Back to Journals » Cancer Management and Research » Volume 12
YAP Triggers Bladder Cancer Proliferation by Affecting the MAPK Pathway
Received 29 July 2020
Accepted for publication 10 November 2020
Published 27 November 2020 Volume 2020:12 Pages 12205—12214
DOI https://doi.org/10.2147/CMAR.S273442
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Dandan Qiu,1 Yan Zhu,1 Zhicheng Cong2
1Department of Urology, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang Province, People’s Republic of China; 2Department of Urology, Zhejiang Hospital, Hangzhou, Zhejiang Province, People’s Republic of China
Correspondence: Zhicheng Cong
Department of Urology, Zhejiang Hospital, Hangzhou 310013 Zhejiang Province, People’s Republic of China
Email [email protected]
Background: The transcriptional regulator YAP is frequently overexpressed in human cancers, such as breast and pancreatic cancers, plays an important role in tumorigenesis and can regulate many factors affecting cancer progression. These observations encouraged us to investigate the effect of YAP expression on bladder cancer.
Methods: The changes in multiple cellular functions associated with tumor progression including cell proliferation, cell migration, cell cycle, and cell apoptosis were assessed after YAP knockdown/overexpression in bladder cancer cell lines. Additionally, Western blot was developed to verify the change of proteins caused by YAP knockdown/overexpression.
Results: YAP had relatively higher expression in bladder cancer tissues than in normal tissues. The proliferation and migration of bladder cancer cells were inhibited by YAP knockdown but were promoted by its overexpression. This promoting effect was accompanied by the increased activity of MAPK/ERK pathway.
Conclusion: Our data established that YAP is an oncogene involved in bladder cancer and thus can be a potential target for treatment.
Keywords: bladder cancer, proliferation, migration, MAPK/ERK pathway
Introduction
Bladder cancer is one of the most frequently diagnosed and lethal cancers and one of the top 10 most common cancers in men with annually increasing global incidence.1,2 The current standard treatment for bladder cancer is surgery combined with platinum-based chemotherapy. However, bladder cancers managed by surgery still recur and progress to locally invasive or metastatic stages. Although bladder cancer has been studied at the molecular level, its underlying mechanism is still unclear, and no effective therapeutic target is currently available.3,4 Therefore, investigating the mechanism of bladder cancer and screening an effective target drug for its treatment are necessary.
YAP is the key transcriptional co-activator of the Hippo pathway and controls cell proliferation, apoptosis, differentiation, and drug resistance in various cancers.5–7 Hippo signaling pathway is a regulator of organ size and consists of a core kinase cascade, in which MST1/2 phosphorylates and activates the Lats1/2.8,9 YAP also plays important roles in bladder cancer tumorigenesis, progression, and drug resistance.10–12 However, the underlying mechanisms on how YAP regulates bladder cancer remain unclear.
This study showed that YAP is upregulated in bladder cancer cell lines, and YAP silencing impairs the proliferating ability and migration of these cells. Furthermore, YAP regulates cell proliferation by influencing the MAPK/ERK pathway. In summary, YAP is required for the tumorigenesis of bladder cancer cells by regulating the MAPK/ERK pathway. Our findings suggest that targeting YAP may be a viable strategy for bladder cancer treatment.
Materials and Methods
Cell Culture
SV-HUC-1, 5637, J82, TCC-SUP, and UMUC-3 bladder cell lines were purchased from the American Type Culture Collection. SV-HUC-1 cell line was maintained in Ham’s F12K medium (Thermo Scientific) supplemented with 10% fetal bovine serum, 5637 was maintained in RPMI 1640 medium (Thermo Scientific) supplemented with 10% fetal bovine serum and 5% glutamine, J82 was maintained in MEM medium (Thermo Scientific) supplemented with 10% fetal bovine serum and 5% glutamine, and TCC-SUP and UMUC-3 were maintained in MEM medium (Thermo Scientific) supplemented with 10% fetal bovine serum and 5% glutamine. These cell lines were grown at 37°C containing 5% CO2 in a humidified chamber.
