Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Xerostomia induced by radiotherapy: an overview of the physiopathology, clinical evidence, and management of the oral damage
Authors Pinna R, Campus G, Cumbo E, Mura I, Milia E
Received 4 July 2014
Accepted for publication 11 September 2014
Published 4 February 2015 Volume 2015:11 Pages 171—188
DOI https://doi.org/10.2147/TCRM.S70652
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh

Roberto Pinna,1 Guglielmo Campus,2 Enzo Cumbo,3 Ida Mura,1 Egle Milia2
1Department of Biomedical Science, 2Department of Surgery, Microsurgery and Medicine, University of Sassari, Sassari, 3Department of Dental Science, University of Palermo, Palermo, Italy
Background: The irradiation of head and neck cancer (HNC) often causes damage to the salivary glands. The resulting salivary gland hypofunction and xerostomia seriously reduce the patient’s quality of life.
Purpose: To analyze the literature of actual management strategies for radiation-induced hypofunction and xerostomia in HNC patients.
Methods: MEDLINE/PubMed and the Cochrane Library databases were electronically evaluated for articles published from January 1, 1970, to June 30, 2013. Two reviewers independently screened and included papers according to the predefined selection criteria.
Results: Sixty-one articles met the inclusion criteria. The systematic review of the literature suggests that the most suitable methods for managing the clinical and pathophysiological consequences of HNC radiotherapy might be the pharmacological approach, for example, through the use of cholinergic agonists when residual secretory capacity is still present, and the use of salivary substitutes. In addition, a modified diet and the patient’s motivation to enhance oral hygiene can lead to a significant improvement.
Conclusion: Radiation-induced xerostomia could be considered a multifactorial disease. It could depend on the type of cancer treatment and the cumulative radiation dose to the gland tissue. A preventive approach and the correct treatment of the particular radiotherapeutic patient can help to improve the condition of xerostomia.
Keywords: radiation-induced xerostomia, salivary gland hypofunction, management strategies
Introduction
Xerostomia is a term used to describe the subjective symptoms of a dry mouth deriving from a lack of saliva. A large variety of causes can lead to xerostomia, eg, radiotherapy and chemotherapy,1–4 the chronic use of drugs,5–7 and rheumatic and dysmetabolic diseases.8,9
Saliva is an important host defense component of the oral cavity. Major salivary glands contribute to most of the secretion volume and electrolyte content of saliva (the parotid, submandibular, and sublingual glands, which account for 90% of saliva production), whereas minor salivary glands contribute little secretion volume and most of the blood-group substance.10 Saliva components interact in related functions in the following general areas:
- bicarbonates, phosphates, and urea act to modulate pH and the buffering capacity of saliva;
- macromolecule proteins and mucins serve to cleanse, aggregate, and/or attach oral microorganisms and contribute to the dental plaque metabolism;
- calcium, phosphates, and proteins work together as an antisolubility factor and modulate demineralization and remineralization of tooth surfaces;
- immunoglobulins, proteins, and enzymes provide antibacterial action.
Objectively, patients affected by xerostomia have a hypofunction of the salivary output11,12 leading to functional oral disorders such as sore throat, altered taste, dental decay, changes in voice quality, and impaired chewing and swallowing function.13 These factors may ultimately cause reduced nutritional intake and weight loss and significantly affect general health and quality of life of the subjects involved.
Head and neck cancer (HNC) actually includes many different malignancies. The most common type of cancer in the head and neck is squamous cell carcinoma, which originates in the cells that line the inside of the paranasal sinuses, nasal cavity, salivary glands, oral cavity, esophagus, pharynx, and larynx.14 Worldwide, lip and oral cavity cancer along with thyroid cancer has the highest incidence; esophagus cancer is the most aggressive presenting a 4.9% mortality rate (Table 1).
  | Table 1 World incidence, mortality, and 5-year prevalence of head and neck cancer |
Similar findings regarding the incidence, mortality, and prevalence of cancer in the European Union have been reported. The highest mortality rate belongs again to esophagus cancer with a predominance of 2.3% (Table 2).
  | Table 2 European Union incidence, mortality, and 5-year prevalence of head and neck cancer |
Other less common types of HNCs include salivary gland tumors, lymphomas, and sarcomas.15
The way a particular HNC behaves depends on the primary site in which it arises, and the spread to the lymph nodes in the neck is relatively common.
A patient may receive radiotherapy before, during, or after surgery. Some patients may receive radiotherapy alone without surgery or any other treatment; others may receive radiotherapy and chemotherapy at the same time. The timing of radiotherapy depends on the type of cancer and on the goal of the treatment (cure or palliation). Radiotherapy treats cancer by using doses of high-energy X-rays to destroy the cancer cells while avoiding as much harm as possible to normal cells. The treatment is usually given every weekday with a pause at the weekend; some protocols are based on more than one irradiation a day, and occasionally include therapy during the weekend. The treatment will usually last 3–7 weeks, depending on the type and size of the cancer. Most of the time, patients with HNC treated with radiotherapy receive a dose between 50 Gy and 70 Gy once a day for 5 days a week (2 Gy per fraction);4 on the other hand, if the radiotherapy protocol is just preoperative, the total amount of radiation is usually lower. Conformal radiotherapy is the most common type of radiotherapy used for the treatment of HNC; a special attachment to the radiotherapy machine carefully arranges the radiation beams to match the shape of the cancer, reducing the radiation to the surrounding healthy cells. Another similar type of radiotherapy used against HNC, known as intensity-modulated radiotherapy, allows a more accurate delivery of specific radiation to be distributed to the tumor mass according to its location and severity, sparing the tissue and organs at risk, eg, salivary glands.10
Aims
The aim of the study is to systematically determine the current treatment option for cancer-/radiation-induced xerostomia among patients treated for HNC, and to describe the strategic prevention and management enhancements.
