Back to Journals » International Journal of General Medicine » Volume 14
Women with PCOS with a History of Early Pregnancy Loss Show a Higher Risk of Gestational Diabetes Mellitus
Authors Liu X, Wang L, Zuo X, Li C, Teng Y
Received 17 August 2021
Accepted for publication 21 September 2021
Published 5 October 2021 Volume 2021:14 Pages 6409—6416
DOI https://doi.org/10.2147/IJGM.S334666
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Xian Liu,1,* Lei Wang,1,* Xiaohang Zuo,2,* Chen Li,3 Yue Teng3
1Department of Reproductive Medicine, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China; 2Department of Endocrinology, Xijing Hospital, Fourth Military Medical University, Xi’an, People’s Republic of China; 3Department of Obstetrics and Gynecology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yue Teng Email [email protected]
Background: Polycystic ovary syndrome (PCOS) is quite common among women of reproductive age and can cause infertility and adverse pregnancy outcomes. Current studies on PCOS mainly focus on the effect of PCOS on pregnancy. So far, it remains unelucidated whether a history of infertility or early pregnancy loss (EPL) has differential effects on obstetric outcomes for PCOS women.
Methods: This is a retrospective case control study. Ninety-two Chinese PCOS patients with a history of EPL or infertility were recruited in our study. A total of 112 Chinese non-PCOS patients with a history of EPL or infertility were taken as control group. Measurements included anthropometric data, serum total testosterone, fasting and two-hour plasma glucose levels, and antral follicle count. After they got pregnant (naturally or via assisted reproductive technology), all obstetric outcomes were observed and analyzed.
Results: PCOS women with a history of EPL showed a much higher cesarean section (CS) rate than PCOS women with primary infertility. PCOS women with a history of EPL showed a much higher possibility of GDM (gestational diabetes mellitus) compared with PCOS women with primary infertility. PCOS women with a history of EPL showed a much higher possibility of GDM compared with non-PCOS women with a history of EPL. PCOS women with a history of EPL showed increased possibility of GDM as their BMI increased. When BMI is above 28, the incidence of GDM is significantly higher in PCOS women with a history of EPL compared with that in PCOS women with infertility.
Conclusion: Both a history of EPL and obesity are risk factors for GDM for PCOS women, and higher BMI indicates a higher possibility of GDM among PCOS women with a history of EPL. Timely intervention is in need for PCOS women with EPL and a higher BMI.
Keywords: polycystic ovarian syndrome, early pregnancy loss, infertility, obstetric complication, gestational diabetes mellitus
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among women of reproductive age. The syndrome is characterized by hyperandrogenism, anovulation, and polycystic ovaries, and there are various psychological, reproductive, and metabolic implications.1,2 PCOS is the most common cause of anovulatory infertility worldwide.3 Meanwhile, women with PCOS have a higher incidence of early pregnancy loss (EPL), defined clinically as the first trimester miscarriage. It is estimated that EPL occurs in 30–50% of PCOS women compared with 10–15% of normal women.4,5
Many studies have been done to study the effect of PCOS on pregnancy. Adverse obstetrical outcomes are commonly reported among infertile PCOS women after ART, including multiple pregnancy, miscarriage, preterm delivery, GDM, pregnancy-induced hypertension (PIH), etc.6 Possible reasons for this include relatively higher maternal age, controlled ovarian hyperstimulation protocol, cycle type, and PCOS status.7,8 A history of EPL is also found to be linked with higher recurrence risk of miscarriage and other adverse outcomes in later pregnancies.9,10 Relevant risk factors include chromosomal abnormalities, uterine abnormalities, antiphospholipid syndrome, obesity, and high risk of thrombosis.11,12 However, whether a history of infertility or EPL has differential effects on obstetric outcome for PCOS women stays unelucidated. This study aims to retrospectively evaluate whether adverse pregnancy history (infertility and EPL) among women with PCOS plays a role in leading to certain obstetric complications and pregnancy outcomes. Since both infertility and EPL are more frequently seen among PCOS women compared to normal women, it is of importance to explore their effects on pregnancy outcomes and provide clinical reference for implementing timely interventions among pregnant women with PCOS in order to improve obstetric and neonatal outcomes.
