Back to Journals » International Journal of General Medicine » Volume 16
Younger Than 55 Years Old and BRAF V600E Mutation are Risk Factors for Lymph Node Metastasis in Papillary Thyroid Carcinomas ≤1.0 cm but Not in >1.0 cm
Authors Lai Y , Gu Y, Yu M, Deng J
Received 28 February 2023
Accepted for publication 13 April 2023
Published 19 April 2023 Volume 2023:16 Pages 1403—1414
DOI https://doi.org/10.2147/IJGM.S408588
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Yeqian Lai,1,2 Yihua Gu,1,2 Ming Yu,1,2 Jiaqin Deng1,2
1Department of Thyroid Surgery, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Precision Medicine and Clinical Translational Research of Hakka Population, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China
Correspondence: Yeqian Lai, Department of Thyroid Surgery, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, 63 Huangtang Road, Meijiang District, Meizhou, People’s Republic of China, Email [email protected]
Background: Studies on the relationship between BRAF V600E mutation and the clinicopathologic features of papillary thyroid carcinoma (PTC), risk of lymph node metastasis in papillary thyroid microcarcinoma (PTMC) have shown inconsistent results.
Methods: In this retrospective analysis, clinicopathological data of the patients were collected, and molecular testing was done for BRAF V600E mutation. PTC patients are divided into PTC≤ 1.0cm (PTMC) and PTC> 1.0cm, and the relationship between BRAF V600E mutation and clinicopathologic features was analyzed respectively.
Results: Of the 520 PTC patients, 432 (83.1%) were female and 416 (80.0%) were < 55 years old. BRAF V600E mutation was detected in 422 (81.2%) tumour samples of PTC. There was no significant difference in the frequency of BRAF V600E mutation between different age groups. There were 250 (48.1%) patients with PTMC and 270 (51.9%) patients with PTC> 1.0cm. BRAF V600E mutation was significantly associated with bilateral cancer (23.0% vs 4.9%, P=0.005) and lymph node metastasis (61.7% vs 39.0%, P=0.009) in PTMC patients, while BRAF V600E mutation was significantly associated with bilateral cancer (24.9% vs 12.3%, P=0.048) in PTC> 1.0cm patients. Logistic regression analysis showed that, after adjusting for gender, Hashimoto’s thyroiditis and calcification, we found that younger age (< 55 years old) (OR: 2.384, 95% CI: 1.241– 4.579, P=0.009) and BRAF V600E mutation (OR: 2.213, 95% CI: 1.085– 4.512, P=0.029) were significantly associated with lymph node metastasis in PTMC, similar results were not obtained in PTC> 1.0cm.
Conclusion: Younger age (< 55 years old) and BRAF V600E mutation was independent risk factor for lymph node metastasis in PTMC.
Keywords: papillary thyroid carcinoma, papillary thyroid microcarcinoma, BRAF, lymph node metastasis
Introduction
Thyroid cancer is the most common malignancy of the endocrine system, and accounts for about 3% of all malignancies in the human.1 The pathological types of thyroid cancer mainly include papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), medullary thyroid carcinoma (MTC), and anaplastic thyroid cancer (ATC). Among them, PTC is the most common.2 While the incidence of thyroid cancer has varied in different populations around the world over the past few decades, the overall trend of PTC incidence has been increasing.3
PTC originates from the follicular epithelial cells of endoderm origin. The disease usually progresses slowly and the prognosis is relatively good.4 At present, the treatment methods for PTC mainly include surgical total thyroidectomy/subtotal thyroidectomy, radiofrequency ablation of radioactive I131 and thyroid hormone therapy, etc., all of which can achieve varying degrees of effect.5 Studies have found that although PTC is often inert, vascular invasion is rare, but the rate of lymph node metastasis is high, and some PTC cases have the risk of progression and/or recurrence.6,7 Regional lymph node metastasis is present in 40–90% of PTC patients upon diagnosis, and 15% of cases with lymph node metastasis exhibit aggressive tumor behavior, which is reflected in regional invasion, distant metastasis, treatment tolerance, and increased mortality.8 Papillary thyroid microcarcinoma (PTMC) is a PTC with the largest tumor diameter ≤1 cm, and its incidence is about 50% of the total incidence.9 Most PTMC patients have low malignant degree and slow progression, but some of them show highly invasive manifestations, the most common being lymph node metastasis in the lateral neck.10 Surgery is the main treatment for PTC, however, prophylactic lymph node dissection is controversial. In some patients without lymph node metastasis, routine prophylactic lymph node dissection may result in hypoparathyroidism and recurrent laryngeal nerve injury, while pure thyroidectomy in high-risk patients may leave metastatic lymph nodes. In order to selectively perform preventive lymph node dissection in high-risk patients, it is important to identify predictors of lymph node metastasis in patients with PTMC.
Mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway is a highly conserved receptor protein kinase signaling pathway in mammals.11 MAPK/ERK plays an important role in cell proliferation, differentiation, cell motility and apoptosis, is one of the most important oncogenic signaling pathways related to tumors.12 The serine/threonine kinase BRAF is an intracellular effector of the MAPK signaling cascade, which helps to carry signals from the extracellular to the nucleus.13 BRAF is encoded by the proto-oncogene BRAF gene, is a member of the RAF protein kinase family.14 BRAF gene mutation causes abnormal activation of MAPK/ERK signaling pathway, which leads to abnormal cell differentiation and proliferation, and is associated with poor prognosis of thyroid cancer.15 BRAF V600E is the replacement of thymine (T) at site 1799 by adenine (A), resulting in the conversion of valine (V) to glutamic acid (E) at the translated amino acid 600 position. BRAF V600E mutation account for more than 80% of all BRAF gene mutations.16 Studies on the relationship between BRAF V600E mutation and the clinicopathologic features of PTC, risk of lymph node metastasis in PTMC have shown inconsistent results.17 We conducted a study of consecutive case series of PTC from a hospital in Meizhou, China, in order to evaluate the association between BRAF V600E mutation and clinicopathological features of PTC.
Materials and Methods
Study Design
In this retrospective analysis, clinicopathological data of the patients were collected, and molecular testing was done for BRAF V600E mutation. PTC patients are divided into PTC≤1.0cm (PTMC) and PTC>1.0cm, and the relationship between of BRAF V600E mutation and clinicopathologic features was analyzed, respectively.
Subjects
A total of 520 PTC patients were recruited from Meizhou People’s Hospital, between January 2018 and December 2021. Inclusion criteria: (1) the patients had complete demographic and clinical data; (2) histologically confirmed diagnosis met the diagnostic criteria for PTC; (3) the tumor localization, size, disease stage, Hashimoto’s thyroiditis, calcification, lymph node metastasis and other conditions of PTC have been determined through clinical examination. Exclusion criteria: (1) patients without PTC; (2) patients with dysfunction of vital organs; (3) patients with serious cardiovascular and cerebrovascular diseases, autoimmune diseases, pregnancy, etc. This study was supported by the Ethics Committee of the Meizhou People’s Hospital.
BRAF V600E Mutation Analysis
Ten pieces of formalin-fixed and paraffin-embedded (FFPE) slices (5 μm thick per slice) were placed into a 1.5mL EP tube. After FFPE slices were deparaffinized, DNA was extracted by Tissue DNA separation Kit (Amoy Diagnostics, Xiamen, China). BRAF V600E mutation was detected by real-time amplification refractory mutation system (ARMS)-PCR with the BRAF V600E Mutation Fluorescence PCR Diagnostic Kit (Amoy Diagnostics, Xiamen, China). PCR was performed with the following procedure: 95°C for 5 min, followed by 15 cycles (95°C for 25s, 64°C for 20s and 72°C for 20s) and 31 cycles (95°C for 25s, 60°C for 35s and 72°C for 20s). When the internal control signal of the sample to be tested should have an obvious amplification curve and the cycle threshold (Ct) value is 13–21, if the FAM signal has an obvious amplification curve and Ct <28, BRAF V600E was positive, if Ct ≥28, BRAF V600E was negative.
Statistical Analysis
Relevant information and medical records of these participants were collected. Clinical information, including gender, age, tumor localization, maximum tumor diameter, disease stage, Hashimoto’s thyroiditis, and calcification was collected. SPSS Statistical Software Version 21.0 (IBM Inc., State of New York, USA) was used for data analysis. Association between BRAF V600E mutation status and the clinical features of PTC patients was evaluated by Fisher’s exact test. Logistic regression analysis was applied to assess the interactions between BRAF V600E mutation and these covariates in risk assessment of lymph node metastasis of PTMC. P <0.05 was set as statistically significant.
Results
Association Between BRAF V600E Mutation and Clinicopathological Features of PTC Patients
Of the 520 PTC patients, 432 (83.1%) were female and 416 (80.0%) were younger than 55 years of age. There were 178 (34.2%), 232 (44.6%) and 110 (21.2%) patients with left, right and bilateral thyroid cancer, respectively. The main disease stage was I–II (n= 470, 90.4%). There were 64 cases (12.3%) with Hashimoto’s thyroiditis and 176 cases (33.8%) with calcification (Table 1).
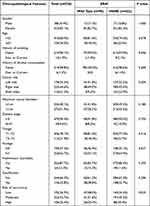 |
Table 1 Association Between BRAF V600E Mutation and Clinicopathological Features of PTC Patients |
BRAF V600E mutation was detected in 422 (81.2%) tumour samples of PTC. There was no significant difference in the frequency of BRAF V600E mutation between different age groups (Figure 1). There was no statistically significant association between BRAF V600E mutation and some clinicopathological features of PTC (such as gender, age, history of smoking, history of alcohol consumption, tumor maximum diameter, disease stage, T-stage, Hashimoto’s thyroiditis, and calcification). In addition, BRAF V600E mutation was significantly associated with bilateral cancer (23.9% vs 9.2%, P=0.004). BRAF V600E mutation was more common in patients with lymph node metastasis (P=0.037). The BRAF V600E mutation was more common in patients with a moderate or high risk of postoperative recurrence (P=0.025) (Table 1).