Cell Transfection
Full YAP cDNA and negative control cDNA were cloned into pc-DNA3.1. Three short-interfering RNAs targeting YAP (Si-YAP#1, Si-YAP #2, and Si-YAP#3) and a relative scrambled siRNA (Si-NC) were designed and purchased from RiboBio (Guangzhou, China). The cells were transfected with plasmids or siRNAs by using Lipofectamine 3000 (Invitrogen, USA) in accordance with the manufacturer’s guidelines.
Western Blot Analysis
For Western blot analysis, the cells were harvested and lysed using RIPA lysis buffer (supplemented with 1× PMSF). Equal protein amounts (20–30 µg) were separated by SDS-PAGE and then blotted onto PVDF membranes. The blots were blocked with 5% non-fat milk for 1 h, probed with primary antibody at 4°C overnight, washed with TBS-Tween-20 three times, and finally incubated with secondary antibody for 1 h at room temperature. Immunoreactive blots were visualized with ECL Plus reagents. The following primary antibodies were purchased from Cell Signaling Technology: YAP (#14074, 1:1000), β-catenin (#8480, 1:1000), E-cadherin (#14472, 1:1000), N-cadherin (#13116, 1:1000), vimentin (#5741, 1:1000), Slug (#9585, 1:1000), P38 (#8690, 1:1000), phosphorylated p38 (pP38) (#4511, 1:1000), ERK (#4695, 1:1000), phosphorylated ERK (pERK) (#9010, 1:1000), c-Jun NH2-terminal kinase (JNK) (#9252, 1:1000), and phosphorylated JNK (pJNK) (#9255, 1:1000).
Cell Viability Assay
The cells were seeded (0.5–1×104) onto a 96-well culture plate for 12, 24, or 72 h with or without the drug to determine the number of living cells. Cell viability was evaluated using CCK8 assay. SCH772984 (MedChem Express) was reconstituted following the manufacturer’s recommendations and used at the indicated doses. Absorbance was measured at the absorbance of color (450 nm) by using a microplate absorbance reader.
Apoptosis and Cell-Cycle Flow Cytometry
The percentage of apoptotic cells and the cell-cycle distribution of cells was determined using the BD Annexin V-FITC/PI Assay Kit and a Cycle Staining Kit, respectively. Both assays were according to the manufacturer’s instructions. Cell apoptosis and cell-cycle analyses were performed by flow cytometry (Accuri model C6).
RNA Isolation and Quantitative Real-Time PCR
Total RNA was isolated from cells and tissues using TRIzol reagent (Invitrogen, Carlsbad, CA) in accordance with the manufacturer’s instructions. Total cDNA was synthesized using a HiFiScript cDNA synthesis kit (Cwbio, Jiangsu, China). Quantitative real-time PCR was performed using the UltraSYBR mixture (CW0957, Cwbio, Jiangsu, China) in ABI 7300. mRNA expression was assessed by evaluating the threshold cycle (CT) values. Relative expression was calculated using the ΔΔCT method, and GAPDH mRNA was employed as an endogenous control for mRNAs. The primers were designed and synthesized in Multi Sciences (Hangzhou, China) using the following primer sequences: GAPDH forward 5′-TGTGGGCATCAATGGATTTGG −3′ and GAPDH reverse 5′-ACACCATGTATTCCGGGTCAAT-3′; YAP forward 5′-TAGCCCTGCGTAGCCAGTTA-3′ and YAP reverse 5′-TCATGCTTAGTCCACTGTCTGT-3′.
Statistical Analysis
Comparisons between groups were performed with multiple comparisons by one‐way ANOVA. Tukey’s after one-way ANOVA was used as the post-hoc test. Student’s t test was used for pairwise comparisons. All data were obtained from at least three independent experiments and presented as mean ± S.D. or mean ± SEM. In all statistical tests, * represents values of P < 0.05, ** represents P< 0.01, and NS represents not significant. Statistical analyses were performed using GraphPad Prism 7.0 (San Diego, CA, USA).
Results
YAP Functions in Human Bladder Cancer Cells
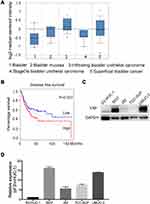
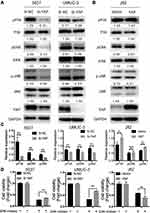
Endogenous YAP expression between bladder cancer and normal tissues by using the oncomine database (https://www.oncomine.org) based on existing cancer microarray data sets. As shown in Figure 1A, YAP expression in cancer tissues was relatively higher than that in normal tissues. The association of survival rate and YAP expression was further examined by using the online survival analysis software GEPIA (http://gepia.cancer-pku.cn/). A high YAP level was significantly associated to a low survival rate (Figure 1B). The protein and mRNA expression of YAP in normal bladder cell line and bladder cancer cell lines were then examined (Figure 1C and D). The level of YAP was higher in bladder cancer cells than in normal bladder cell line. These data strongly suggested that YAP is involved in bladder cancer progression.