Materials and Methods
Participants
This research is a retrospective case-control study. Two hundred and four women were enrolled in total. All the participants were either diagnosed with infertility or had a history of EPL, visited the Department of Reproductive Medicine, and later delivered at the Department of Obstetrics, in the First Affiliated Hospital of Xi’an Jiaotong University (Xi’an, China) from July 2016 to December 2017. All participants were divided into two groups, PCOS group (92 women), and control group (112 women). Among all PCOS women, 37 women had a history of EPL, and 55 women were diagnosed as primary infertility. The diagnosis of PCOS was based on the 2003 Rotterdam consensus.13 PCOS was confirmed by excluding other disorders with a similar clinical presentation such as congenital adrenal hyperplasia, Cushing’s syndrome and androgen‐secreting tumors. Serum 17‐hydroxyprogesterone, thyroid‐stimulating hormone, FSH/LH, prolactin and cortisol were measured to exclude related disorders.14 A diagnosis of female infertility is defined as the inability to establish a clinical pregnancy after 12 months of regular unprotected sexual intercourse with a healthy partner.15
Among all non-PCOS women, 77 women had a history of EPL, and 35 women were diagnosed as primary infertility. None of the control subjects had clinical and/or biochemical hyperandrogenism.
All PCOS women were divided into the overweight (24<BMI<28), obese (BMI ≥28) and lean (BMI ≥ 24) groups.16 All subjects were Han nationality. The exclusion criteria for all subjects were: (a) use of hormone medication (including oral contraceptives) within the past month; or (b) use of medication affecting insulin sensitivity (eg, metformin or thiazolidinediones) within the past three months; or (c) application of ART before visiting our clinic.15 Informed consent was obtained from all participants.
Data Collection
Anthropometric data were measured and recorded from all patients including height, weight, waist circumference (WC) and hip circumference (HC). BMI was determined as the ratio between weight in kilograms and the square of height in metres. Waist‐hip ratio (WHR) was determined as the ratio between WC and HC, in centimetres.
Blood Test and Antral Follicle Count
All patients underwent serum total testosterone measurement (T). The fasting blood was sampled between days 2 and 4 of the menstrual cycle or during amenorrhoea after excluding pregnancy. An OGTT test (75g glucose protocol) was done in each patient, with fasting plasma glucose level (mmol/L) two-hour plasma glucose level measured. Antral follicle count was performed in each patient via transvaginal ultrasound (frequency of 5‐9 MHz) between days 2 and 4 of the menstrual cycle or during amenorrhoea.
Observational Indexes
All subjects in both PCOS and control groups were observed until they gave birth. Some conceived naturally, and some got pregnant under the assistance of assisted reproductive technology (ART). All subjects received regular antepartum examinations and gave birth in the Department of Obstetrics and Gynecology in the First Affiliated Hospital of Xi’an Jiaotong University. Obstetric complications and adverse pregnancy outcomes were carefully monitored, treated, recorded, and analyzed. Recorded indexes are categorized into 3 types. (1). Pregnancy complications: include gestational hypertension, gestational diabetes mellitus (GDM), premature labor, and intrahepatic cholestasis of pregnancy (ICP); (2) Abnormalities of the fetus: include fetal growth restriction, macrosomia, and fetal distress; (3) Abnormalities of fetal appendage: include polyhydramnios/ oligohydramnios, placental abruption, premature rupture of membranes (PROM), and placenta previa (PP).
Statistical Analysis
All statistical analyses and graph plots were performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). Data were presented as means ± SD. The independent sample t-test was used for comparisons between two groups. Total testosterone, fasting plasma glucose level, and two-hour plasma glucose level were SQRT‐transformed before analysis due to non‐normal distribution. Categorical variables were analyzed using the Chi‐square test and logistic regression analysis. P value <0.05 was considered statistically significant.