Materials and methods
Systematic review methodology
Search strategy
A first systematic literature search for articles published between January 1, 1970 and June 30, 2013 was conducted in the databases MEDLINE/PubMed and the Cochrane Library, using combinations of the MeSH terms: [Saliva] OR [Salivary Glands] OR [Saliva Flow] OR [Salivation] OR [Salivary Gland Diseases] OR [Xerostomia] OR [Saliva in Xerostomia] OR [Dry Mouth] OR [Oral Dryness] OR [Composition Saliva Xerostomia] AND [Head and Neck Cancer] OR [Radiotherapy] OR [Radiation-induced Xerostomia] OR [Parotid-Sparing Intensity-Modulated Radiotherapy] AND [Quality of Life Analysis-Xerostomia] OR [Management Strategies Salivary Gland Hypofunction] OR [Prevention Xerostomia] OR [Treatment Xerostomia]. The search results were imported into a computerized database Review Manager 5.2. The search results from each of the electronic databases of MEDLINE/PubMed and the Cochrane Library were combined, and duplicated publications were eliminated. Subsequently, an update to include studies published up to June 30, 2013, was performed.
Criteria for selecting studies
After completing the search, articles for review were selected based on:
- English language
- Original data of cancer therapy protocols
- Oral complications associated with cancer therapies
- Human.
Exclusion criteria
The reasons for exclusion were defined as follows:
- Studies without original and/or actual data
- Studies with data from previous publications
- Opinion papers
- Editorials.
In this way, a preliminary set of potentially relevant publications, removing irrelevant citations according to the criteria, was created. Two reviewers (RP and GC) independently screened the registered title and abstracts, and author and references in two separate files (one for included abstracts and one for excluded abstracts) using a screening guide based on eligibility criteria. Studies rejected at this stage or subsequent stages were reported in the table of excluded studies (Table 3). The full text of all potentially eligible studies in at least one screening was retrieved. Reviewers then evaluated the full text for inclusion using a screening guide and a second reviewer (RP) screened all the findings. When disagreement occurred, a third reviewer (IM) was consulted. For each review, the following information was recorded: year, authors, journal, aim, and number of papers reviewed (Table 4); and for clinical trial papers included: year, authors, journal, aim, number of patients, and results (Table 5). All studies meeting the inclusion criteria then underwent validity assessment. Two examiners (RP and GC) read the papers independently. The qualities and relevance of each study were graded as follows: high (+++), medium (++), or low (+) using a study-quality checklist. External validity, internal validity, and study precision were analyzed to obtain an overall assessment of quality. The assessment was used as a basis for the discussion between the two examiners to grade the studies. In the case of disagreement, all authors discussed the paper until a consensus was reached.
  | Table 3 Papers excluded |
  | Table 4 Reviews included |
Results
The electronic searches identified about 1,000 titles and abstracts, and after reviewing the titles, 411 studies were evaluated. Subsequently, during the review of the abstract, 336 studies were excluded. The final analysis included 70 articles that conformed to the criteria for the present review (Figure 1). Although animal studies have been excluded, important information regarding the experimental results on two of the papers was considered useful and therefore they were discussed.
  | Figure 1 Search flowchart. |
Physiopathological and clinical consequences in cancer therapy
Each of the reviews concerning the physiopathological and clinical consequences are listed in Table 6.
  | Table 6 Reviews included related to physiopathological and clinical consequences in cancer therapy |
Physiopathological consequences
A total of six articles reported the physiopathological effects of radiotherapy on salivary glands parenchyma: one systematic review, one narrative review, one pilot study, one animal experimentation study, and one cohort study.16–20 Radiotherapy-induced xerostomia could be considered a multifactorial disease. On the one hand, the damage to the oral cavity has been strongly related to the radiation dose, fraction size, volume of irradiated tissue, fractionation scheme, and type of ionizing irradiation, but on the other, it may be difficult to distinguish changes caused by radiotherapy itself from those related to the malignant disease, the concomitant systemic diseases, and the medication needed for the treatment of the cancer.16,17
The salivary glands are superficially located compared to most head and neck tumors, and thus, the ionizing radiation has to pass through the salivary glands to effectively treat the tumor.18 Tissues with a rapid turnover rate are more susceptible than tissues with a slow one and even with the most accurate therapeutic protocol, X-rays cause unwanted changes in non-tumoral tissues. Despite the fact that salivary gland cells turnover is slow, production and quality of saliva change after radiation, so they are not as radioresistant as they are supposed to be.19 There are differences among the various types of salivary glands; in fact, the submandibular gland is less radiosensitive than the parotid gland.20 From this point of view, the most severe and irreversible forms of salivary gland hypofunction result from the damage/loss of salivary acinar cells, giving rise to rapid and predictable compositional changes, and reduction in saliva production and in the quality of the flow.