YAP Promotes Cell Proliferation and Induces Cell Death in Bladder Cancer
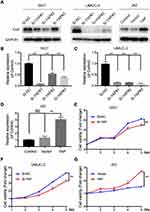
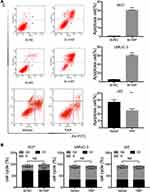
YAP expression was relatively high in 5637 and UMUC-3 cells but low in J82 cells (Figure 1C and D). Therefore, these cell lines were selected for further experiments. Western blot and qRT-PCR analyses revealed that YAP level was altered in 5637 and UMUC-3 cells transfected with either YAP siRNA (Si-YAP #1, Si-YAP #2, and Si-YAP #3) or non-targeting control (Si-NC) (Figure 2A–C). J82 cells transfected with either YAP or vector plasmids were analyzed (Figure 2A and D). Therefore, Si-YAP#2 was employed in latter experiments. Cell proliferation assays revealed that YAP silencing attenuated the viability of 5637 cells (Figure 2E). Consistent with that in 5637 cells, YAP inhibition could reduce UMUC-3 cell proliferation (Figure 2F). However, YAP overexpression promoted the cell proliferation of J82 cells (Figure 2G). Furthermore, we determined the cell apoptosis and cell cycle whether were related with the change of YAP. The results revealed that YAP knockdown significantly induced apoptosis in 5637 and UMUC-3 cells, whereas YAP-overexpressed J82 cells inhibited apoptosis (Figure 3A). However, additional or depleted expression of YAP did not significantly influence cell cycle progression (Figure 3B). In summary, YAP promotes bladder cancer cell proliferation though inhibiting cell apoptosis.
YAP Knockdown Suppresses Cell Migration Ability
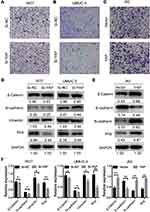
The effects of YAP on the migration ability of 5637, UMUC-3, and J82 cells were studied via Transwell assay to further investigate its biological potential in bladder cancer. The number of migrating cells increased in the YAP overexpression group but decreased in the YAP knockdown group (Figure 4A–C). The expression of EMT-related markers was also analyzed through Western blot. E-cadherin could not be detected in 5637 and UMUC-3 cells, and vimentin was not determined in J82 cells (data not shown). These results might be related to cell migration ability. As shown in Figure 4D, YAP knockdown inhibited Slug and vimentin expression in 5637 and UMUC-3 cells. YAP overexpression for 48 h significantly inhibited E-cadherin expression but increased β-catenin, N-cadherin, and Slug expression in J82 cells (Figure 4E and F). These findings indicated that a decreased YAP expression inhibits cell migration ability.
YAP Regulates Bladder Cancer Progression by Influencing the MAPK Pathway
How YAP regulates bladder cancer cell progression was studied by controlling the expression of different target genes. The putative downstream targets of YAP were examined to further confirm the mechanisms underlying its effect on the proliferation and migration of bladder cancer cells. The activated MAPK signaling pathway is oncogenic-related and is responsible for different cellular processes, such as proliferation, differentiation, and development, in various cancers including bladder cancer.3,15,16 The levels of MAPK pathway-associated molecules and their phosphorylated forms were examined to determine whether YAP regulates bladder cancer proliferation via this pathway. Western blot analyses revealed that pP38, pERK, and pJNK levels were significantly high in the YAP-related high group (Figure 5A and C). In addition, YAP overexpression could induce the expression of pP38, pERK, and pJNK (Figure 5B and C). Cell proliferation in response to YAP overexpression significantly attenuated by ERK inhibitor (Figure 5D). These results indicated that YAP activates MAPK/ERK pathway and thus promotes bladder cancer proliferation.
