Back to Journals » Drug Design, Development and Therapy » Volume 17
Withaferin A: A Dietary Supplement with Promising Potential as an Anti-Tumor Therapeutic for Cancer Treatment - Pharmacology and Mechanisms
Authors Xing Z, Su A, Mi L, Zhang Y, He T, Qiu Y , Wei T, Li Z, Zhu J, Wu W
Received 23 May 2023
Accepted for publication 18 August 2023
Published 21 September 2023 Volume 2023:17 Pages 2909—2929
DOI https://doi.org/10.2147/DDDT.S422512
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Zhichao Xing,1,* Anping Su,1,* Li Mi,1 Yujie Zhang,1 Ting He,1 Yuxuan Qiu,2 Tao Wei,1 Zhihui Li,1 Jingqiang Zhu,1 Wenshuang Wu1
1Division of Thyroid Surgery, Department of General Surgery and Laboratory of Thyroid and Parathyroid Disease, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Department of Ultrasound, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jingqiang Zhu; Wenshuang Wu, Division of Thyroid Surgery, Department of General Surgery and Laboratory of Thyroid and Parathyroid Disease, Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University, Chengdu, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Cancer, as the leading cause of death worldwide, poses a serious threat to human health, making the development of effective tumor treatments a significant challenge. Natural products continue to serve as crucial resources for drug discovery. Among them, Withaferin A (WA), the most active phytocompound extracted from the renowned dietary supplement Withania somnifera (L.) Dunal, exhibits remarkable anti-tumor efficacy. In this manuscript, we aim to comprehensively summarize the pharmacological characteristics of WA as a potential anti-tumor drug candidate, with the objective of contributing to its further development and the discovery of prospective drugs. Through an extensive review of literature from PubMed, Science Direct, and Web of Science, we have gathered substantial evidence showcasing WA’s significant anti-tumor effects against a wide range of cancers in both in vitro and in vivo studies. Mechanistically, WA exerts its anti-tumor influence by inducing cell cycle arrest, apoptosis, autophagy, and ferroptosis. Additionally, it inhibits cell proliferation, cancer stem cells, tumor metastasis, and also suppresses epithelial-mesenchymal transition (EMT) and angiogenesis. Several studies have identified direct target proteins of WA, such as vimentin, Hsp90, annexin II and mFAM72A, while BCR-ABL, Mortalin (mtHsp70), Nrf2, and c-MYB are potential targets of WA. Notwithstanding its remarkable anti-tumor efficacy, there are some limitations associated with WA, including potential toxicity and poor oral bioavailability, which need to be addressed when considering it as an anti-tumor candidate agent. Nevertheless, I given its promising anti-tumor attributes, WA remains an encouraging candidate for future drug development. Unveiling the exact target and comprehensive mechanism of WA’s action represents a crucial research direction to pursue in the future.
Keywords: Withaferin A, Withania somnifera, dietary supplement, anti-cancer activity, pharmacological mechanism, direct target
Graphical Abstract:
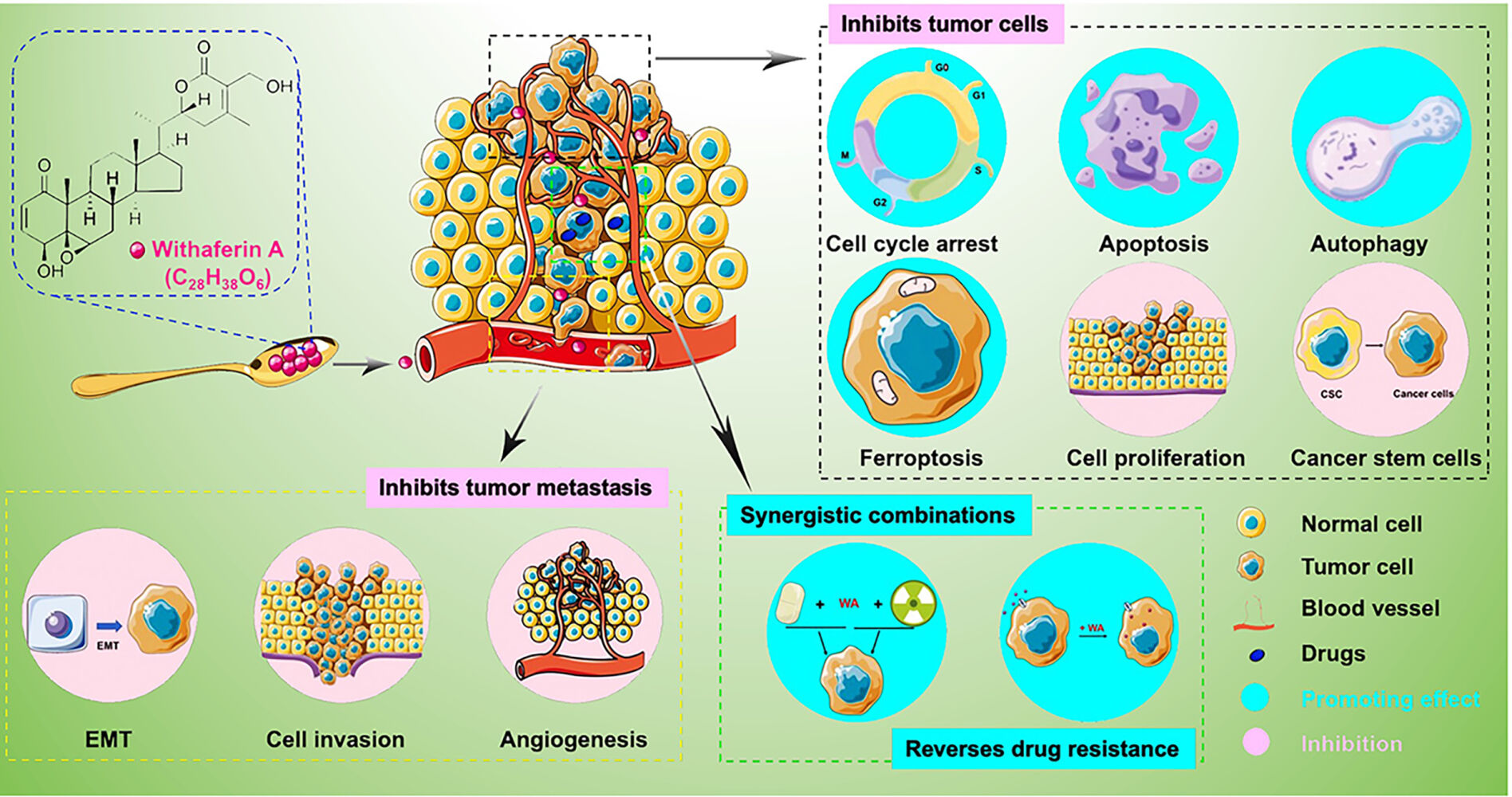
Introduction
Cancer poses a severe threat to human health and has emerged as a leading cause of death in many countries worldwide, particularly those experiencing rapid population growth and aging. According to the 2020 global cancer statistics, there were 19.3 million new cases and almost 10.0 million cancer-related deaths. It is projected that the number of cancer cases will rise to 28.4 million by the year 2040.1 Consequently, there is an urgent need for novel anti-cancer drugs and treatment approaches.