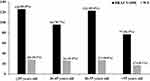 |
Figure 1 The frequency of BRAF V600E mutation between different age groups. |
Association Between BRAF V600E Mutation and Clinicopathological Features of PTMC and PTC Patients with Maximum Tumor Diameter >1.0cm
In this study, there were 250 patients (48.1%) with PTMC. Among them, 208 cases (83.2%) were female and 201 cases (80.4%) were under 55 years old. There were 200 cases (80.0%) of unilateral thyroid cancer and 50 cases (20.0%) of bilateral thyroid cancer; 234 patients (93.6%) with stage I–II; 27 patients (10.8%) with Hashimoto’s thyroiditis, and 69 patients (27.6%) with calcification (Table 2). There was no statistically significant association between BRAF V600E mutation and clinicopathological features of PTMC (such as gender, age, history of smoking, history of alcohol consumption, disease stage, T-stage, Hashimoto’s thyroiditis, and calcification). In addition, BRAF V600E mutation was significantly associated with bilateral cancer (23.0% vs 4.9%, P=0.005), and lymph node metastasis (61.7% vs 39.0%, P=0.009) in PTMC patients (Table 2).
 |
Table 2 Association Between BRAF V600E Mutation and Clinicopathological Features of Papillary Thyroid Microcarcinoma (PTMC) (≤1.0 cm) Patients |
There were 270 (51.9%) PTC patients with maximum tumor diameter >1.0 cm in this study. Among them, 224 cases (83.0%) were female and 215 cases (79.6%) were <55 years old. There were 210 cases (77.8%) with unilateral thyroid cancer and 60 cases (22.2%) with bilateral thyroid cancer; 236 patients (87.4%) with stage I–II; 37 patients (13.7%) with Hashimoto’s thyroiditis, and 107 patients (39.6%) with calcification (Table 3). There was no statistically significant association between BRAF V600E mutation and clinicopathological features of PTMC, including gender, age, history of smoking, history of alcohol consumption, disease stage, T-stage, lymph node metastasis, Hashimoto’s thyroiditis, and calcification). In addition, BRAF V600E mutation was significantly associated with bilateral cancer (24.9% vs 12.3%, P=0.048) in PTC patients with tumor diameter >1.0 cm (Table 3).
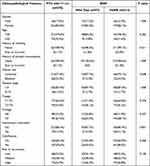 |
Table 3 Association Between BRAF V600E Mutation and Clinicopathological Features of PTC Patients with Maximum Tumor Diameter >1.0cm |
Logistic Regression Analysis of Risk Factors of Lymph Node Metastasis of PTC
To investigate the effect of BRAF V600E mutation, gender, age, bilateral, Hashimoto’s thyroiditis, and calcification on lymph node metastasis in all PTC, PTMC, and PTC patients with >1.0cm, respectively, we performed a univariate analysis to measure the association between these parameters and the presence of lymph node metastasis. The results showed that younger age (<55 years old) (OR: 2.385, 95% CI: 1.261–4.509, P=0.007), bilateral (OR: 2.147, 95% CI: 1.091–4.226, P=0.027), and BRAF V600E mutation (OR: 2.520, 95% CI: 1.268–5.007, P=0.008) were significantly associated with lymph node metastasis in PTMC, but not PTC patients with >1.0cm. Finally, we performed a multivariate regression logistic analysis in order to investigate whether these features could be considered as independent predictors of lymph node metastasis. After adjusting for gender, Hashimoto’s thyroiditis and calcification, we found that younger age (<55 years old) (OR: 2.384, 95% CI: 1.241–4.579, P=0.009) and BRAF V600E mutation (OR: 2.213, 95% CI: 1.085–4.512, P=0.029) were still significantly associated with lymph node metastasis in PTMC (Table 4).
 |
Table 4 Logistic Regression Analysis of Risk Factors of Lymph Node Metastasis |
Discussion
In this study, 520 patients with PTC were collected to analyze the relationship between the BRAF V600E mutation status and the clinicopathologic features of the patients. Risk factors for lymph node metastasis in all PTC, PTMC, and PTC>1.0cm patients were also analyzed respectively. The results showed that younger age (<55 years old) and BRAF V600E mutation was independent risk factor for lymph node metastasis in PTMC. BRAF gene is an important member of RAF protease family. BRAF V600E mutation has been considered as an important molecular marker of PTC,14 but the mutation rate of BRAF V600E in PTC varies in different countries, ranging from about 30% to 90%.18–20 In a study from Korea, the prevalence of the BRAF V600E mutation was 84.0%.21 BRAF V600E mutation prevalence was 38.46% in PTC in a Filipino population.22 The frequency of BRAF V600E mutation increased with age,23 but not showed in this study. BRAF V600E is considered to have a higher specificity in differentiating PTC from benign lesions.24 Several studies have reported on the relationship between BRAF gene mutation status and the clinicopathologic features of PTC, but their conclusions are not entirely consistent.