Radiation-induced changes in saliva
Nineteen articles analyzed the effects of the radiotherapy on salivary flow and composition, and the changes in microbial population: one narrative review, four randomized controlled trials, nine cohort studies, and five cross-sectional studies.21–40
Salivary flow
One of the main problems resulting from tissue damage generated by radiotherapy is the reduction of salivary flow. The radiation level necessary to cause severe dysfunction to gland tissue is >52 Gy. Below this threshold, the radiation damage generally has a transient and reversible duration.12 Routinely, HNC patients receive a total of 50–70 Gy, the radiation dose normally used to destroy malignant cells, which very often leads to the onset of chronic xerostomia.21 The major reduction in salivation after radiotherapy is observed in the period from the onset of radiotherapy to 3 months after completion. During radiotherapy, the first 10 days are the worst ones as a massive decrease in saliva production occurs; especially in the first week, it could reduce by 50%–60%.22 After this period, the flow rate is reduced by <10% of the initial conditions.23
Chemical and immunochemical alterations
Radiotherapy can also induce alterations in electrolytes and antibacterial systems. Salivary electrolyte levels are altered, with an increase in the concentrations of sodium, chloride, calcium, and magnesium, while potassium is only slightly affected.24 Saliva also reduces the buffering capacity in irradiated patients due to a reduction of bicarbonate concentration in parotid saliva.25,26 Saliva becomes, moreover, highly viscous, and reduces its pH from about 7.0 to 5.025,27 with slow recovery to the neutral pH in dental plaque after a sugar rinse.28,29 The permanence of the acidic pH in dental plaque was related to the reduced buccal gland saliva flow30 as the secretion from the buccal glands, closely delivered to the teeth surfaces, could affect the dental plaque more than the whole saliva during resting conditions.
Changes also involve the nonimmune and immune antibacterial systems. The concentrations of immunoproteins (eg, secretory immunoglobulin A), lysozyme, and lactoferrin are increased, as well as the serum and non-salivary components.31–34 However, the decrease in salivary flow rate is greater than the increase in immunoprotein and lysozyme levels, and this results in significant immunoprotein deficit. Since the reduction of the oral clearance, immunologic mechanisms and buffering capacity of saliva are altered, and the host protection decreases giving rise to changes in the oral flora.35
Microbial changes
All of these radiation-induced changes cause a different oral flora growth and acidogenic, cariogenic microorganisms are more present than non-cariogenic microorganisms. Unfortunately, even if there is an increase in immunoprotein and lysozyme levels, a significant immunoprotein deficit occurs due to the decrease in salivary flow rate. Streptococcus mutans, Lactobacillus spp., and Candida spp. are the most prevalent in the plaque of irradiated patients.30,36–38 In a longitudinal study, Brown et al assessed the effects of radiation-induced xerostomia on the human oral microflora and on the subsequent development of dental caries.35 Five intraoral specimens consisting of resting saliva, gingival sulcus fluid, dental plaque, lingual swabs, and stimulated whole saliva were collected from each patient two times during 1 week before radiation, one time per week during radiotherapy, at 3-month intervals during the first postradiation year, and at 6-month intervals thereafter. During irradiation, the development of xerostomia was matched by a parallel and pronounced shift in certain microbial populations at each intraoral site assessed. The most prominent changes were the increase in S. mutans and species of Lactobacillus, Candida (primarily Candida albicans), and Staphylococcus, with parallel decreases in Streptococcus sanguis and species of Neisseria and Fusobacterium. Microbial differences were relatively minimal between the groups of patients receiving radiotherapy who used a fluoride gel and a nonfluoride gel during the irradiation period. However, there was a more rapid decrease in the level of S. sanguis in the plaque of the patients using the nonfluoride gel compared with those patients using the fluoride gel, and the subsequent development of dental caries differed greatly. The increased number of Lactobacilli was correlated to a high acidic potential of the plaque and the use of fluoride was associated with a protective effect in the prevention of dental decay during xerostomia.
The findings that a high frequency, number, and proportion of Lactobacillus spp. occur in irradiated patients were strengthened by a study of Almståhl et al who analyzed the saliva oral microbiota in subjects with hyposalivation using a rinsing technique and a cultivation technique. Results indicated that the salivary secretion rate, pH, and buffer capacity were the more important factors in the increase in Lactobacillus spp. A marked increase in C. albicans was also characteristic of the irradiated patients.39
In a more recent study, Almståhl et al evaluated the frequency of different Lactobacillus spp. in relation to the pH-lowering potential of the supra-gingival plaque in irradiated patients in comparison to primary Sjögren’s syndrome patients and controls with normal salivary secretion.40 The irradiated subjects had finished their bilateral radiation treatment (64.6 Gy) 3–5 years before participating in the study. Interproximal plaque pH was measured by the microtouch method30 before and up to 60 minutes after a 10% sugar rinse.29 The measurements were performed at two sites: in the anterior and in the premolar/molar region. Data indicated that the most common species were Lactobacillus fermentum, Lactobacillus rhamnosus, and Lactobacillus casei. In anterior sites, both the hyposalivated group subjects with high Lactobacillus counts had an increased plaque acidogenicity compared to those with low counts. In posterior sites, subjects with high Lactobacillus counts had a lower final pH compared with those with low counts. Authors concluded that hyposalivation patients often harbor several different Lactobacillus spp. in their supragingival plaque. There were, however, large differences in number and proportion of Lactobacilli between individuals and between anterior and posterior dental sites, but no specific species could be related to plaque acidogenicity.