Natural products have long been vital in drug discovery due to their unique biocompatibility, novel structural backbones, and diverse pharmacological activities. Many anti-cancer agents are either natural products or direct synthetic derivatives of natural products,2 such as paclitaxel, colchicine, irinotecan (a derivative of camptothecin). However, chemotherapeutic agents often come with undesirable side effects. As a result, dietary compounds derived from food sources are being explored as potential alternatives for anti-cancer drug discovery.
One such traditional medicine is Withania somnifera (L.) Dunal (WS), also kwon as Indian ginseng (Ashwagandha) and considered the king of Ayurvedic herbs, which has been utilized for 6, 000 years in Indian.3 WS finds wide application in treating various conditions, including cancers, epilepsy, depression, arthritis, diabetes, Parkinson’s disease, schizophrenia insomnia, and hypothyroidism, and palliative effects, such as analgesic, rejuvenating, regenerating, and growth-promoting effects, as well as improvement in sexual function.4–9 Numerous research groups have investigated the chemical constituents of Ashwagandha to identify its bioactive entities. Withaferin A (WA) is a major bioactive lactone of WS (Figure 1). WA displays a wide range of activities, including anti-inflammatory, anticancer, anticoagulant, neuroprotective, hypoglycemic, hepatoprotective, and antiarthritic effects. Furthermore, recent study have reported that WA can potentially treat or prevent the COVID-19 transmission by inhibiting the virus’s S protein from binding to the host receptor.10
 |
Figure 1 Role for different cancer types of WA isolated from Withania somnifera. |
Of particular note is WA’s highly sensitive and broadly applicable anticancer efficacy. Studies have indicated that WA is a promising candidate and may become a potential treatment option for various cancers. In this review, we provide a comprehensive examination of the pharmacology and mechanism of WA as a potential drug candidate for cancer therapy. Additionally, we discuss viable strategies to overcome limitations and enhance the feasibility of WA as a novel anti-tumor agent.
The Anti-Tumor Efficacy of WA Against Various Cancers
Despite WA’s popularity as a small molecule compound with diverse bioactivities, its antitumor activity has raised significant concerns. Nevertheless, WA shows promise in the treatment of multisystem tumors (Figure 1). In this study, we demonstrated the remarkable antitumor activity of WA in cancer cells (Table 1) and animal models (Table 2). Furthermore, we provided a comprehensive summary of the progress of WA in clinical trials.
 |
Table 1 Anti-Cancerous Activities of WA in vitro |
 |
Table 2 Anti-Cancerous Activities of WA in vivo |
In vitro Anti-Tumor Effects of WA
WA exhibits significant anti-tumor efficacy against almost all type cancers, Notably, it demonstrates promising results in reproductive system tumors, such as breast cancer,11–13 cervical cancer,33 ovarian cancer,38,46 and endometrial cancer,119 as well as urinary system tumors, including prostate cancer48,49,125 and renal carcinoma.59 Additionally, WA shows potential in combating digestive system tumors, such as oral cancer,88,89,131,132 colorectal cancer (CRC),61,62 pancreatic cancer,68,69 hepatocellular carcinoma (HCC)74,128 and gastric cancer,118 as well as respiratory system tumors, including lung cancer.76,77,141 Furthermore, it displays anti-tumor effects in endocrine system tumors like adrenocortical carcinoma,116 and thyroid cancers,87,130 circulatory system tumors like lymphoma96 and leukemia97,134 nervous system tumors including glioblastoma (GBM)103 and neuroblastoma,101 and motor system tumors like osteosarcoma.109,110,142 Moreover, WA exhibits significant tumor inhibition in other types of tumors, including in uveal melanoma,93 melanoma,112 mesothelioma,111 head and neck squamous carcinoma cells117 and Ehrlich ascites.138 The anti-tumor efficacy of WA is attributed to its ability to modulate multiple signaling pathways. It inhibits cell proliferation, migration, angiogenesis, and cancer stem cells (CSCs), while also inducing cell cycle arrest, apoptosis, autophagy, ferroptosis (Table 1).
In vivo Anti-Tumor Effects of WA
Various animal models have been utilized to evaluate the in vivo anti-tumor efficacy of WA (Table 2). The results consistently demonstrate that WA exhibits potent inhibitory effect on tumor growth and metastasis across multiple cancers when administered intraperitoneal (i.p.) or per os (p.o.) at doses ranging from 1–20 mg/kg. Moreover, WA has been showed to have synergistic effects when combinations with chemotherapeutics. In in vivo experiments, observations of mouse mortality and body weight suggest that WA is well-tolerated and safe, further indicating its potential for future development.
Clinical Research of WA
Given the significant antitumor activity of WA in cancers, several clinical trials have been conducted to investigate its safety and pharmacokinetics in the clinical treatment of cancer patients. Notably, a Phase I trial conducted by Pires N et al found that WA was generally well-tolerated in patients with advanced stage high-grade osteosarcoma at doses of 72, 108, 144 and 216 mg.142 Furthermore, a recent clinical study titled “Combination therapy with liposomal doxorubicin and WA in recurrent ovarian cancer” aims to assess the feasibility and tolerance of WA in phase I and evaluate the treatment response (complete response (CR), partial response (PR), and stable disease (SD)) in recurrent ovarian cancer patients in Phase II (ClinicalTrials.gov Identifier: NCT05610735, 2022).