In this study, BRAF V600E mutation was significantly associated with bilateral cancer and lymph node metastasis in PTC patients, but not gender, age, tumor localization, maximum tumor diameter, disease stage, Hashimoto’s thyroiditis, and calcification. BRAF V600E mutation was significantly associated with the bilaterality of PTC,25 our results are consistent with this study. In addition, no significant association between BRAF V600E mutation and age, gender and tumor localization was detected.26,27 Studies showed that BRAF V600E mutation was associated with lymph node metastasis but not with other clinicopathological features.28,29 BRAF V600E mutation was not associated with central lymph node metastasis in PTC patients.30 In addition, BRAF V600E mutation was associated with age,31,32 advanced tumor stage,33,34 and a reduced prevalence of Hashimoto’s thyroiditis34 in PTC patients, however, similar results were not obtained in this study.
PTC is characterized by early lymph node metastasis, among which occult metastasis is the direct cause of recurrence, which greatly increases the probability of recurrent laryngeal nerve and parathyroid gland injury.35 Surgery is the main treatment for PTC patients, however, prophylactic lymph node dissection is controversial. In some patients without lymph node metastasis, routine prophylactic lymph node dissection may result in hypoparathyroidism and recurrent laryngeal nerve injury, while pure thyroidectomy in high-risk patients may leave metastatic lymph nodes. PTMC is one of the subtypes of PTC, although most of them are less invasive and have a well prognosis, the incidence of lymph node metastasis is still not low. Some studies have confirmed that the incidence of central lymph node metastasis and cervical lymph node metastasis in PTMC patients was 30.2–48.6% and 4.6%–12.2%, respectively.36–38 However, whether there are some factors affecting lymph node metastasis in PTMC has not been fully elucidated.
In this study, BRAF V600E mutation was an independent risk factor for lymph node metastasis in PTMC. BRAF V600E mutation was related to the lymph node metastasis of PTMC.39–42 The BRAF V600E mutation was associated with central lymph node metastasis when the tumor was less than 0.5 cm.43 In addition, BRAF V600E mutation was associated with lymph node metastasis of PTMC, however the proportion of lymph node metastasis in PTMC patients with BRAF V600E mutation and Hashimoto’s thyroiditis is significantly reduced.44 Moreover, BRAF V600E mutation was not associated with lymph node metastasis of PTMC.45–48 Younger age (<55 years old) was an independent risk factor for lymph node metastasis in PTMC in this study. Studies have showed that age ≤30 years old,43 age < 45 years old,36,49,50 < 50 years old51 and < 55 years old52,53 was an independent predictor of central lymph node metastasis. There are also studies that have found that age was not associated with lymph node metastasis of PTMC.54,55 On the contrary, LNM was more common in older patients (age >55 years old,56 age≥45 years old57). In addition, independent risk factors for LNM in patients with PTMC included age <45 years, nodule size ≥6mm, tall cell variant of PTC, extrathyroidal extension, and angioinvasion.58 Bilaterality and gross extrathyroidal extension (ETE) were independent influencing factors of LNM in PTMC patients.59 Calcification,52,60 male,50,53,61 and bilaterality62 have been found to be risk factors for lymph node metastasis, however, similar results were not obtained in this study.
In tumor-node-metastasis (TNM) classification, BRAF V600E is not related to T-stage except for N-stage. There was no correlation between BRAF V600E mutation and T stage or disease stage of PTC. A study has showed that BRAF V600E mutation was associated with T4 stage.31 It suggests that the TNM stage and disease stage of PTC may also be related to other factors, except for the mutation status of BRAF gene. In patients with thyroid carcinoma, the expression of naked cuticle homolog 2 (NKD2) gradually increases with the increase of TNM classification, and the high expression of NKD2 may be related to the progression and poor prognosis of thyroid carcinoma.63 The patients with higher expression levels of programmed death ligand 1 (PD-L1) and phosphoinositide-dependent protein kinase 1 (PDK1) had higher rates of TNM III–IV, lymph node metastasis, and recurrence.64 PD-1 is a cell surface inhibitory receptor, which plays an important physiological role in the maintenance of peripheral tolerance and characteristics of tumor cells.65 PD-L1 can be used as a potential prognostic biomarker for disease recurrence in PTC patients.66 At present, oncologists and pathologists are constantly improving indications, scoring and reporting systems for PD-L1 immunohistochemical tests.67 In addition, study has also shown that TNM stage is related to noncoding RNA.68
In summary, the differences in these results may be related to the differences in sample size and tumor heterogeneity, and the relationship between BRAF V600E and clinical features needs to be further verified with the data from multiple populations and multiple research centers. This study had several limitations. First, as a single-center study taking place in urban Meizhou in China, which is limited by geographical selection bias and small number of study cases. Future studies should include patients from multiple institutions. Second, some patients with thyroid nodules ≤1.0 cm were not biopsied. Therefore, the conclusion that PTMC carrying the BRAF V600E mutation has a higher risk of lymph node metastasis may not fully represent the true situation. Third, this study only tested for the BRAF V600E mutation and did not analyze other mutations. There is evidence suggesting other gene mutations, such as tumor protein p53 (TP53) and telomerase reverse transcriptase (TERT) mutations,69 contribute even more to lymph node metastasis. Future studies should analyze more genetic variants and the interaction between these genetic variations. Lastly, this study could not evaluate the long-term clinical outcomes of PTC patients with the BRAF V600E mutation. In the future, the prognostic value of the BRAF V600E mutation should be further evaluated based on long-term outcomes.