Radiotherapy clinical consequences
In eleven articles, the clinical consequences that may arise as a result of HNC radiotherapy have been described: three narrative reviews, one randomized clinical trial, one animal experimentation study, four cohort studies, and two cross-sectional studies.20,36,41–50 Radiotherapy can cause some temporary side effects. Although these may be worse if the treatment is combined with chemotherapy, they gradually disappear after the treatment has finished. Most radiotherapy side effects occur toward the middle and end of the course of treatment and continue during the first couple of weeks after the treatment. The effects can be mild or more troublesome, depending on the dose of radiotherapy and the length of treatment. Thus, the quantitative and qualitative salivary changes predispose the irradiated patient to a variety of problems.
Radiotherapy in HNC is inevitably associated with damages to the oral tissues and, in addition, the clinical consequences of radiotherapy include also dermatitis and osteoradionecrosis.41 In fact, salivary glands are often involved and, as a result, patients may have a salivary gland hypofunction, even if 3D planning and unilateral irradiation have considerably reduced the side effects by minimizing the dose to normal tissues. However, the final degree of damage to gland tissue depends on individual patient characteristics, such as pretreatment already done, age, and sex.
Xerostomia may affect 80% of the patients who need radiotherapy as a primary treatment, as an adjunct to surgery, in combination with chemotherapy, or as palliation.42–44 Hyposalivation represents the biggest acute side effect in HNC radiotherapy and, in general, is always associated with oral function problems, such as chewing and swallowing, or caries at a later stage. Normally, during radiotherapy, salivary composition may change and it becomes more viscous than usual, so its color may turn yellow, brown, or even white (Figure 2). Furthermore, salivary glands with high flow rates before the initiation of radiotherapy show less reduction in salivary flow rate. As a consequence of the reduction in the rate of saliva flow, which is correlated to the amount of radiation given to the patient, oral complications occur.20
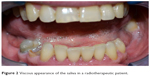  | Figure 2 Viscous appearance of the saliva in a radiotherapeutic patient. |
The buccal mucosa has a dry and sticky appearance (Figure 3). The normally moist, glistening appearance of the oral cavity is often replaced with a thin, pale, cracked appearance that is more susceptible to gingivitis and bleeding. Another frequent acute side effect is oral mucositis, which can be experienced by >50% of patients receiving HNC radiotherapy. Some typical side effects are onset of erythema, edema, and pain in the oral mucosa.41 Patients may also exhibit a dry irritated tongue with an erythematous appearance of the dorsal surface, the hard or soft palate, the commissures of the mouth, and under-removable prostheses.43 Furthermore, the lack of saliva may lead to angular cheilitis, cracked lips (Figure 4), periodontal disease, aching of the mouth, and halitosis.
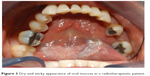  | Figure 3 Dry and sticky appearance of oral mucosa in a radiotherapeutic patient. |
  | Figure 4 Cheilitis and cracked lips, and teeth cervical caries in a radiotherapeutic patient. |
When part or all of the mouth is treated, the sense of taste may change quickly during the radiotherapy, and some patients may even either lose their sense of taste completely or find that everything tastes the same (usually rather metallic or salty). Changes in taste are correlated to the direct irradiation of the taste buds, and also to the reduction in salivary flow rate that alters the ionic composition of saliva that is related to the sensation of taste.46
Moreover, the loss of saliva compromises mastication and nutrition. Some patients lose their appetite as a general effect of radiotherapy. Dryness of the mouth and lips can cause discomfort, ranging from a mild irritation to a severe burning sensation with difficulties in normal eating habits, particularly eating spicy or acidic food. A sore, dry mouth can also make eating and swallowing difficult because moistening of food is insufficient and oral mucosa surfaces are not wet and not lubricated enough.47
Furthermore, an insufficient lubrication, due to a diminished salivary output, causes intolerance to prosthetic appliances, so more friction is present between the mucosa and the resin that can injure the delicate irradiated epithelial layer. In addition, the inadequate presence of saliva weakens the stability of prostheses in the mouth. Ulceration is more likely because the dry mucosa is more vulnerable to trauma.
A further complication that tends to occur later in irradiated patients is the increased risk of developing dental caries and oral infections, due to the alterations in the saliva flow and consequently in oral microflora.36 The decay is most often recurrent or primary and located at sites generally not usually susceptible to caries such as the cervical margins, incisal margins, or the tips of teeth (Figure 4).
Another issue is the high incidence of yeast infections during xerostomia.48 An example being the C. albicans infection, which is very common in both dentate and edentulous individuals and allows a colonization of oral mucosae49 increasing the risk of oral mucosal infections.50
Another acute side effect of radiotherapy is dermatitis, which can be experienced by up to 95% of patients.41 The skin over the face and neck is very likely to gradually redden or darken and become sore. At the same time, the mouth and throat become sore and inflamed after a couple of weeks of treatment and mouth ulcers may occur; the voice may also become hoarse.