The Reported Direct Binding Target of WA in Cancers
Identifying the specific targets of compounds plays a crucial role in elucidating their mechanism of action and in drug development. Active natural compounds often act on multiple targets. Several studies have identified candidate targets of WA through computer calculations and molecular simulations (Table 3). BCR-ABL,143 ACE2,144 Mortalin (mtHsp70) and Nrf2145 were proposed as potential targets of WA based on these approaches. Furthermore, Clesham et al utilized the Connectivity Map (CMAP) database and identified c-MYB as a potential target of WA in acute myeloid leukemia.97
 |
Table 3 The Direct Target Proteins of WA in Cancers |
Additionally, a few articles have demonstrated that vimentin, Hsp90, and annexin II proteins directly bind to WA using WA-biotin analogs (Table 3). Paola BM et al revealed a covalent bonding between the C3 or C6 electrophilic carbon centers of WA and Cys328 of the vimentin A-helix. Moreover, hydrogen bonding was observed between the C1 position oxygen atom and Gln324 of the vimentin A-helix, as well as between the C4 hydroxyl group and Asp331 of the vimentin A’-helix.146 Yanke et al identified that WA-biotin binds to the C-terminus of Hsp90.69 Additionally, both Falsey RR et al and Gabriel Ozorowski et al demonstrated that WA directly binds to annexin II protein.147,148 However, Falsey RR et al found that WA binds covalently to the N-terminal domain of annexin A2, not Cys133. Furthermore, Jessica et al reported WA binds to mFAM72A with micromolar affinity in HeLa and HEK293T cells using biolayer interferometry.149
Molecular Mechanism of the Antitumor Effect of WA in Various Cancers
The complete understanding of the mechanisms underlying WA’s antitumor activity remains elusive; nevertheless, it appears to involve multiple effects, such as inducting cell cycle arrest, apoptosis, autophagy, ferroptosis, and suppressing invasion, metastasis, angiogenesis, and cancer stem cells.150–152 In light of this, we conducted a comprehensive review of the molecular mechanism of the antitumor activity of WA in various cancers (Figure 2).
 |
Figure 2 Mechanisms and signaling pathway of WA on cancer inhibition. |
Inducing Cell Cycle Arrest
In various cancers, including human breast cancer cells, ovarian cancer cells, cervical cancer cells,34,46 uveal melanoma cells,93 human head and neck cancer cells,117 and GBM cells,104,105 WA has been reported to induce G2/M phase arrest. The process of WA-induced G2/M arrest involves multiple cell cycle-related proteins, such as cyclin-dependent kinase 1 (Cdk1), cyclin B1, cell division cycle 25C (Cdc25C) and Cdc25B. In MCF-7 and MDA-MB-231 cells, WA treatment led to a concentration-dependent and time-dependent decrease in the expression levels of Cdk1, Cdc25C and Cdc25B, ultimately inducing G2/M arrest.153 Moreover, overexpression of Cdc25C in breast cancer cells partially prevented the G2/M arrest induced by WA.153 Similarly, WA treatment downregulated Cdc25C and induced cyclin B1 in GBM and ovarian cancer cells CaOV3 and SKOV3.34,105 Consistent with Stan’s findings,153 WA-treated CaSki cells showed an accumulation of cyclin B1, downregulation of Cdk1, decreased complex formation between cyclin B1 and Cdk1,34 and induction of the Cdk inhibitor p21.125 Additionally, clues for G2/M arrest induced by WA emerged from the observation of mitotic spindle disruption microtubules.109
Inducing Apoptosis
Apoptosis, a form of programmed cell death, is a common mechanism employed by antitumor drugs. In human cells, there are two well-characterized pathways that trigger apoptosis. One is the intrinsic pathway, which is induced by mitochondrial changes. The activation of caspases is regulated by mitochondrial Bcl-2 family proteins.154,155 Another is the extrinsic pathway, activated by death domain-containing receptors, such as CD95, TNF receptors and TNF-related apoptosis-inducing ligands.
Inducing apoptosis is the primary anti-tumor effect of WA, observed in breast cancer,13 prostate cancer, leukemia,156 melanoma,113 and head and neck cancer. WA induces the generation of the reactive oxygen species (ROS) in many cancer cells, leading to increased expression of Bak and Bax, which in turn induces mitochondrial apoptosis. Additionally, WA triggers Bak and Bax protein activation by reducing laminin and integrin gene expression.14 In CRC cells, WA induces apoptosis through ROS-mediated mitochondrial dysfunction and the JNK pathway.61 Tang’s study revealed that WA triggered intrinsic apoptosis in GBM cells via the ATF4-ATF3-CHOP axis.103 Another study demonstrated that WA induced apoptosis by inhibiting the AKT/mTOR pathway. Silvia’s research indicated that by knocking down Bim and FOXO3a levels in breast cancer cell lines, WA-induced apoptosis was significantly attenuated in vivo.13
Moreover, it has been documented that WA enhances TNFα-related apoptosis-inducing ligand-induced apoptosis (TRAIL) by decreasing the expression of c-FLIPL and c-FLIPs (cellular FLICE-like inhibitory protein), the negative regulators of apoptosis.157–160 In Seon’s study, WA induced cell apoptosis by inhibiting the phosphorylation of Axl and STAT3 induced by the growth inhibition specific protein 6 (rhGas6).161 The inhibition of STAT3 (on Tyr705) phosphorylation also results in inhibition of Janus-activated kinase 2 (JAK2) activity. Furthermore, WA causes apoptosis by downregulating STAT3-regulated genes, including Bcl-2, Bcl-xL, survivin and cyclin D1, in human renal carcinoma Caki cells.59
Inhibiting Proliferation
Additionally, WA has demonstrated the ability to inhibit tumor cell proliferation through various mechanisms. For instance, in human endometrial carcinoma, WA inhibits tumor cell proliferation by blocking the phosphorylation of TGF-β-dependent Smad2 and the expression of other TGF-β-related proteins.119 In the case of myeloma cells, WA inhibits proliferation through ROS-mediated intrinsic apoptosis.162 Moreover, WA has been reported to inhibit proliferation in chronic myeloid leukemia by targeting BCR-ABL oncogenic signaling.143 Furthermore, WA exerts its inhibitory effects on proliferation by regulating c-MYB target gene expression. This occurs through the induction of c-MYB protein ablation, resulting in reduced viability, impaired colony formation, and hindered progression of acute myeloid leukemia cells.97 Additionally, combined with blocking SGs by targeting G3BP1, WA-induced oxidative stress combined with blocking SGs by targeting G3BP1 results in reduced survival of prostate cancer.50
Inducing Autophagy