In conclusion, younger age (<55 years old) and BRAF V600E mutation was independent risk factor for lymph node metastasis in PTMC. In other words, lymph node dissection and routine excision of lymph and adipose tissue are important for young PTMC patients with preoperative BRAF V600E mutation. However, the need for prophylactic lymph node dissection in the patients with BRAF V600E mutation and with tumor diameter >1.0cm needs to be reassessed. On the other hand, the results of this study also suggest that molecular detection of the BRAF gene can help guide treatment decisions and identify PTMC at high risk of lymph node metastasis.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of Medicine, Meizhou People’s Hospital. All participants signed informed consent in accordance with the Declaration of Helsinki.
Acknowledgments
The author would like to thank other colleagues whom were not listed in the authorship of Department of Thyroid Surgery, Meizhou People’s Hospital for their helpful comments on the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Guangdong Provincial Key Laboratory of Precision Medicine and Clinical Translation Research of Hakka Population (Grant No.: 2018B030322003); the Science and Technology Program of Meizhou (Grant No.: 2019B0202001).
Disclosure
The authors declare that they have no competing interests.
References
1. Sung H, Ferlay J, Siegel RL. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Kurczyk A, Gawin M. Classification of thyroid tumors based on mass spectrometry imaging of tissue microarrays; a single-pixel approach. Int J Mol Sci. 2020;21(17):6289. doi:10.3390/ijms21176289
3. Miranda-Filho A, Lortet-Tieulent J, Bray F, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. 2021;9(4):225–234. doi:10.1016/S2213-8587(21)00027-9
4. Guo L, Ma YQ, Yao Y. Role of ultrasonographic features and quantified BRAFV600E mutation in lymph node metastasis in Chinese patients with papillary thyroid carcinoma. Sci Rep. 2019;9(1):75. doi:10.1038/s41598-018-36171-z
5. Wang W, Su X, He K, et al. Comparison of the clinicopathologic features and prognosis of bilateral versus unilateral multifocal papillary thyroid cancer: an updated study with more than 2000 consecutive patients. Cancer. 2016;122(2):198–206. doi:10.1002/cncr.29689
6. Dadafarin S, Carnazza M, Islam HK, Moscatello A, Tiwari RK, Geliebter J. Noncoding RNAs in papillary thyroid cancer: interaction with cancer-associated fibroblasts (CAFs) in the tumor microenvironment (TME) and regulators of differentiation and lymph node metastasis. Adv Exp Med Biol. 2021;1350:145–155. doi:10.1007/978-3-030-83282-7_7
7. Pu W, Shi X, Yu P, Zhang M, Liu Z. Single-cell transcriptomic analysis of the tumor ecosystems underlying initiation and progression of papillary thyroid carcinoma. Nat Commun. 2021;12(1):6058. doi:10.1038/s41467-021-26343-3
8. Zhang J, Yang Y, Zhao J, et al. Investigation of BRAF mutation in a series of papillary thyroid carcinoma and matched-lymph node metastasis with ARMS PCR. Pathol Res Pract. 2019;215(4):761–765. doi:10.1016/j.prp.2019.01.006
9. Shafique K, LiVolsi VA, Montone K, Baloch ZW. Papillary thyroid microcarcinoma: reclassification to non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a retrospective clinicopathologic study. Endocr Pathol. 2018;29(4):339–345. doi:10.1007/s12022-018-9546-3
10. Sugitani I, Ito Y, Takeuchi D, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the japan association of endocrine surgery task force on management for papillary thyroid microcarcinoma. Thyroid. 2021;31(2):183–192. doi:10.1089/thy.2020.0330
11. Albert-Gascó H, Ros-Bernal F. MAP/ERK signaling in developing cognitive and emotional function and its effect on pathological and neurodegenerative processes. Int J Mol Sci. 2020;21(12):4471. doi:10.3390/ijms21124471
12. Asl ER, Amini M, Najafi S, et al. Interplay between MAPK/ERK signaling pathway and MicroRNAs: a crucial mechanism regulating cancer cell metabolism and tumor progression. Life Sci. 2021;278:119499. doi:10.1016/j.lfs.2021.119499