Discussion
The treatment of xerostomia has four aims: increasing existing saliva flow or replacing lost secretions, the control of the state of oral health, the control of dental caries, and the treatment of possible infections.51
Therapy options in xerostomia depend on the presence of residual secretion or the absence of it. When residual secretory capacity is present, it is advisable to regularly stimulate the salivary glands by mechanical or gustatory stimuli as supportive oral care. The use of sugarless chewing gum or candy-containing xylitol or sorbitol can be recommended as a means of stimulating extra salivary flow to aid caries management and lubrication. Nocturnal oral dryness can be alleviated by applying a small amount of dentifrice on smooth dental surfaces, especially using anti-xerostomia dentifrices that contain three salivary enzymes, lactoperoxidase, glucose oxidase, and lysozyme, specifically formulated to activate intraoral bacterial systems.
The salivary flow can also be stimulated by the use of cholinergic pharmaceutical preparations, such as pilocarpine or cevimeline. These two parasympathomimetic drugs are approved by the Food and Drug Administration for treatment of xerostomia; pilocarpine is approved for Sjögren’s syndrome and radiotherapy-induced xerostomia, while cevimeline seems to be more specific for Sjögren’s syndrome. Pilocarpine, a natural alkaloid, is a parasympathomimetic agent with β-adrenergic effects that activates cholinergic receptors,52 stimulating the residual function of the salivary glands. The recommended dose is 5 mg orally three times a day.53 Severe adverse effects are rare, but side effects associated with the use of the drug are vomiting, sweating, headache, increased urinary frequency, wheezing, watery eyes, and nausea, and gastrointestinal intolerance. Hypotension, rhinitis, diarrhea, and visual disturbances can also occur.54,55 Normally, these are moderate in intensity and last for a short period of time. Patients with asthma, high blood pressure, heart diseases, and in therapy with β-blockers cannot use pilocarpine because this drug is a nonselective antagonist of muscarinic receptors and, therefore, it can interfere with the cardiac and respiratory functions in those patients. For the same reason, pilocarpine, stimulating muscarinic receptors in the central nervous system, can cause onset of agitation, confusion, and parkinsonian-like syndromes.56
Cevimeline is analogous to acetylcholine, which binds to muscarinic acetylcholine receptors in exocrine glands, specifically the M1 and M3 subtypes present, for instance, in the epithelium of the salivary and lachrymal glands, leading to an increase in exocrine gland secretion including saliva and sweat. M2 and M4 receptor sites predominate in cardiac and respiratory tissues. This receptor subtype selectivity is presumed to mitigate the systemic adverse effects of muscarinic–cholinergic stimulation.56 It is rapidly absorbed from the gastrointestinal tract, reaching peak concentrations in approximately 90 minutes without food. The duration of its sialogogic effect seems to be unclear. Clinical trials have shown it to be more effective than placebos in relieving the symptoms of a dry mouth. The recommended dose is 30 mg orally three times a day, but in two clinical trials, it has been shown that the use of cevimeline in treating radiation-induced xerostomia, increasing the dose to 45 mg, was well tolerated by patients, with an increase of unstimulated salivary flow.57,58 This medication is not recommended for patients with uncontrolled asthma, narrow-angle glaucoma, or iritis. Excessive sweating and nausea are the most frequently reported adverse effects with cevimeline. Rhinitis, diarrhea, and visual disturbances, especially at night, can also occur.
Another medication is anethole trithione that is a bile secretion-stimulating drug, or cholagogue. It increases the secretion of acetylcholine and stimulates the parasympathetic nervous system, and so as a result, we have the stimulation of salivation from serous acinar cells. This medication has been used for many years in the treatment of chronic xerostomia, but reports differ regarding its efficacy. While some studies report improvements in salivary flow rates in drug-induced xerostomia, trials in patients with Sjögren’s syndrome show conflicting results. Side effects reported include abdominal discomfort and flatulence. Dosages of 75 mg three times daily may be effective in treating patients with mild-to-moderate symptoms of xerostomia, but further research is needed to establish its safety and efficacy in this setting.59
Yohimbine has also been used in patients with xerostomia and it is an alpha-2 adrenergic antagonist, which induces an increase in cholinergic activity peripherally. In one randomized, double-blind, crossover study, the effect of this medication was compared to that of anethole trithione in patients treated with psychotropic medications. Patients given yohimbine 6 mg three times daily for 5 days showed significantly increased saliva flow (P<0.01) when compared with anethole trithione 25 mg three times daily.60
Human interferon alfa (IFN-α) is currently undergoing clinical trials to determine the safety and efficacy of low-dose lozenges in the treatment of salivary gland dysfunction and xerostomia. In one study, IFN-α lozenges at dosages of 150 IU given three times daily for 12 weeks resulted in a significant increase in stimulated whole saliva (P=0.04) when compared with placebos.61
If some residual function of salivary glands remains, acupuncture could be a good alternative treatment for alleviating radio-induced xerostomia.62 The way this works remains poorly understood, but it seems that acupuncture modulates central nervous system processes,63 increasing the concentration of salivary neuropeptides, which seem capable of modulating the complex process of salivary secretion.64 There are some studies that provide encouraging results, suggesting an effective increase in salivary flow, while others do not detect statistically significant differences in the increase in salivary flow between the treated subjects and controls.65 However, the results of systematic reviews do not indicate the efficacy of acupuncture in the treatment of xerostomia due to the current lack of relevant randomized clinical trials.64–67
When stimulation of salivary secretion fails, patients can be given palliative oral care in the form of application of mouthwashes and saliva substitutes. Although the daily use of a mouthwash or one of the saliva substitutes that are formulated to mimic natural saliva, is strongly recommended, they do not stimulate salivary gland production. Commercially available products come in a variety of formulations including solutions, sprays, gels, and lozenges. In general, they contain an agent to increase viscosity, such as carboxymethylcellulose or hydroxypropylmethylcellulose, hydroxyethylcellulose, and polyglycerylmethacrylate,65 minerals such as calcium and phosphate ions and fluoride, preservatives such as methyl or propylparaben, and flavoring and related agents.