Physiologically, autophagy is a cellular process responsible for degrading macromolecules and organelles and has been shown to contribute to cell death.163,164 Inducing autophagy is an important anti-tumor mechanism of WA. In three WA-treated prostate cancer cell lines (22Rv1, PC-3 and LNCaP), autophagy induction was confirmed through the results of transmission electron microscopy and Western blot analysis. The key autophagy markers, including LC3BII, SQSTM1, Atg7 and Beclin-1, were robustly increased in WA-induced cancer cells. Moreover, GABARAPL1 was found to be involved in the cytoprotective autophagy induced by WA in prostate cancer cells.48
Additionally, WA inhibits the expression of β-catenin and p-GSK-3β proteins, leading to autophagy.165 Recent studies have shown that WA induces autophagy in both a spontaneously immortalized and nontumorigenic normal human mammary epithelial cell line (MCF-10A) and human breast cancer cells (MCF-7 and MDA-MB-231). Furthermore, WA induces autophagy in MDA-MB-231 xenograft mice.15
Inducing Ferroptosis
Ferroptosis is characterized by uncontrolled lipid peroxidation of cell membranes, resulting in membrane damage and cell death.166,167 Recent studies have reported that WA can induce a form of nonapoptotic cell death known as ferroptosis in cancer cells. This induction is attributed to the excessive activation of heme oxygenase-1 (HMOX1) by WA, which elevates intracellular labile ferrous iron (Fe2+) levels, consequently leading to the accumulation of toxic lipid radicals and triggering ferroptosis.101 Additionally, WA has been found to induce ferroptosis through directly targeting and inhibiting glutathione peroxidase 4 (GPX4), which plays a critical role in detoxifying membrane hydrogen peroxide.168 Notably, according to Emilie, due to considerable overlap in ferroptosis and apoptosis kinome activity, WA induces mixed ferroptosis and apoptosis in multiple myeloma cells.169
Suppressing Cancer Stem Cells
Cancer stem cells (CSCs) are a subpopulation of cells within tumors that possess self-renewal and differentiation capabilities, and they are believed to play a significant role in tumor initiation, growth, metastasis, and therapy resistance.39 WA has demonstrated properties in suppressing CSC as well. Kakar et al reported the role of WA in suppressing putative CSCs in ovarian cancer.38 They observed a remarkable 70–80% reduction in tumor metastasis and growth of cancerous cells. WA significantly inhibited the expressing of CSC markers, including CD24, CD44, CD117, CD34 and Oct 4, and downregulated the Hey 1, Notch 1, and Hes 1 genes. Similarly, Jade et al demonstrated that WA effectively inhibits the growth of lung CSCs, the formation of lung cancer spheroids, and decreases side population cells.76 Additionally, Kim and Singh reported that FoxQ1 is a target of WA for inhibiting breast CSCs in vivo.11 Moreover, Mayuko et al found that WA is a potent inhibitor of CSC stemness, leading to cellular senescence primarily via the induction of p21Cip1 expression.170 Therefore, WA holds promise for providing potential therapeutic benefit in various cancers by suppressing CSCs through diverse pathways, making it a promising candidate for further investigation as a potential therapeutic agent for different types of cancer.
Inhibiting Cancer Metastasis and Angiogenesis
Epithelial mesenchymal transition (EMT) is a biological process in which cells lose their epithelial characteristics and acquire mesenchymal properties, facilitating the migration and invasion of tumor cells. Studies have demonstrated that WA decreases cellular mobility by countering EMT, such as decreasing Slug (SNAI2) and Twist expression, while increasing the adhesion molecule E-cadherin expression.63,170,171 In human non-small cell lung cancer (NSCLC) cells, WA suppresses TGFβ1- and TNFα-induced EMT and inhibited cell adhesion, invasion and migration of H1299 and A549 cells. Moreover, WA inhibits EMT by preventing the nuclear translocation and phosphorylation of Smad2/3 and nuclear factor kappa B (NF-κB) in H1299 and A549 cells.77 Additionally, Chen et al reported that WA inhibits the migration of lung cancer cells by downregulating miR-27a and miR-10b, which regulate the expression of Bax and E-cadherin.78 Furthermore, WA suppresses the AK4-HIF-1α signaling axis and acts as a potent antimetastatic agent in lung cancer.79 In MMTV-neu mouse and breast cancer xenografts, WA increases E-cadherin expression and reduces vimentin expression.16 Moreover, at a concentration of 700 nM, WA suppresses breast cancer metastasis and relapse by inhibiting the urokinase-like plasminogen activator (uPA) protease, which promotes cell migration and proliferation by remodeling the extracellular matrix.17,18 Another study demonstrated that 3-azido-WA upregulates TIMP-1 and E-cadherin expression in prostate cancer cells.135 As a vimentin inhibitor, WA suppresses the migration and invasion activity of glioblastoma cells by inhibiting vimentin.172 Additionally, WA interacts with vimentin and heterogeneous nuclear ribonucleoprotein hnRNP-K to downregulate the expression of proteins involved in tumor cell metastasis, such as MMPs, VEGF, N-cadherin, and u-PA.173
Furthermore, WA exerts potent anti-angiogenic activity in vivo.174 In the Ehrlich ascites tumor model, WA exerts its anti-angiogenic activity by reducing the binding of the transcription factor specificity protein 1 (Sp1) to VEGF.175 S Saha et al demonstrated that WA has a favorable binding affinity with vascular endothelial growth factor (VEGF), leading to decreased angiogenesis.176 In another study, WA reduces macrophage infiltration and inhibits the expression of protein tyrosine kinase-2 (Pyk2), rho-associated kinase 1 (ROCK1), and VEGF in a hepatocellular carcinoma xenograft model, thereby suppressing tumor invasion and angiogenesis.177 WA can also directly interact with the hnRNP residue domain through hydrogen bonding and hydrophobic interactions, disrupting the binding between RNA-binding protein hnRNP-k (hnRNP-k) and single-stranded DNA (ssDNA). This inhibits hnRNP-k from binding to ssDNA and subsequently lowers the downstream effects of hnRNP-k, including the expression of VEGF, PERK, and MMP2, thus suppressing the migration and invasion of HT1080 fibrosarcoma cells.178 Moreover, a recent study found that WA treatment downregulates the secretion of many angiogenesis proteins in HCC.74 According to Bilal’s findings, 3-azido WA dose-dependently suppresses the expression of p-ERK and p-AKT, which may play a significant role in inhibiting angiogenesis in mouse.179 By reducing Akt signaling and MMP-9 expression, WA also decreases the invasion and migration capabilities of CasKi cells.35
Synergistic Combinations
Since the first approval of synergistic combination drugs in the 1940s, the number of approved synergistic combination has experienced significant growth. The ideal synergistic combination can improve clinical efficacy, reduce drug toxicity, and delay or prevent the development of drug resistance. As a highly effective antitumor agent, WA has been studied in combination with several other drugs.