Discussion
Experimental results revealed that YAP modulates bladder cancer. The Hippo pathway regulates cell fate and stem cell differentiation during normal organogenesis and tumorigenesis and is also involved in drug resistance.17–19 YAP is the major protein in the Hippo pathway, and its overexpression is involved in many diseases, especially cancer.10,19–22 The reciprocal antagonism between Hippo-YAP/TAZ and NF-κB signaling is important in osteoarthritis pathogenesis and regulates matrix-degrading enzymes and cartilage degradation.23 In breast cancer, YAP activity is maintained in a certain level by TRPS1 to regulate tumor-infiltrating immune cells.24 YAP1-NMU expression can regulate pancreatic cancer metastasis and is related to poor prognosis.25 YAP is associated with bladder cancer progression, ALDH1A1 expression, and bladder cancer stem cell properties and thus is a promising target for the prevention and treatment of bladder cancer. YAP/Nrf2 crosstalk is critical for bladder cancer chemoresistance; however, the mechanism underlying YAP regulation in bladder cancer remains unclear.
These results provide direct evidence for the protumorigenic role of YAP in bladder cancer. YAP expression in bladder cancer tissues is higher than that in normal tissues and is related to poor survival. For confirmation, cell proliferation assay and Transwell assay were performed, and the results showed that YAP enhanced tumor growth and induced cell migration in vitro as indicated by the increase in the proliferation capacity and migration of tumor cells. On the contrary, YAP knockdown significantly inhibited these abilities. These results revealed that YAP is involved in bladder cancer progression. The mitogen-activated protein kinase family, which includes ERK, p38, and JNK, modulates tumor progression by upregulating downstream kinases and transcription factors.3,15,16,26 ERK and p38 MAPK activate cell growth and invasive ability in bladder cancer cells. Further assays were performed to determine whether YAP can regulate the MAPK pathway, and the results revealed that the protein levels of MMP-9, XIAP, VEGF, and Cyclin-D1 were significantly reduced by PD98059 or SB203580 treatment. In addition, the protein levels of pERK, pP38, and pJNK were diminished by YAP silencing but promoted by its overexpression.
In summary, YAP is an oncogene that could actively promote cell viability and proliferation by acting as an upstream regulator and activating the MAPK pathway in bladder cancer. The YAP/MAPK pathway is a novel axis in bladder cancer tumorigenesis. This work provides novel insights into the molecular pathogenesis of bladder cancer.
Data Sharing Statement
All data analyzed during this study are included in this published article.
Acknowledgments
We thank the First Affiliated Hospital of Zhejiang Chinese Medical University for supporting our research and this study was supported by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (N0.2021423910).
Author Contributions
Dandan Qiu, Yan Zhu and Zhicheng Cong designed the study and wrote the manuscript. Dandan and Yan Zhu performed all of the experiments. Dandan Qiu and Zhicheng Cong conducted the statistical analysis. All authors read and approved the final manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing financial interests.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
3. Chiang CH, Chung JG, Hsu FT. Regorefenib induces extrinsic/intrinsic apoptosis and inhibits MAPK/NF-κB-modulated tumor progression in bladder cancer in vitro and in vivo. Environ Toxicol. 2019;34(6):679–688. doi:10.1002/tox.22734
4. Hua X, Xu J, Deng X, et al. New compound ChlA-F induces autophagy-dependent anti-cancer effect via upregulating Sestrin-2 in human bladder cancer. Cancer Lett. 2018;436:38–51. doi:10.1016/j.canlet.2018.08.013
5. Zhou Y, Wang Y, Zhou W, et al. YAP promotes multi-drug resistance and inhibits autophagy-related cell death in hepatocellular carcinoma via the RAC1-ROS-mTOR pathway. Cancer Cell Int. 2019;19:179. doi:10.1186/s12935-019-0898-7
6. Noguchi S, Saito A, Nagase T. YAP/TAZ signaling as a molecular link between fibrosis and cancer. Int J Mol Sci. 2018;19(11). doi:10.3390/ijms19113674
7. Ciamporcero E, Shen H, Ramakrishnan S, et al. YAP activation protects urothelial cell carcinoma from treatment-induced DNA damage. Oncogene. 2016;35(12):1541–1553. doi:10.1038/onc.2015.219
8. Rausch V, Bostrom JR, Park J, et al. The Hippo pathway regulates caveolae expression and mediates flow response via caveolae. Curr Biol. 2019;29(2):242–255.e246. doi:10.1016/j.cub.2018.11.066
9. Zhang Z, Du J, Wang S, et al. OTUB2 promotes cancer metastasis via Hippo-independent activation of YAP and TAZ. Mol Cell. 2019;73(1):7–21.e27. doi:10.1016/j.molcel.2018.10.030
10. Zhao AY, Dai YJ, Lian JF, et al. YAP regulates ALDH1A1 expression and stem cell property of bladder cancer cells. Onco Targets Ther. 2018;11:6657–6663. doi:10.2147/OTT.S170858
11. Wu Y, Zheng Q, Li Y, et al. Metformin targets a YAP1-TEAD4 complex via AMPKα to regulate CCNE1/2 in bladder cancer cells. J Exp Clin Cancer Res. 2019;38(1). doi:10.1186/s13046-019-1346-1.
