). However, there was 1 patient developed grade 3 radiation pneumonitis in our study, with lung dose and lung shunt fraction being 8.6 Gy and 12.1, respectively.
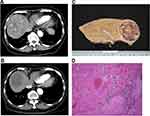
Recent studies showed that patients who received curative treatment after Y90 radioembolization had favorable outcomes.9,24 In our study, 9 of 32 (28.1%) patients received curative treatment including LT and surgical resection after Y90 radioembolization with excellent 2-year OS being 100%. Complete tumor necrosis could be achieved in some of these patients (Figure 4). This finding emphasizes the importance of having subsequent curative treatment after Y90 radioembolization.
The results of our study are limited by the retrospective nature, relatively small sample size and limited follow-up time. Furthermore, the various treatments following Y90 treatment could have affected the survival analysis.
Our study highlights that a favorable long-term outcome and survival can be anticipated with Y90 radioembolization even in advanced HCC, especially in those who received subsequent curative treatment. The large tumor size being the main limitation for OS.
Conclusion
Y90 Radioembolization can control HCC well even in advanced diseases. Patients successfully downstaging/bridging to resection or transplantation have excellent OS.
Ethical Approval and Informed Consent
The institutional review board (IRB) of Kaohsiung Chang-Gung Memorial Hospital approved the protocol for this retrospective study of patient medical records. Because of the retrospective design, the IRB waived informed consent from included patients (IRB:202002180B0). We confirmed that all the data was anonymized or maintained with confidentiality and the study was performed in accordance with the ethical standards of the Declaration of Helsinki.
Acknowledgments
This work was supported by Grant CMRPG8L0421, CMRPG8L0422 from the Chang Gung Memorial Hospital, R.O.C and Grants from health and welfare surcharge of tobacco products, Ministry of Health and Welfare, Taiwan MOHW111-TDU-B-221-014009 (PMRPG3M0091), and MOST 110-2321-B-182A-003 (NZRPG8L0031) from the Ministry of Science and Technology, R.O.C. We appreciated the Biostatistics Center Kaohsiung Cheng Gung Memorial Hospital for statistics work.
Funding
No funding was received for this study.
Disclosure
Hsin-You Ou and Yu-Fan Cheng are co-correspondence authors for this study. The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Shao YY, Wang SY, Lin SM, Diagnosis G, Systemic Therapy G. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2021;120(4):1051–1060. doi:10.1016/j.jfma.2020.10.031
3. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
4. Mittal S, Kanwal F, Ying J, et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: a United States cohort. J Hepatol. 2016;65(6):1148–1154. doi:10.1016/j.jhep.2016.07.025
5. Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(6):1155–63 e2. doi:10.1053/j.gastro.2016.08.029
6. Salem R, Gabr A, Riaz A, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1000-patient 15-year experience. Hepatology. 2018;68(4):1429–1440. doi:10.1002/hep.29691
7. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
8. Tabone M, Calvo A, Russolillo N, et al. Downstaging unresectable hepatocellular carcinoma by radioembolization using 90-yttrium resin microspheres: a single center experience. J Gastrointest Oncol. 2020;11(1):84–90. doi:10.21037/jgo.2019.06.01
9. Gabr A, Kulik L, Mouli S, et al. Liver transplantation following Yttrium-90 radioembolization: 15-year experience in 207-patient cohort. Hepatology. 2021;73(3):998–1010. doi:10.1002/hep.31318
10. Kamarajah SK, Frankel TL, Sonnenday C, Cho CS, Nathan H. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with Hepatocellular Carcinoma (HCC): a Surveillance, Epidemiology, End Results (SEER) analysis. J Surg Oncol. 2018;117(4):644–650. doi:10.1002/jso.24908
11. Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68(1):13–23. doi:10.1016/j.ijrobp.2006.11.060
12. Levillain H, Bagni O, Deroose CM, et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur J Nucl Med Mol Imaging. 2021;48(5):1570–1584. doi:10.1007/s00259-020-05163-5
13. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
14. Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421–430. doi:10.1016/S0168-8278(01)00130-1
15. Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr. 2021;112(1):90–92. doi:10.1016/j.ad.2019.05.009
16. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled Phase 3 trial. Lancet Oncol. 2017;18(12):1624–1636. doi:10.1016/S1470-2045(17)30683-6
17. Levillain H, Duran Derijckere I, Ameye L, et al. Personalised radioembolization improves outcomes in refractory intra-hepatic cholangiocarcinoma: a multicenter study. Eur J Nucl Med Mol Imaging. 2019;46(11):2270–2279. doi:10.1007/s00259-019-04427-z
18. Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28(23):3687–3694. doi:10.1200/JCO.2010.28.5643
19. Saini A, Wallace A, Alzubaidi S, et al. History and evolution of Yttrium-90 radioembolization for hepatocellular carcinoma. J Clin Med. 2019;8(1). doi:10.3390/jcm8010055
20. De la Garza-Ramos C, Overfield CJ, Montazeri SA, et al. Biochemical safety of ablative Yttrium-90 radioembolization for hepatocellular carcinoma as a function of percent liver treated. J Hepatocell Carcinoma. 2021;8:861–870. doi:10.2147/JHC.S319215
21. Lee SY, Ou HY, Yu CY, Huang TL, Tsang LL, Cheng YF. Drug-eluting bead transarterial chemoembolization for hepatocellular carcinoma: does size really matter? Diagn Interv Radiol. 2020;26(3):230–235. doi:10.5152/dir.2019.19261
22. Chaminda SR, Suchintha T, Anuk NM, et al. Pre-treatment alphafeto protein in hepatocellular carcinoma with non-viral aetiology - a prospective study. BMC Gastroenterol. 2017;17(1):142. doi:10.1186/s12876-017-0710-x
23. Kao YH, Tan EH, Ng CE, Goh SW. Clinical implications of the body surface area method versus partition model dosimetry for yttrium-90 radioembolization using resin microspheres: a technical review. Ann Nucl Med. 2011;25(7):455–461. doi:10.1007/s12149-011-0499-6
24. Yim SY, Chun HS, Lee JS, et al. Transarterial radioembolization for unresectable hepatocellular carcinoma: real-life efficacy and safety analysis of Korean patients. Cancers. 2022;14(2). doi:10.3390/cancers14020385
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.