In one combination of WA and cisplatin, WA produced ROS, while cisplatin caused DNA damage, suggesting that lower doses of cisplatin combined with suboptimal doses of WA could achieve the same effect.40 Kendra’s study demonstrated the combinatorial of sulforaphane and WA showed synergistic effects on epigenetic modifiers and cell proliferation in breast cancer cells.180 Cohen et al reported that a synergistic combination of WA and sorafenib caused G2/M arrest in anaplastic and papillary thyroid cancer cells.87 Abdullah et al reported that the combination of WA and 5-FU executed PERK axis-mediated endoplasmic reticulum (ER) stress-induced autophagy and apoptosis by upregulating the expression of ER stress sensors, such as PERK, BiP, CHOP, eIF2α, and ATF-4.64 The combination also modulated ER stress and significantly induced antiproliferative effect and cell death in CRC cells.64 Additionally, the combination of cisplatin and pemetrexed with WA synergistically inhibited wild-type epidermal growth factor receptor (EGFR) lung cancer cell viability. Moreover, WA further enhanced the cytotoxic effect of cisplatin in lung CSCs.76 The combination of WA with tumor treating fields (TTFields) showed obvious synergistic effects by significantly inhibiting tumor growth in glioma cells (GBM2, GBM39, U87-MG).106 WA and carnosol also exhibit a synergistic effect on pancreatic cancer cells through targeting pancreatic cancer stem cells by phosphorylating c-met and downregulating pluripotency maintenance genes (Oct-4 and Nanog).70
Reversing Drug Resistance
Over the past two decades, drug development in the field of oncology has predominantly focused on molecular targeted drugs. However, the rapid emergence of drug resistance due to target mutations has significantly reduced drug efficacy, making overcoming drug resistance a major challenge for current antitumor drugs. Multiple studies have found that WA has the ability to reverse drug resistance in various cancers.
Kunimasa et al demonstrated that the combination of WA and phloretin (a glucose transport inhibitor) led to a significant reduction in tumor size in gefitinib-induced drug- tolerant lung cancer, indicating that EGFR-resistant lung cancer could be effectively treated with a combination of WA and metabolism-targeting therapies.129 In sorafenib-resistant HCC cells (HepG2 and SNU449 cells), WA enhanced ferroptosis and increased Keap1 expression to counteract the effects of Nrf2 signaling activation on the ferroptosis-related protein xCT and EMT. Moreover, blockade of Keap1/Nrf2 signaling facilitated sorafenib resistance and reversed WA-induced ferroptosis. Consequently, WA attenuated sorafenib resistance and metastatic potential by regulating Keap1/Nrf2-associated ferroptosis and EMT.181 In sorafenib-resistant HepG2 cells, WA induced a dose-dependent reduction in vimentin expression, followed by a reduction in ABCG2 expression and a decrease in cell viability, induced by the inhibition of vimentin in both parental and sorafenib-resistant HepG2 cells.182 WA also repressed the invasiveness of cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) chemotherapeutic regimen-resistant DLBCL cells in collagen matrices.183 In GBM, WA could resensitize temozolomide-resistant GBM cells by depleting O6-methylguanine-DNA methyltransferase (MGMT) and inducing apoptosis through the AKT/mTOR pathway.107
Limitations of WA as a Potential Anti-Cancer Candidate and Corresponding Solutions
Despite the broad antitumor effects of WA, there were also some limitations, including potential toxicity, poor oral bioavailability, and low production.
Potential Toxicity
The toxicity of natural active compounds is often unavoidable, and WA is no exception. However, the toxicity of WA has been a subject of controversial and potential concern. As the main bioactive component of WS, there have been articles demonstrating the safety of WS extract in all tested groups.184,185 An acute and sub-acute toxicity study of oral WA also yielded similar results, showing that the LD50 of WA in mice was above 2000 mg/kg body weight.186 On the other hand, other studies found that WA exhibited certain toxic side effects in mice, with an LD50 of 54 mg/kg body weight.187,188 To address these toxicity concerns, researchers have explored structural modifications to obtain derivatives of WA with comparable but safer activity. For instance, the 2-thiophene ester-linked derivative of WA, ASR488, selectively inhibited bladder cancer cells while showing no toxicity to normal cells.189 Furthermore, a range of IC50 values for withanolides, as reported by Zhang et al, suggests the existence of various compounds within this class with potential anti-cancer activity, warranting further exploration and development.190 Notably, the ability of another WA derivate, ASR490, to inhibit the growth of colon cancer cell lines and xenotransplanted tumors without causing systemic toxicity is encouraging.191
Overall, these studies highlight the potential of WA as a source of lead compounds for the development of new anti-cancer drugs. However, further research in this area is essential to fully understand and address the potential toxicity concerns associated with WA and its derivatives.
Poor Oral Bioavailability
In addition to the potential toxicity issues, another limitation of WA is its poor oral bioavailability. Saurabh by Saurabh et al and Tianming et al reported oral bioavailability values 1.8% and 32.4 ± 4.8%, respectively, in male rats.186,192 The poor oral bioavailability of WA limits its effectiveness as a drug for cancer prevention and treatment.
To address this challenge, Farrukh et al proposed an improvised implant formulation known as “coated” implants to enhance the bioavailability of WA.193 The method involves coating polycaprolactone implants with 20–30 layers of polycaprolactone solution containing 0.5–2% of WA dissolved in dichloromethane. When compared with the ineffective intraperitoneal administration of the same total dose of WA, the drug-eluting implant significantly inhibited the growth of human lung cancer A549 xenografts in athymic nude mice. Another study by Ramesh et al also explored the use of polycaprolactone implants embedded with WA for controlled systemic delivery, resulting in nearly 60% inhibition of lung cancer A549 cell xenografts in mice.141
Low Production
Although WA can be isolated from the leaves, berry (winter cherry) and root of WS, its content is relatively low. The data showed that the concentration of major active compounds known as withanolides, represented by WA, in the leaves typically ranges from 0.001% to 0.5% of the dry weight.194 Considering the broad pharmacological effects of WA and WS, the demand for the plant in the market continues to increase. According to data, approximately 9127 tons of dried plant material are required for the production of WA in India, while the annual yield is around 5905 tons.195 Clearly, the current cultivation of the plant cannot meet the market demand. Therefore, increasing the production of withanolides in the plant has become a significant focus of attention.