Also homeopathic remedies such as olive oil, aloe vera gel, and rape oil spray may be effective alternatives in the palliative management of xerostomic patients.68,69
In order to minimize problems related to the absence of or reduced secretion of saliva, all patients should be encouraged to take an active role in the management of their xerostomia; so a daily mouth examination, checking for red, white, or dark patches, ulcers, or tooth decay, is highly recommended.
Patients with reduced saliva should also be encouraged to consider visiting their dentist more frequently because they have got a greater susceptibility to dental problems. Teeth should be cleaned at least twice a day, so brushing and flossing regularly and the daily use of fluoride and chlorhexidine rinses may also be useful in preventing caries by reducing amounts of Streptococcus and Lactobacillus in the mouth. For daily use, a special dentifrice (eg, children’s toothpaste or anti-xerostomia dentifrices) is recommended, since the taste of a regular dentifrice may be too strong for these patients.
Dentures and acrylic appliances should not be worn during sleep and they should be kept clean by soaking them overnight in chlorhexidine. Sometimes, lubricants, Vaseline and/or glycerin based, put on the lips and under dentures, may relieve drying, cracking, soreness, and mucosal trauma.61
Patients with decreased salivary flow should also be made aware of the necessity to comply with suggested oral hygiene regimens after exposure to acid-producing food sources. Recommendations for professional and home fluoride treatments should be considered carefully for patients with salivary dysfunction, especially those with high caries rates and exposed root surfaces. A modified diet can be useful to minimize the effects of xerostomia; for instance, they should avoid sugary or acidic foods and also avoid dry, spicy, astringent, or excessively hot or cold foods that are more irritating, while eating foods such as carrots or celery may also help patients with residual salivary gland function. The addition of flavor enhancers such as herbs, condiments, and fruit extracts may make food more palatable to patients complaining of their food tasting bland, papery, salty, or otherwise unpleasant; at the same time, taking frequent sips of water throughout the day and sucking on ice chips are helpful.70
Conclusion
The resulting salivary gland hypofunction and xerostomia arising from radiotherapy for HNC can cause a serious diseased condition. The stomatologic complications could depend on the type of cancer treatment and the cumulative radiation dose to the gland tissue. They can be reversible or irreversible, transient, or enduring. The best approach to manage the radiotherapeutic patient begins with a careful clinical assessment of the individual case, followed by preventive therapy aimed to reduce oral complications when possible. Therefore, the clinician must keep this kind of patients under careful control in order to palliate the symptoms of xerostomia and improve their quality of life.
Disclosure
The authors report no conflicts of interest in this work.
References
Bivona PL. Xerostomia. A common problem among the elderly. N Y State Dent J. 1998;64:46–52. | ||
Cassolato SF, Turnbull RS. Xerostomia: clinical aspects and treatment. Gerodontology. 2003;20:64–77. | ||
Waltimo T, Christen S, Meurman JH, Filippi A. Dental care of patients with leukemia. Schweiz Monatsschr Zahnmed. 2005;115:308–315. | ||
Brand HS, Bots CP, Raber-Durlacher E. Xerostomia and chronic oral complications among patients treated with haematopoietic stem cell transplantation. Br Dent J. 2009;207:E17. | ||
Gupta A, Epstein JB, Sroussi H. Hyposalivation in elderly patients. J Can Dent Assoc. 2006;72:841–846. | ||
Villa A, Abati S. Risk factors and symptoms associated with xerostomia: a cross-sectional study. Aust Dent J. 2011;56:290–295. | ||
Villa A, Polimeni A, Strohmenger L, Cicciù D, Gherlone E, Abati S. Dental patients’ self-reports of xerostomia and associated risk factors. J Am Dent Assoc. 2011;142:811–816. | ||
Busato IM, Ignácio SA, Brancher JA, Grégio AM, Machado MA, Azevedo-Alanis LR. Impact of xerostomia on the quality of life of adolescents with type 1 diabetes mellitus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:376–382. | ||
Moore P, Guggenheimer J, Etzel JK, Weyant RJ. Trevor orchard type 1 diabetes mellitus, xerostomia, and salivary flow rates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:281–291. | ||
Jensen SB, Pedersen AM, Vissink A, et al; Salivary Gland Hypofunction/Xerostomia Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. 2010;18: 1039–1060. | ||
Fox PC, Bunch KA, Baum BJ. Subjective reports of xerostomia and objective measures of salivary gland performance. J Am Dent Assoc. 1987;115:581–584. | ||