12. Dong L, Lin F, Wu W, Liu Y, Huang W. Verteporfin inhibits YAP-induced bladder cancer cell growth and invasion via Hippo signaling pathway. Int J Med Sci. 2018;15(6):645–652. doi:10.7150/ijms.23460
13. Xu X, Gu J, Ding X, et al. LINC00978 promotes the progression of hepatocellular carcinoma by regulating EZH2-mediated silencing of p21 and E-cadherin expression. Cell Death Dis. 2019;10(10). doi:10.1038/s41419-019-1990-6.
14. Zhao T, Jiang W, Wang X, et al. ESE3 inhibits pancreatic cancer metastasis by upregulating E-cadherin. Cancer Res. 2017;77(4):874–885. doi:10.1158/0008-5472.CAN-16-2170
15. Zhang Y, Hu Q, Li G, et al. ONZIN upregulation by mutant p53 contributes to osteosarcoma metastasis through the CXCL5-MAPK signaling pathway. Cell Physiol Biochem. 2018;48(3):1099–1111. doi:10.1159/000491976
16. Ye Y, Guo J, Xiao P, et al. Macrophages-induced long noncoding RNA H19 up-regulation triggers and activates the miR-193b/MAPK1 axis and promotes cell aggressiveness in hepatocellular carcinoma. Cancer Lett. 2019.
17. Chen D, Sun Y, Wei Y, et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18(10):1511–1517. doi:10.1038/nm.2940
18. Han H, Qi R, Zhou JJ, et al. Regulation of the Hippo pathway by phosphatidic acid-mediated lipid-protein interaction. Mol Cell. 2018;72(2):328–340 e328.
19. Chen W, Wang H, Liu Y, et al. Linc-RoR promotes proliferation, migration, and invasion via the Hippo/YAP pathway in pancreatic cancer cells. J Cell Biochem. 2019.
20. Calvo F, Ege N, Grande-Garcia A, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15(6):637–646. doi:10.1038/ncb2756
21. Yang CS, Stampouloglou E, Kingston NM, Zhang L, Monti S, Varelas X. Glutamine-utilizing transaminases are a metabolic vulnerability of TAZ/YAP-activated cancer cells. EMBO Rep. 2018;19(6). doi:10.15252/embr.201643577
22. Bottini A, Wu DJ, Ai R, et al. PTPN14 phosphatase and YAP promote TGFβ signalling in rheumatoid synoviocytes. Ann Rheum Dis. 2019:
23. Deng Y, Lu J, Li W, et al. Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat Commun. 2018;9(1). doi:10.1038/s41467-018-07022-2.
24. Elster D, Tollot M, Schlegelmilch K, et al. TRPS1 shapes YAP/TEAD-dependent transcription in breast cancer cells. Nat Commun. 2018;9(1):3115.
25. Yoo W, Lee J, Jun E, et al. The YAP1-NMU axis is associated with pancreatic cancer progression and poor outcome: identification of a novel diagnostic biomarker and therapeutic target. Cancers. 2019;11(10):1477. doi:10.3390/cancers11101477
26. Murali B, Ren Q, Luo X, et al. Inhibition of the stromal p38MAPK/MK2 pathway limits breast cancer metastases and chemotherapy-induced bone loss. Cancer Res. 2018;78(19):5618–5630. doi:10.1158/0008-5472.CAN-18-0234
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.