Currently, there are various methods to increase withanolides production in plants. Firstly, concerning plant cultivation, it has been reported that compared to Kunapa jala, farmyard manure, and inorganic fertilizer, the application of Pancha gavya can increase withanolides production in plants.196 There also are some new technologies to be developed to increase the production of withanolides in plants by optimizing conditions to enhance the accumulation of effective metabolites or secondary metabolites. Studies have shown that inducers such as 100 ppm salicylic acid, 50 ppm jasmonic acid, and 100 ppm chitosan can be sprayed on the leaf surface,197 short-term UV-B radiation (less than 3 hours),198 or low concentrations of WcAgNPs to improve root organogenesis,199 all of which promote the synthesis of withanolides compounds in plants. Endophytes can also be used to regulate the expression of withanolides biosynthetic genes in plant leaves and roots,200 overexpress squalene synthase (SQS)201 or cycloartenol synthase,202 or use treatments involving ultrasound, vacuum infiltration, and thiol compounds (l-cys at 100 mg/l, STS at 125 mg/l, DTT at 75 mg/l) to promote the integration and expression of the gusA gene in transgenic plants,203 thereby increasing withanolides content in the transgenic plants. In addition, researchers have established a multiple shoot culture system of WS using single shoot apices as explants,204 and examined the withanolides production from adventitious root cultures,205 hairy roots,206 and cell suspension of WS,207,208 all of which aim to provide theoretical basis for efficient withanolides production in the industry.
Overall, the use of polycaprolactone implants embedded with WA shows promise as a potential therapeutic approach for the treatment of cancers. However, further research is needed to determine its safety, effectiveness, and production in humans before it can be used as a clinical treatment.
Conclusions and Outlook
The exploration of various plant extracts for their potential anti-tumor properties has been extensive. Among these extracts, WA, the primary bioactive component of the Ayurvedic herb WS, is a promising anti-tumor agent. While some studies have identified potential target proteins of WA, such as vimentin and heat shock proteins, the exact mechanism of action of WA and its comprehensive effects on cancer cells remain active areas of research. Understanding the systematic effects of WA on cancer cells is crucial for its development as an anti-tumor agent. A critical aspect in the development of WA as an anti-tumor agent is assessing its toxicity and safety. While animal studies have shown that WA is well-tolerated, further research is required to thoroughly evaluate its toxicity and potential side effects in humans. Safety is paramount in the development of any therapeutic agent. Moreover, to enhance its therapeutic effectiveness, innovative approaches to improve the bioavailability of WA are needed. Methods like drug-eluting implants and other delivery systems show promise in enhancing the delivery and targeting of WA to cancer cells.
In conclusion, WA holds immense potential as an anti-tumor agent, and its pharmacological properties have shown significant antitumor efficacy in various cancers. As research in this field continues to progress, we expect a better understanding of the precise mechanisms of WA’s action, its toxicity profile, and advancements in delivery strategies. These efforts will contribute to establishing WA as a potent and safe candidate for cancer treatment, opening new possibilities for clinical applications and improving the overall outlook in the fight against cancer.
Abbreviations
ACC, Adrenocortical carcinoma; AKT, Protein kinase B; Cdc25C, Cell division cycle 25C; Cdk1, Cyclin-dependent kinase 1; CRC, Colorectal cancer; CSCs, Cancer stem cells; DLBCL, Diffuse large B-cell lymphoma; DMBA, Dimethyl butanoic acid; EMT, Epithelial mesenchymal transition; ER, Endoplasmic reticulum; EGFR, Epidermal growth factor receptor;GBM, Glioblastoma; GPX4, Glutathione peroxidase 4; HCC, Hepatocellular carcinoma; HMOX1, Heme oxygenase-1; IC50, Half maximal inhibitory concentration; IKK, IκB kinase; IL-6, Interleukin-6; i.p., Intraperitoneal injection; JAK2, Janus-activated kinase 2; LXR-α, Liver X receptor-α; MAPK, Mitogen-activated protein kinase; MGMT, O6-methylguanine-DNA methyltransferase; NF-κB, Nuclear factor kappa B; NSCLC, Non-small cell lung cancer; rhGas6, Recombinant human growth inhibition specific protein 6; ROS, Reactive oxygen species; SO, Sorafenib; T-ALL, Lymphoblastic leukemia; TTFields, Tumor treating fields; uPA, Urokinase-like plasminogen activator; VEGF, Vascular endothelial growth factor; WA, Withaferin A; WS, Withania somnifera (L.) Dunal.
Data Sharing Statement
Data will be made available from the corresponding author on request.
Funding
This work was financially supported by the Support Program for Science and Technology Department of Sichuan Province (2021YFS0230), the Projects of Sichuan University (2018SCUH0067) and West China Hospital (19HXFH101).