Results
General Characteristics
As shown in Table 1, the women with PCOS had higher BMI, WHR, total testosterone, fasting plasma glucose, two-hour plasma glucose, and fasting serum insulin levels, compared with control subjects (all P < 0.05). The women with PCOS also had a lower gestational age at birth compared with those non-PCOS women (P < 0.05). Classical morphology of PCOS includes ovarian cortical thickening, multiple tiny capsular follicular cysts, luteinized inner theca, stromal hyperplasia and multiple immature follicles, which indicate cessation of folliculogenesis.17 Antral follicle count (AFC) on ultrasound (number of small antral follicles 2–10 mm) measured during the follicular phase can serve as a useful marker of ovarian reserve. PCOS women had much higher AFC than non-PCOS women (P < 0.0001). CS (cesarean section) rate is defined as having at least one time of cesarean delivery. No difference in age and CS rate was found between two groups.
 |
Table 1 General Characteristics of PCOS Women and Control Subjects |
Among PCOS women, no significant difference was found in age, BMI, WHR, total testosterone, fasting plasma glucose, two-hour plasma glucose, fasting serum insulin, and antral follicle count between infertility and EPL subgroups (all P > 0.05) (Table 2). Intriguingly, PCOS women with a history of EPL showed a much higher CS rate than PCOS women with primary infertility (P= 0.0067, Table 2).
 |
Table 2 General Characteristics of PCOS Subgroups |
Obstetric Complications Between PCOS Subgroups
Obstetric complications for PCOS women are shown in Table 3. There was no significant difference between the two groups with respect to PIH (pregnancy-induced hypertension), preeclampsia, premature delivery, ICP (intrahepatic cholestasis of pregnancy), FGR (fetal growth restriction), macrosomia, fetal distress, polyhydramnios/oligohydramnios, placental abruption, PROM (premature rupture of membrane), and placenta previa. It has been previously reported that macrosomia may not be detectable in PCOS because of higher rate of CS and preterm birth in PCOS.18 Thus, we also compared the gestational age at birth between the subgroups in our study. The result was similar to that of the index of macrosomia: no significant difference was found (P=0.3975). However, PCOS women with a history of EPL showed a much higher possibility of GDM (gestational diabetes mellitus) compared with PCOS women with primary infertility (p=0.0296).
 |
Table 3 Obstetric Complications of PCOS Subgroups |
Obstetric Complications Between EPL Subgroups
Next, we analyzed the data of obstetric complications between EPL subgroups. Intriguingly, the result is very similar to that of PCOS subgroups. As shown in Table 4, no significant difference was found between the two groups with respect to PIH, preeclampsia, premature delivery, ICP, FGR, macrosomia, fetal distress, polyhydramnios/oligohydramnios, placental abruption, PROM, and placenta previa. Similar to results in Table 3, PCOS women with a history of EPL showed a much higher possibility of GDM compared with non-PCOS women with EPL (p=0.0006).
 |
Table 4 Obstetric Complications of EPL Subgroups |
Higher BMI Indicates a Higher Possibility of GDM Among PCOS Women with EPL
Since significant difference was found in GDM incidence between different subgroups, we then performed stratified analysis and divided all PCOS women based on their BMI, aiming to explore whether PCOS women with different BMIs show different possibility to suffer GDM when they get pregnant. As shown in Table 5 and Figure 1, the prevalence of GDM was dramatically increased with increasing BMI among PCOS patients with a history of EPL and infertile PCOS patients with a BMI < 28. Intriguingly, there is no GDM patient in the subgroup of PCOS+infertility+BMI>28. Possible reason may be the small sample size in this subgroup: there were only 4 patients with a BMI over 28 in the subgroup of PCOS with infertility. No significant difference was found between two PCOS subgroups when BMI is less than 28 (defined as overweight, 24<BMI<28; and lean, BMI≤24). However, when BMI is above 28 (defined as obese), the incidence of GDM is significantly higher in PCOS women with EPL compared with that in PCOS women with infertility (p=0.0476) (Table 5 and Figure 1). No significant difference was found between the two BMI-subgroups of PCOS patients with infertility (p=0.1792, BMI≤24 vs 24<BMI<28) (Table 5 and Figure 1).