13. Dain Md Opo FA, Alsaiari AA, Rahman Molla MH, et al. Identification of novel natural drug candidates against BRAF mutated carcinoma; An integrative in-silico structure-based pharmacophore modeling and virtual screening process. Front Chem. 2022;10:986376. doi:10.3389/fchem.2022.986376
14. Scheffel RS, Dora JM, Maia AL. BRAF mutations in thyroid cancer. Curr Opin Oncol. 2022;34(1):9–18. doi:10.1097/CCO.0000000000000797
15. Cuomo F, Giani C, Cobellis G. The role of the kinase inhibitors in thyroid cancers. Pharmaceutics. 2022;14(5):1040. doi:10.3390/pharmaceutics14051040
16. Almubarak H, Qassem E, Alghofaili L, Alzahrani AS, Karakas B. Non-invasive molecular detection of minimal residual disease in papillary thyroid cancer patients. Front Oncol. 2019;9:1510. doi:10.3389/fonc.2019.01510
17. Silver JA, Bogatchenko M, Pusztaszeri M, et al. BRAF V600E mutation is associated with aggressive features in papillary thyroid carcinomas ≤ 1.5 cm. J Otolaryngol Head Neck Surg. 2021;50(1):63. doi:10.1186/s40463-021-00543-9
18. Chen D, Qi W, Zhang P, et al. Investigation of BRAF V600E detection approaches in papillary thyroid carcinoma. Pathol Res Pract. 2018;214(2):303–307. doi:10.1016/j.prp.2017.09.001
19. Lee SE, Hwang TS, Choi YL, et al. Molecular profiling of papillary thyroid carcinoma in Korea with a high prevalence of BRAF(V600E) mutation. Thyroid. 2017;27(6):802–810. doi:10.1089/thy.2016.0547
20. Kim JK, Seong CY, Bae IE, et al. Comparison of immunohistochemistry and direct sequencing methods for identification of the BRAF(V600E) mutation in papillary thyroid carcinoma. Ann Surg Oncol. 2018;25(6):1775–1781. doi:10.1245/s10434-018-6460-3
21. Kim MJ, Kim JK, Kim GJ, Kang SW. TERT promoter and BRAF V600E mutations in papillary thyroid cancer: a single-institution experience in Korea. Cancers. 2022;14(19):4928. doi:10.3390/cancers14194928
22. Navarro-Locsin CG, Chang AM, Daroy ML, Alfon AC, Andal JJ, Padua PF. Clinical and histopathological profile of BRAF V600E mutation in conventional papillary thyroid carcinoma in a Filipino population. Malays J Pathol. 2016;38(2):141–148. PMID: 27568671.
23. Kure S, Ishino K. Incidence of BRAF V600E mutation in patients with papillary thyroid carcinoma: a single-institution experience. J Int Med Res. 2019;47(11):5560–5572. doi:10.1177/0300060519873481
24. Wu Y, Xu T, Cao X, et al. BRAF (V600E) vs. TIRADS in predicting papillary thyroid cancers in Bethesda system I, III, and V nodules. Cancer Biol Med. 2019;16(1):131–138. doi:10.20892/j.issn.2095-3941.2018.0291
25. Liu Z, Lv T, Xie C, Di Z. BRAF V600E gene mutation is associated with bilateral malignancy of papillary thyroid cancer. Am J Med Sci. 2018;356(2):130–134. doi:10.1016/j.amjms.2018.04.012
26. Celik M, Bulbul BY, Ayturk S, et al. The relation between BRAFV600E mutation and clinicopathological characteristics of papillary thyroid cancer. Med Glas. 2020;17(1):30–34. doi:10.17392/1086-20
27. Chakraborty D, Shakya S, Ballal S, Agarwal S, Bal C. BRAF V600E and TERT promoter mutations in paediatric and young adult papillary thyroid cancer and clinicopathological correlation. J Pediatr Endocrinol Metab. 2020;33(11):1465–1474. doi:10.1515/jpem-2020-0174
28. Gao J, Ma XP, Deng FS, Jiang L, Jia WD, Li M. Associations of the BRAF V600E mutation and PAQR3 protein expression with papillary thyroid carcinoma clinicopathological features. Pathol Oncol Res. 2020;26(3):1833–1841. doi:10.1007/s12253-019-00779-x
29. Sahin S, Daglar G. The effect of BRAF (V600E) mutation on lymph node involvement in papillary thyroid cancer. Turk J Surg. 2020;36(3):249–255. doi:10.47717/turkjsurg.2020.4696
30. Dong SY, Zeng RC, Jin LP, et al. BRAF(V600E) mutation is not associated with central lymph node metastasis in all patients with papillary thyroid cancer: different histological subtypes and preoperative lymph node status should be taken into account. Oncol Lett. 2017;14(4):4122–4134. doi:10.3892/ol.2017.6694
31. Özçelik S, Bircan R, Sarıkaya Ş, et al. BRAF V600E mutation in papillary thyroid cancer is correlated with adverse clinicopathological features but not with iodine exposure. Endokrynol Pol. 2019;70(5):401–408. doi:10.5603/EP.a2019.0025