Disclosure
Zhichao Xing and Anping Su are co-first authors for this study. The authors declare no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803. doi:10.1021/acs.jnatprod.9b01285
3. Dutta R, Khalil R, Green R, et al. (Ashwagandha) and Withaferin A: potential in Integrative Oncology. Int J Mol Sci. 2019;20(21):5310. doi:10.3390/ijms20215310
4. Farooqui AA, Farooqui T, Madan A, et al. Ayurvedic Medicine for the Treatment of Dementia: mechanistic Aspects. Evid Based Complement Alternat Med. 2018;2018:2481076. doi:10.1155/2018/2481076
5. Pratte MA, Nanavati KB, Young V, et al. An Alternative Treatment for Anxiety: a Systematic Review of Human Trial Results Reported for the Ayurvedic Herb Ashwagandha (Withania somnifera). J Altern Complement Med. 2014;20(12):901–908. doi:10.1089/acm.2014.0177
6. Singh RH, Narsimhamurthy K, Singh G. Neuronutrient impact of Ayurvedic Rasayana therapy in brain aging. Biogerontology. 2008;9(6):369–374. doi:10.1007/s10522-008-9185-z
7. Rege NN, Thatte UM, Dahanukar SA. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytother Res. 1999;13(4):275–291. doi:10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S
8. Dar NJ, Hamid A, Ahmad M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell Mol Life Sci. 2015;72(23):4445–4460. doi:10.1007/s00018-015-2012-1
9. Bhat JA, Akther T, Najar RA, et al. Withania somnifera (L.) Dunal (Ashwagandha); current understanding and future prospect as a potential drug candidate. Front Pharmacol. 2022;13:1029123. doi:10.3389/fphar.2022.1029123
10. Straughn AR, Kakar SS. Withaferin A: a potential therapeutic agent against COVID-19 infection. J Ovarian Res. 2020;13(1):79. doi:10.1186/s13048-020-00684-x
11. Kim S-H, Singh SV. Mammary cancer chemoprevention by withaferin A is accompanied by in vivo suppression of self-renewal of cancer stem cells. Cancer Prevent Res. 2014;7(7):738–747. doi:10.1158/1940-6207.CAPR-13-0445
12. Sehrawat A, Samanta SK, Hahm E-R, et al. Withaferin A-mediated apoptosis in breast cancer cells is associated with alterations in mitochondrial dynamics. Mitochondrion. 2019;47:282–293. doi:10.1016/j.mito.2019.01.003
13. Stan SD, Hahm E-R, Warin R, et al. Withaferin A Causes FOXO3a- and Bim-Dependent Apoptosis and Inhibits Growth of Human Breast Cancer Cells In vivo. Cancer Res. 2008;68(18):7661–7669. doi:10.1158/0008-5472.CAN-08-1510
14. Hahm E-R, Moura MB, Kelley EE, et al. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One. 2011;6(8):e23354. doi:10.1371/journal.pone.0023354
15. Hahm E-R, Singh SV. Autophagy fails to alter withaferin A-mediated lethality in human breast cancer cells. Curr Cancer Drug Targets. 2013;13(6):640–650. doi:10.2174/15680096113139990039
16. Lee J, Hahm E-R, Marcus AI, et al. Withaferin A inhibits experimental epithelial-mesenchymal transition in MCF-10A cells and suppresses vimentin protein level in vivo in breast tumors. Mol Carcinog. 2015;54(6):417–429. doi:10.1002/mc.22110
17. Szarc Vel Szic K, Op de Beeck K, Ratman D, et al. Pharmacological levels of Withaferin A (Withania somnifera) trigger clinically relevant anticancer effects specific to triple negative breast cancer cells. PLoS One. 2014;9(2):e87850. doi:10.1371/journal.pone.0087850
18. Lee J, Sehrawat A, Singh SV. Withaferin A causes activation of Notch2 and Notch4 in human breast cancer cells. Breast Cancer Res Treat. 2012;136(1):45–56. doi:10.1007/s10549-012-2239-6
19. Thaiparambil JT, Bender L, Ganesh T, et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer. 2011;129(11):2744–2755. doi:10.1002/ijc.25938
20. Nagalingam A, Kuppusamy P, Singh SV, et al. Mechanistic elucidation of the antitumor properties of withaferin a in breast cancer. Cancer Res. 2014;74(9):2617–2629. doi:10.1158/0008-5472.CAN-13-2081
21. Lu L, Shi W, Deshmukh RR, et al. Tumor necrosis factor-α sensitizes breast cancer cells to natural products with proteasome-inhibitory activity leading to apoptosis. PLoS One. 2014;9(11):e113783. doi:10.1371/journal.pone.0113783
22. Liu W, Barnette AR, Andreansky S, et al. ERBB2 Overexpression Establishes ERBB3-Dependent Hypersensitivity of Breast Cancer Cells to Withaferin A. Mol Cancer Ther. 2016;15(11):2750–2757. doi:10.1158/1535-7163.MCT-15-0932
23. Hahm ER, Lee J, Huang Y, et al. Withaferin a suppresses estrogen receptor-α expression in human breast cancer cells. Mol Carcinog. 2011;50(8):614–624. doi:10.1002/mc.20760
24. Zhang X, Mukerji R, Samadi AK, et al. Down-regulation of estrogen receptor-alpha and rearranged during transfection tyrosine kinase is associated with withaferin a-induced apoptosis in MCF-7 breast cancer cells. BMC Complement Altern Med. 2011;11(1):84. doi:10.1186/1472-6882-11-84
25. Antony ML, Lee J, Hahm E-R, et al. Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of β-tubulin. J Biol Chem. 2014;289(3):1852–1865. doi:10.1074/jbc.M113.496844
26. Samanta SK, Lee J, Hahm E-R, et al. Peptidyl-prolyl cis/trans isomerase Pin1 regulates withaferin A-mediated cell cycle arrest in human breast cancer cells. Mol Carcinog. 2018;57(7):936–946. doi:10.1002/mc.22814
27. Zhang X, Timmermann B, Samadi AK, et al. Withaferin a induces proteasome-dependent degradation of breast cancer susceptibility gene 1 and heat shock factor 1 proteins in breast cancer cells. ISRN Biochem. 2012;2012:707586. doi:10.5402/2012/707586
28. Hahm ER, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is associated with suppression of inhibitor of apoptosis family protein expression. Cancer Lett. 2013;334(1):101–108. doi:10.1016/j.canlet.2012.08.026
29. Hahm ER, Lee J, Singh SV. Role of mitogen-activated protein kinases and Mcl-1 in apoptosis induction by withaferin A in human breast cancer cells. Mol Carcinog. 2014;53(11):907–916. doi:10.1002/mc.22050
30. Ghosh K, De S, Das S, et al. Withaferin A Induces ROS-Mediated Paraptosis in Human Breast Cancer Cell-Lines MCF-7 and MDA-MB-231. PLoS One. 2016;11(12):e0168488. doi:10.1371/journal.pone.0168488