 |
Table 5 BMI-Stratified Incidence of GDM in PCOS Subgroups |
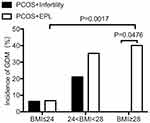 |
Figure 1 BMI-stratified incidence of GDM in PCOS subgroups. |
Next, we analyzed the incidence of GDM in two subgroups by the stratification of WHR (waist-to-hip ratio). As indicated in Table 6, no significant difference was found in prevalence of GDM between PCOS subgroups with different WHRs (Table 6, all P > 0.05).
 |
Table 6 WHR-Stratified Incidence of GDM in PCOS Subgroups |
Discussion
Our study finds PCOS women had much higher BMI, WHR, total testosterone, fasting and two-hour glucose, fasting serum insulin and antral follicle count compared with non-PCOS women, indicating PCOS women had a higher risk of metabolic disturbance compared to non-PCOS women. This is in accordance with many previous studies.19–21 Whether these metabolic disorders are connected with adverse pregnancy outcomes remains unclear. A recent study found that women with hyperandrogenism prior to pregnancy were more likely to suffer preeclampsia and preterm delivery and that a greater serum insulin and serum testosterone were predictive of adverse pregnancy outcomes.22 Whereas another study gave the opposite result: there was no difference in the obstetric outcomes when a potential influence of hyperandrogenism was sought.23
In previous studies, PCOS women were always observed and analyzed as a whole unit. In our study, we divided PCOS patients into two groups, those with infertility and those with a history of EPL. We did this for two reasons. On the one hand, primary infertility and early pregnancy loss are two main adverse complications for PCOS women at reproductive age. However, no study is available on whether a history of infertility or EPL differentially contributes to adverse pregnancy outcomes for PCOS women when they get pregnant. On the other hand, previous studies gave inconsistent conclusion on the correlation between PCOS and adverse pregnancy outcomes. Considering the heterogeneity within different PCOS patients, we aim to explore whether a history of EPL or infertility is a decisive factor for the result difference of previous studies.
Previous studies found women with PCOS are at an increased risk of developing GDM, pregnancy-induced hypertension and preeclampsia.24,25 In our study, no significant difference was found in all subgroups for all listed obstetric complications except for GDM. Specifically, we found that PCOS women with EPL showed a much higher incidence of GDM than PCOS women with infertility (p=0.0296). We also found PCOS women with EPL showed a much higher incidence of GDM than non-PCOS women with EPL (p=0.0006). Together, this indicates that both a history of EPL and the state of PCOS are independent predictive factors for GDM, and that PCOS women with a history of EPL showed highest possibility of GDM.
In a previous review, the authors suggested the link between PCOS and EPL was through hyperandrogenemia caused by the hyperinsulinemic characteristics in PCOS, which lead to a decrease in the receptivity of the uterus for implantation.26 In another review on pregnancy outcomes of PCOS women, the authors concluded that insulin resistance, with compensatory hyperinsulinemia that will lead to hyperandrogenemia, may be the link to the association between PCOS and EPL.27 Both of these indicated a close link between insulin resistance and EPL among PCOS women, which may possibly explain the higher incidence of GDM among PCOS women with a history of EPL. The reasons for no difference in other pregnancy complications between EPL and infertility subgroups remain unclear. Future studies with larger sample size are in need to certify our findings and further explore the underlying mechanism.