32. Pessôa-Pereira D, Medeiros M, Lima VMS, et al. Association between BRAF (V600E) mutation and clinicopathological features of papillary thyroid carcinoma: a Brazilian single-centre case series. Arch Endocrinol Metab. 2019;63(2):97–106. doi:10.20945/2359-3997000000120
33. Park AY, Son EJ, Kim JA, et al. Associations of the BRAF(V600E) mutation with sonographic features and clinicopathologic characteristics in a large population with conventional papillary thyroid carcinoma. PLoS One. 2014;9(10):e110868. doi:10.1371/journal.pone.0110868
34. Ye Z, Xia X, Xu P, et al. The prognostic implication of the BRAF V600E mutation in papillary thyroid cancer in a Chinese population. Int J Endocrinol. 2022;2022:6562149. doi:10.1155/2022/6562149
35. Tao Y, Wang F, Shen X, et al. BRAF V600E status sharply differentiates lymph node metastasis-associated mortality risk in papillary thyroid cancer. J Clin Endocrinol Metab. 2021;106(11):3228–3238. doi:10.1210/clinem/dgab286
36. Zheng X, Peng C, Gao M, et al. Risk factors for cervical lymph node metastasis in papillary thyroid microcarcinoma: a study of 1587 patients. Cancer Biol Med. 2019;16(1):121–130. doi:10.20892/j.issn.2095-3941.2018.0125
37. Luo Y, Zhao Y, Chen K, et al. Clinical analysis of cervical lymph node metastasis risk factors in patients with papillary thyroid microcarcinoma. J Endocrinol Invest. 2019;42(2):227–236. doi:10.1007/s40618-018-0908-y
38. Lee YH, Lee YM, Sung TY, et al. Is male gender a prognostic factor for papillary thyroid microcarcinoma? Ann Surg Oncol. 2017;24(7):1958–1964. doi:10.1245/s10434-017-5788-4
39. Zhou C, Li J, Wang Y, Xue S, Zhang Y. Association of BRAF gene and TSHR with cervical lymph node metastasis of papillary thyroid microcarcinoma. Oncol Lett. 2019;17(1):183–194. doi:10.3892/ol.2018.9572
40. Chen BD, Zhang Z, Wang KK, et al. A multivariable model of BRAF(V600E) and ultrasonographic features for predicting the risk of central lymph node metastasis in cN0 papillary thyroid microcarcinoma. Cancer Manag Res. 2019;11:7211–7217. doi:10.2147/CMAR.S199921
41. Jin WX, Ye DR, Sun YH, et al. Prediction of central lymph node metastasis in papillary thyroid microcarcinoma according to clinicopathologic factors and thyroid nodule sonographic features: a case-control study. Cancer Manag Res. 2018;10:3237–3243. doi:10.2147/CMAR.S169741
42. Sun Y, Shi C, Shi T, Yu J, Li Z. Correlation between the BRAF(v600E) gene mutation and factors influencing the prognosis of papillary thyroid microcarcinoma. Int J Clin Exp Med. 2015;8(12):22525–22528. PMID: 26885238.
43. Zhou SL, Guo YP, Zhang L, et al. Predicting factors of central lymph node metastasis and BRAF(V600E) mutation in Chinese population with papillary thyroid carcinoma. World J Surg Oncol. 2021;19(1):211. doi:10.1186/s12957-021-02326-y
44. Issa PP, Omar M, Buti Y, et al. Hashimoto’s thyroiditis minimizes lymph node metastasis in BRAF mutant papillary thyroid carcinomas. Biomedicines. 2022;10(8):2051. doi:10.3390/biomedicines10082051
45. Ren H, Shen Y, Hu D, et al. Co-existence of BRAF(V600E) and TERT promoter mutations in papillary thyroid carcinoma is associated with tumor aggressiveness, but not with lymph node metastasis. Cancer Manag Res. 2018;10:1005–1013. doi:10.2147/CMAR.S159583
46. Jeon MJ, Chun SM, Lee JY, et al. Mutational profile of papillary thyroid microcarcinoma with extensive lymph node metastasis. Endocrine. 2019;64(1):130–138. doi:10.1007/s12020-019-01842-y
47. Dong Y, Wang D, Luo Y, et al. Comprehensive evaluation of risk factors for lymph node metastasis in patients with papillary thyroid carcinoma. Oncol Lett. 2021;21(3):188. doi:10.3892/ol.2021.12449
48. Kim K, Zheng X, Kim JK, et al. The contributing factors for lateral neck lymph node metastasis in papillary thyroid microcarcinoma (PTMC). Endocrine. 2020;69(1):149–156. doi:10.1007/s12020-020-02251-2
49. Ji W, Xie H, Wei B, et al. Relationship between BRAF V600E gene mutation and the clinical and pathologic characteristics of papillary thyroid microcarcinoma. Int J Clin Exp Pathol. 2019;12(9):3492–3499. PMID: 31934195.