Porter SR, Scully C, Hegarty AM. An update of the etiology and management of xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:28–46. | ||
De Graeff A, De Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. A prospective study on quality of life of patients with cancer of the oral cavity or oropharynx treated with surgery with or without radiotherapy. Oral Oncol. 1999;35:27–32. | ||
Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. | ||
Globocan.iarc.fr [homepage on the Internet]. Lyon; International Agency for Research on Cancer; World Health Organization; 2012 [updated January 9, 2014]. Available from: http://globocan.iarc.fr/Default.aspx. Accessed May 1, 2014. | ||
Maciejewski B, Zajusz A, Pilecki B, et al; Salivary Gland Hypofunction/Xerostomia Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). Acute mucositis in the stimulated oral mucosa of patients during radiotherapy for head and neck cancer. Radiother Oncol. 1991;22:7–11. | ||
Scully C, Epstein JB. Oral health care for the cancer patient. Eur J Cancer B Oral Oncol. 1996;32:281–292. | ||
Sreebny LM. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50:140–161. | ||
Vissink A, Kalicharan D, S-Gravenmade EJ, et al. Acute irradiation effects on morphology and function of rat submandibular glands. J Oral Pathol Med. 1991;20:449–456. | ||
Burlage FR, Coppes RP, Meertens H, Stokman MA, Vissink A. Parotid and submandibular/sublingual flow during high dose radiotherapy. Radiother Oncol. 2001;61:271–274. | ||
Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson-Moore P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck. 2001;23:389–398. | ||
Franzén L, Funegård U, Ericson T, Henriksson R. Parotid gland function during and following radiotherapy of malignancies in the head and neck. A consecutive study of salivary flow and patient discomfort. Eur J Cancer. 1992;28:457–462. | ||
Dreizen SA, Daly TE, Drane JB, Brown LR. Oral complications of cancer radiotherapy. Postgrad Med. 1977;61:85–92. | ||
Ben-Aryeh H, Gutman D, Szargel R, Laufer D. Effects of irradiation on saliva in cancer patients. Int J Oral Surg. 1975;14:205–210. | ||
Dreizen S, Brown LR, Handler S, Levy BM. Radiation-induced xerostomia in cancer patients. Effect on salivary and serum electrolytes. Cancer. 1976;38:273–278. | ||
Marks JE, Davis CC, Gottsman VL, Purdy JE, Lee F. The effects of radiation of parotid salivary function. Int J Radiat Oncol Biol Phys. 1981;7:1013–1019. | ||
Ben-Aryeh H, Miron D, Berdicevsky I, Szargel R, Gutman D. Xerostomia in the elderly: prevalence, diagnosis, complications and treatment. Gerodontology. 1985;4:77–82. | ||
Abelson DC, Mandel ID. The effect of saliva on plaque pH in vivo. J Dent Res. 1981;60:1634–1638. | ||
Lingström P, Birkhed D. Plaque pH and oral retention after consumption of starchy snack products at normal and low salivary secretion rate. Acta Odontol Scand. 1993;51:379–388. | ||
Eliasson L, Birkhed D, Osterberg T, Carlén A. Minor salivary gland secretion rates and immunoglobulin A in adults and the elderly. Eur J Oral Sci. 2006;114:494–499. | ||
Almståhl A, Wikström M. Oral microflora in subjects with reduced salivary secretion. J Dent Res. 1998;78:1410–1416. | ||
Valdez IH, Wolff A, Atkinson JC, Macynski AA, Fox PC. Use of pilocarpine during head and neck radiation therapy to reduce xerostomia and salivary dysfunction. Cancer. 1993;71:1848–1851. | ||
Makkonen TA, Tenovuo J, Vilja P, Heimdahl A. Changes in the protein composition of whole saliva during radiotherapy in patients with oral or pharyngeal cancer. Oral Surg Oral Med Oral Pathol. 1986;62:270–275. | ||
Izutsu KT, Truelove EL, Bleyer WA, Anderson WM, Schubert MM, Rice JC. Whole saliva albumin as an indicator of stomatitis in cancer therapy patients. Cancer. 1981;48(6):1450–1454. | ||
Brown LR, Dreizen S, Handler S, Johnston DA. Effect of radiation-induced xerostomia on human oral microflora. J Dent Res. 1974;54(4):740–750. | ||
Keene HJ, Daly T, Brown LR, et al. Dental caries and Streptococcus mutans prevalence in cancer patients with irradiation-induced xerostomia: 1–13 years after radiotherapy. Caries Res. 1981;15:416–427. | ||
Hase JC, Birkhed D. Salivary glucose clearance, dry mouth and pH changes in dental plaque in man. Arch Oral Biol. 1988;33:875–880. | ||
Ravald N, List T. Caries and periodontal conditions in patients with primary Sjögren’s syndrome. Swed Dent J. 1998;22(3):97–103. | ||
Almståhl A, Wikström M, Groenink J. Lactoferrin, amylase and mucin MUC5B and their relation to the oral microflora in hyposalivation of different origins. Oral Microbiol Immunol. 2001;16:345–352. | ||
Almståhl A, Carlén A, Eliasson L, Lingström P. Lactobacillus species in supragingival plaque in subjects with hyposalivation. Arch Oral Biol. 2010;55:255–259. | ||