Our study also found that as BMI increases, the incidence of GDM among PCOS women with a history of EPL also increased. When patients were defined as obese (with a BMI above 28), the incidence of GDM is significantly higher in PCOS women with a history of EPL compared with that in PCOS women with infertility. EPL is also found to be linked with obesity in other studies. As discovered in a previous study, increased risk of EPL among PCOS women has been attributed to obesity, hyperinsulinaemia, elevated luteinizing hormone concentrations, and endometrial dysfunction.28 Thus, obesity, EPL, and PCOS may form a vicious cycle, all of which contribute to the incidence of GDM. Those findings indicate the importance of timely intervention in obstetric clinical practice. For PCOS patients with a history of EPL, a careful, strict, and dynamic monitoring and management of their BMIs is urgently important. For those high-risk women, close monitoring and management of plasma glucose level are also very important.
It should also be noted that some limitations exist in our present study. First, the sample sizes in both obesity subgroups were both small (5 and 4 patients, respectively), which may influence the statistical power to some extent. Second, only two categories of PCOS women (infertility and EPL) were enrolled and analyzed in the present study, whereas PCOS women with other features like conception modes (natural conception or conceived by Assisted Reproductive Technology), the usage of medication during pregnancy, etc. In our future study, more patients will be enrolled, which may help to enlarge the sample size and specify the features of subgroups as well.
Conclusions
Collectively, our study found PCOS women with a history of EPL showed a much higher possibility of GDM compared with PCOS women with primary infertility. PCOS women with a history of EPL showed increased possibility of GDM as their BMI increased. When BMI is above 28, the incidence of GDM is significantly higher in PCOS women with EPL compared with that in PCOS women with infertility. Both EPL and obesity are risk factors for GDM for PCOS women, and higher BMI indicates a higher possibility of GDM among PCOS women with EPL. Timely intervention is in need for PCOS women with both a history of EPL and a higher BMI.
Data Sharing Statement
All data were analyzed by SPSS 22.0 software and presented as mean ± SD.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University, PR China. Written consent was obtained from each study participant enrolled. The study complied with the requirements of the 1975 Helsinki guidelines.
Acknowledgments
The authors gratefully thank the Natural Science Basic Research Plan in Shaanxi Province of China for funding this research.
Funding
This work was supported by the Natural Science Basic Research Plan in Shaanxi Province of China (No. 2018JM7014).
Disclosure
The authors declare that they have no competing interests.
References
1. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. doi:10.1186/1741-7015-8-41
2. Anagnostis P, Tarlatzis BC, Kauffman RP. Polycystic ovarian syndrome (PCOS): long-term metabolic consequences. Metabolism. 2018;86:33–43. doi:10.1016/j.metabol.2017.09.016
3. Zeng XL, Zhang YF, Tian Q, Xue Y, An RF. Effects of metformin on pregnancy outcomes in women with polycystic ovary syndrome: a meta-analysis. Medicine. 2016;95(36):e4526. doi:10.1097/MD.0000000000004526
4. Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Roberts KA, Nestler JE. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(2):524–529. doi:10.1210/jcem.87.2.8207
5. Gray RH, Wu LY. Subfertility and risk of spontaneous abortion. Am J Public Health. 2000;90(9):1452–1454.
6. Han AR, Kim HO, Cha SW, et al. Adverse pregnancy outcomes with assisted reproductive technology in non-obese women with polycystic ovary syndrome: a case-control study. Clin Exp Reprod Med. 2011;38(2):103–108. doi:10.5653/cerm.2011.38.2.103
7. Wang JX, Davies MJ, Norman RJ. Polycystic ovarian syndrome and the risk of spontaneous abortion following assisted reproductive technology treatment. Hum Reprod. 2001;16(12):2606–2609. doi:10.1093/humrep/16.12.2606
8. Hu L, Du J, Lv H, et al. Influencing factors of pregnancy loss and survival probability of clinical pregnancies conceived through assisted reproductive technology. Reprod Biol Endocrinol. 2018;16(1):74. doi:10.1186/s12958-018-0390-6
9. Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ. 2019;364:l869.