50. Yu X, Song X, Sun W, Zhao S, Zhao J, Wang YG. Independent risk factors predicting central lymph node metastasis in papillary thyroid microcarcinoma. Horm Metab Res. 2017;49(3):201–207. doi:10.1055/s-0043-101917
51. Cupisti K, Lehwald N, Anlauf M, et al. Encapsulation status of papillary thyroid microcarcinomas is associated with the risk of lymph node metastases and tumor multifocality. Horm Metab Res. 2014;46(2):138–144. doi:10.1055/s-0033-1361158
52. Liu W, Wang S, Xia X. Risk factor analysis for central lymph node metastasis in papillary thyroid microcarcinoma. Int J Gen Med. 2021;14:9923–9929. doi:10.2147/IJGM.S346143
53. Yin Y, Xu X, Shen L, Zhao W, Diao H, Li C. Influencing factors and cumulative risk analysis of cervical lymph node metastasis of papillary thyroid microcarcinoma. Front Oncol. 2021;11:644645. doi:10.3389/fonc.2021.644645
54. Yang Y, Chen C, Chen Z, et al. Prediction of central compartment lymph node metastasis in papillary thyroid microcarcinoma. Clin Endocrinol. 2014;81(2):282–288. doi:10.1111/cen.12417
55. Ş A, Yazgan Aksoy D, Akın S, Kılıç M, Yetişir F, Bayraktar M. Prediction of central lymph node metastasis in patients with thyroid papillary microcarcinoma. Turk J Med Sci. 2017;47(6):1723–1727. doi:10.3906/sag-1702-99
56. Shi Y, Yang Z, Heng Y. Clinicopathological findings associated with cervical lymph node metastasis in papillary thyroid microcarcinoma: a retrospective study in China. Cancer Control. 2022;29:10732748221084926. doi:10.1177/10732748221084926
57. Zhao W, Chen S, Hou X, Liao Q, Chen G. Predictive factors of lateral lymph node metastasis in papillary thyroid microcarcinoma. Pathol Oncol Res. 2019;25(3):1245–1251. doi:10.1007/s12253-018-0511-8
58. Medas F, Canu GL, Cappellacci F, et al. Predictive factors of lymph node metastasis in patients with papillary microcarcinoma of the thyroid: retrospective analysis on 293 cases. Front Endocrinol. 2020;11:551. doi:10.3389/fendo.2020.00551
59. Parvathareddy SK, Siraj AK, Annaiyappanaidu P, Siraj N. Risk factors for cervical lymph node metastasis in middle eastern papillary thyroid microcarcinoma. J Clin Med. 2022;11(15):4613. doi:10.3390/jcm11154613
60. Wang Y, Nie F, Wang G, Liu T, Dong T, Sun Y. Value of combining clinical factors, conventional ultrasound, and contrast-enhanced ultrasound features in preoperative prediction of central lymph node metastases of different sized papillary thyroid carcinomas. Cancer Manag Res. 2021;13:3403–3415. doi:10.2147/CMAR.S299157
61. Gu JH, Zhao YN, Xie RL, et al. Analysis of risk factors for cervical lymph node metastasis of papillary thyroid microcarcinoma: a study of 268 patients. BMC Endocr Disord. 2019;19(1):124. doi:10.1186/s12902-019-0450-8
62. Zou Q, Ma S, Zhou X. Association of sonographic features and clinicopathologic factors of papillary thyroid microcarcinoma for prevalence of lymph node metastasis: a retrospective analysis. Arch Endocrinol Metab. 2021;64(6):803–809. doi:10.20945/2359-3997000000297
63. Gao Y, Wang Y, Guo R. NKD2 is correlated with the occurrence, progression and prognosis of thyroid carcinoma. Eur J Med Res. 2022;27(1):235. doi:10.1186/s40001-022-00853-2
64. Wang H, Zhang Z, Yan Z, Ma S. PD-L1, PDK-1 and p-Akt are correlated in patients with papillary thyroid carcinoma. Adv Clin Exp Med. 2020;29(7):785–792. doi:10.17219/acem/121518
65. Munari E, Mariotti FR. PD-1/PD-L1 in cancer: pathophysiological, diagnostic and therapeutic aspects. Int J Mol Sci. 2021;22(10):5123. doi:10.3390/ijms22105123
66. Girolami I, Pantanowitz L, Mete O, et al. Programmed death-ligand 1 (PD-L1) is a potential biomarker of disease-free survival in papillary thyroid carcinoma: a systematic review and meta-analysis of PD-L1 immunoexpression in follicular epithelial derived thyroid carcinoma. Endocr Pathol. 2020;31(3):291–300. doi:10.1007/s12022-020-09630-5
67. Marletta S, Fusco N. Atlas of PD-L1 for pathologists: indications, scores, diagnostic platforms and reporting systems. J Pers Med. 2022;12(7):1073. doi:10.3390/jpm12071073
68. Xu Y, Lu J, Lou N, et al. Long noncoding RNA GAS5 inhibits proliferation and metastasis in papillary thyroid carcinoma through the IFN/STAT1 signaling pathway. Pathol Res Pract. 2022;233:153856. doi:10.1016/j.prp.2022.153856
69. Perera D, Ghossein R, Camacho N, et al. Genomic and transcriptomic characterization of papillary microcarcinomas with lateral neck lymph node metastases. J Clin Endocrinol Metab. 2019;104(10):4889–4899. doi:10.1210/jc.2019-00431
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