Radvansky LJ, Pace MB, Siddiqui A. Prevention and management of radiation-induced dermatitis, mucositis, and xerostomia. Am J Health Syst Pharm. 2013;70:1025–1032. | ||
Wijers OB, Levendag PC, Braaksma MM, Boonzaaijer M, Visch LL, Schmitz PI. Patients with head and neck cancer cured by radiation therapy: a survey of the dry mouth syndrome in long-term survivors. Head Neck. 2002;24:737–747. | ||
Sher DJ, Balboni TA, Haddad RI, et al. Efficacy and toxicity of chemoradiotherapy using intensity-modulated radiotherapy for unknown primary of head and neck. Int J Radiat Oncol Biol Phys. 2011;80:1405–1411. | ||
Schoenfeld JD, Sher DJ, Norris CM Jr, et al. Salivary gland tumors treated with adjuvant intensity-modulated radiotherapy with or without concurrent chemotherapy. Int J Radiat Oncol Biol Phys. 2012;82:308–314. | ||
Atkinson JC, Wu AJ. Salivary gland dysfunction: causes, symptoms, treatment. J Am Dent Assoc. 1994;125:409–416. | ||
Spielman AI. Gustducin and its role in taste. J Dent Res. 1998;77:539–544. | ||
Pedersen AM, Bardow A, Jensen SB, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002;8:117–129. | ||
Ramirez-Amador V, Silverman S Jr, Mayer P, Tyler M, Quivey J. Candidal colonization and oral candidiasis in patients undergoing oral and pharyngeal radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:149–153. | ||
Chen TY, Webster JH. Oral monilia study on patients with head and neck cancer during radiotherapy. Cancer. 1974;34:246–249. | ||
Samaranayake LP, Lamey PJ. Oral candidosis: 1. clinicopathological aspects. Dent Update. 1988;15(6):227–228; 230–231. | ||
Kuntz R, Allen M, Osburn J. Xerostomia. Int J Pharm Compd. 2000;4:1176–1177. | ||
Almeida JP, Kowalski LP. Pilocarpine used to treat xerostomia in patients submitted to radioactive iodine therapy: a pilot study. Braz J Otorhinolaryngol. 2010;76(5):659–662. | ||
Koseki M, Maki Y, Matsukubo T, Ohashi Y, Tsubota K. Salivary flow and its relationship to oral signs and symptoms in patients with dry eyes. Oral Dis. 2004;10(2):75–80. | ||
Tomiita M, Takei S, Kuwada N, et al. Efficacy and safety of orally administered pilocarpine hydrochloride for patients with juvenile-onset Sjögren’s syndrome. Mod Rheumatol. 2010;20(5):486–490. | ||
Ramos-Casals M, Tzioufas AG, Stone JH, Sisó A, Bosch X. Treatment of primary Sjögren syndrome: a systematic review. JAMA. 2010;304(4):452–460. | ||
Hendrickson RG, Morocco AP, Greenberg MI. Pilocarpine toxicity and the treatment of xerostomia. J Emerg Med. 2004;26(4):429–432. | ||
Chambers MS, Jones CU, Biel MA, et al. Open-label, long-term safety study of cevimeline in the treatment of postirradiation xerostomia. Int J Radiat Oncol Biol Phys. 2007;69(5):1369–1376. | ||
Chambers MS, Posner M, Jones CU, et al. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68(4):1102–1109. | ||
Hamada T, Nakane T, Kimura T, et al. Treatment of xerostomia with the bile secretion-stimulant drug anethole trithione: a clinical trial. Am J Med Sci. 1999;318:146–151. | ||
Bagheri H, Schmitt L, Berlan M, Montastruc JL. A comparative study of the effects of yohimbine and anetholtrithione on salivary secretion in depressed patients treated with psychotropic drugs. Eur J Clin Pharmacol. 1997;52:339–342. | ||
Dyke S. Clinical management and review of Sjögren’s syndrome. Int J Pharm Compd. 2000;4:338–341. | ||
Johnstone PA, Peng YP, May BC, Inouye WS, Niemtzow RC. Acupuncture for pilocarpine-resistant xerostomia following radiotherapy for head and neck malignancies. Int J Radiat Oncol Biol Phys. 2001;50:353–357. | ||
Sagar SM. Acupuncture as an evidence-based option for symptom control in cancer patients. Curr Treat Options Oncol. 2008;9:117–126. | ||
Jedel E. Acupuncture in xerostomia – a systematic review. J Oral Rehabil. 2005;32:392–396. | ||
Jensen SB, Pedersen AM, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010;18(8):1061–1079. | ||
O’Sullivan EM, Higginson IJ. Clinical effectiveness and safety of acupuncture in the treatment of irradiation-induced xerostomia in patients with head and neck cancer: a systematic review. Acupunct Med. 2010;28:191–199. | ||
Zhuang L, Yang Z, Zeng X, et al. The preventive and therapeutic effect of acupuncture for radiation-induced xerostomia in patients with head and neck cancer: a systematic review. Integr Cancer Ther. 2013;12: 197–205. | ||
Diaz-Arnold AM, Marek CA. The impact of saliva on patient care: a literature review. J Prosthet Dent. 2002;88:337–343. | ||
Momm F, Volegova-Neher NJ, Schulte-Monting J, Guttenberger R. Different saliva substitutes for treatment of xerostomia following radiotherapy. A prospective crossover study. Strahlenther Onkol. 2005;181:231–236. | ||
Davies A. Clinically proved treatments for xerostomia were ignored. BMJ. 1998;316:1247. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