10. San Lazaro Campillo I, Meaney S, McNamara K, O’Donoghue K. Psychological and support interventions to reduce levels of stress, anxiety or depression on women’s subsequent pregnancy with a history of miscarriage: an empty systematic review. BMJ Open. 2017;7(9):e017802. doi:10.1136/bmjopen-2017-017802
11. Zhang LM, Yang YN, Zhang RX, et al. [Comparison of the etiological constitution of two and three or more recurrent miscarriage]. Zhonghua Fu Chan Ke Za Zhi. 2018;53(12):855–859. Chinese.
12. Cavalcante MB, Sarno M, Cavalcante C, Araujo Júnior E, Barini R. Coagulation biomarkers in women with recurrent miscarriage and polycystic ovarian syndrome: systematic review and meta-analysis. Geburtshilfe Frauenheilkd. 2019;79(7):697–704. doi:10.1055/a-0884-3212
13. Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47. doi:10.1093/humrep/deh098
14. Li W, Chen Q, Xie Y, Hu J, Yang S, Lin M. Prevalence and degree of insulin resistance in Chinese Han women with PCOS: results from euglycemic-hyperinsulinemic clamps. Clin Endocrinol. 2019;90(1):138–144. doi:10.1111/cen.13860
15. Yatsenko SA, Rajkovic A. Genetics of human female infertility. Biol Reprod. 2019;101(3):549–566. doi:10.1093/biolre/ioz084
16. Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17:1–36.
17. Ghafurniyan H, Azarnia M, Nabiuni M, Karimzadeh L. The effect of green tea extract on reproductive improvement in estradiol valerate-induced polycystic ovarian syndrome in rat. Iran J Pharm Res. 2015;14(4):1215–1233.
18. Wang T, Fu H, Chen L, Xu Y. [Pregnancy complications among women with polycystic ovary syndrome in China: a meta-analysis]. Zhong Nan Da xue Xue Bao Yi Xue Ban = J Cent South Univ Med Sci. 2017;42(11):1300–1310. Chinese.
19. Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab. 2015;100(3):911–919. doi:10.1210/jc.2014-3886
20. Gilbert EW, Tay CT, Hiam DS, Teede HJ, Moran LJ. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol. 2018;89(6):683–699. doi:10.1111/cen.13828
21. Doherty DA, Newnham JP, Bower C, Hart R. Implications of polycystic ovary syndrome for pregnancy and for the health of offspring. Obstet Gynecol. 2015;125(6):1397–1406. doi:10.1097/AOG.0000000000000852
22. Christ JP, Gunning MN, Meun C, et al. Pre-conception characteristics predict obstetrical and neonatal outcomes in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(3):809–818. doi:10.1210/jc.2018-01787
23. Mumm H, Jensen DM, Sørensen JA, et al. Hyperandrogenism and phenotypes of polycystic ovary syndrome are not associated with differences in obstetric outcomes. Acta Obstet Gynecol Scand. 2015;94(2):204–211. doi:10.1111/aogs.12545
24. Hart R. PCOS and infertility. Panminerva Med. 2008;50(4):305–314.
25. Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132(2):321–336. doi:10.1097/AOG.0000000000002698
26. Jakubowicz D, Sharma ST. Insulin resistance and early pregnancy loss in polycystic ovary syndrome. In: Diumanti-Kandarakis E, Nestler JE, Panidis D, Pasquali R, editors. Contemporary Endocrinology: Insulin Resistance and Polycystic Ovarian Syndrome: Pathogenesis, Evaluation, and Treatment. Totowa, NJ: Humana Press Inc.; 2007:451–466.
27. Ghazeeri GS, Nassar AH, Younes Z, Awwad JT. Pregnancy outcomes and the effect of metformin treatment in women with polycystic ovary syndrome: an overview. Acta Obstet Gynecol Scand. 2012;91(6):658–678. doi:10.1111/j.1600-0412.2012.01385.x
28. Homburg R. Pregnancy complications in PCOS. Best Pract Res Clin Endocrinol Metab. 2006;20(2):281–292. doi:10.1016/j.beem.2006.03.009
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