31. Muniraj N, Siddharth S, Nagalingam A, et al. Withaferin A inhibits lysosomal activity to block autophagic flux and induces apoptosis via energetic impairment in breast cancer cells. Carcinogenesis. 2019. doi:10.1093/carcin/bgz015
32. Grossman EA, Ward CC, Spradlin JN, et al. Covalent Ligand Discovery against Druggable Hotspots Targeted by Anti-cancer Natural Products. Cell Chem Biol. 2017;24(11):1368–1376.e4. doi:10.1016/j.chembiol.2017.08.013
33. Sari AN, Bhargava P, Dhanjal JK, et al. Combination of Withaferin-A and CAPE Provides Superior Anticancer Potency: bioinformatics and Experimental Evidence to Their Molecular Targets and Mechanism of Action. Cancers. 2020;12(5):1160. doi:10.3390/cancers12051160
34. Munagala R, Kausar H, Munjal C, et al. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32(11):1697–1705. doi:10.1093/carcin/bgr192
35. Lee DH, LIM I-H, SUNG E-G, et al. Withaferin A inhibits matrix metalloproteinase-9 activity by suppressing the Akt signaling pathway. Oncol Rep. 2013;30(2):933–938. doi:10.3892/or.2013.2487
36. Sherwood LC, Aqil F, Vadhanam MV, et al. Development of a goat model for evaluation of withaferin A: cervical implants for the treatment of cervical intraepithelial neoplasia. Exp Mol Pathol. 2017;103(3):320–329. doi:10.1016/j.yexmp.2017.11.008
37. Nile SH, Nile A, Gansukh E, et al. Subcritical water extraction of withanosides and withanolides from ashwagandha (Withania somnifera L) and their biological activities. Food Chem Toxicol. 2019;132:110659. doi:10.1016/j.fct.2019.110659
38. Kakar SS, Ratajczak MZ, Powell KS, et al. Withaferin a alone and in combination with cisplatin suppresses growth and metastasis of ovarian cancer by targeting putative cancer stem cells. PLoS One. 2014;9(9):e107596. doi:10.1371/journal.pone.0107596
39. Kakar SS, Parte S, Carter K, et al. Withaferin A (WFA) inhibits tumor growth and metastasis by targeting ovarian cancer stem cells. Oncotarget. 2017;8(43):74494–74505. doi:10.18632/oncotarget.20170
40. Kakar SS, Jala VR, Fong MY. Synergistic cytotoxic action of cisplatin and withaferin A on ovarian cancer cell lines. Biochem Biophys Res Commun. 2012;423(4):819–825. doi:10.1016/j.bbrc.2012.06.047
41. Straughn AR, Kelm NQ, Kakar SS. Withaferin A and Ovarian Cancer Antagonistically Regulate Skeletal Muscle Mass. Front Cell Dev Biol. 2021;9:636498. doi:10.3389/fcell.2021.636498
42. Straughn AR, Kakar SS. Withaferin A ameliorates ovarian cancer-induced cachexia and proinflammatory signaling. J Ovarian Res. 2019;12(1):115. doi:10.1186/s13048-019-0586-1
43. Pistollato F, Calderón Iglesias R, Ruiz R, et al. The use of natural compounds for the targeting and chemoprevention of ovarian cancer. Cancer Lett. 2017;411:191–200. doi:10.1016/j.canlet.2017.09.050
44. Kakar SS, Worth CA, Wang Z, et al. DOXIL when combined with Withaferin A (WFA) targets ALDH1 positive cancer stem cells in ovarian cancer. J Cancer Stem Cell Res. 2016;4.
45. Fong MY, Jin S, Rane M, et al. Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer. PLoS One. 2012;7(7):e42265. doi:10.1371/journal.pone.0042265
46. Zhang X, Samadi AK, Roby KF, et al. Inhibition of cell growth and induction of apoptosis in ovarian carcinoma cell lines CaOV3 and SKOV3 by natural withanolide Withaferin A. Gynecol Oncol. 2012;124(3):606–612. doi:10.1016/j.ygyno.2011.11.044
47. Perestelo NR, Llanos GG, Reyes CP, et al. Expanding the Chemical Space of Withaferin A by Incorporating Silicon To Improve Its Clinical Potential on Human Ovarian Carcinoma Cells. J Med Chem. 2019;62(9):4571–4585. doi:10.1021/acs.jmedchem.9b00146
48. Hahm ER, Singh SV. Cytoprotective autophagy induction by withaferin A in prostate cancer cells involves GABARAPL1. Mol Carcinog. 2020;59(10):1105–1115. doi:10.1002/mc.23240
49. Moselhy J, Suman S, Alghamdi M, et al. Withaferin A Inhibits Prostate Carcinogenesis in a PTEN-deficient Mouse Model of Prostate Cancer. Neoplasia. 2017;19(6):451–459. doi:10.1016/j.neo.2017.04.005
50. Kumar R, Nayak D, Somasekharan SP. SILAC-based quantitative MS approach reveals Withaferin A regulated proteins in prostate cancer. J Proteomics. 2021;247:104334. doi:10.1016/j.jprot.2021.104334
51. Roy RV, Suman S, Das TP, et al. Withaferin A, a Steroidal Lactone from Withania somnifera, Induces Mitotic Catastrophe and Growth Arrest in Prostate Cancer Cells. J Nat Prod. 2013;76(10):1909–1915. doi:10.1021/np400441f
52. Nishikawa Y, Okuzaki D, Fukushima K, et al. Withaferin A Induces Cell Death Selectively in Androgen-Independent Prostate Cancer Cells but Not in Normal Fibroblast Cells. PLoS One. 2015;10(7):e0134137. doi:10.1371/journal.pone.0134137
53. Kim SH, Singh KB, Hahm E-R, et al. Withania somnifera root extract inhibits fatty acid synthesis in prostate cancer cells. J Tradit Complement Med. 2020;10(3):188–197. doi:10.1016/j.jtcme.2020.02.002
54. Setty Balakrishnan A, Nathan AA, Kumar M, et al. Withania somnifera targets interleukin-8 and cyclooxygenase-2 in human prostate cancer progression. Prostate Int. 2017;5(2):75–83. doi:10.1016/j.prnil.2017.03.002
55. Kim SH, Hahm E-R, Singh KB, et al. RNA-seq reveals novel mechanistic targets of withaferin A in prostate cancer cells. Carcinogenesis. 2020;41(6):778–789. doi:10.1093/carcin/bgaa009
56. Srinivasan S, Ranga RS, Burikhanov R, et al. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67(1):246–253. doi:10.1158/0008-5472.CAN-06-2430
57. Das TP, Suman S, Alatassi H, et al. Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer. Cell Death Dis. 2016;7(2):e2111. doi:10.1038/cddis.2015.403
58. Siddique AA, Joshi P, Misra L, et al. 5,6-De-epoxy-5-en-7-one-17-hydroxy withaferin A, a new cytotoxic steroid from Withania somnifera L. Dunal leaves. Nat Prod Res. 2014;28(6):392–398. doi:10.1080/14786419.2013.871545
59. Um HJ, Min K-J, Kim DE, et al. Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of human renal carcinoma Caki cells. Biochem Biophys Res Commun. 2012;427(1):24–29. doi:10.1016/j.bbrc.2012.08.133
60. Choi MJ, Park EJ, Min KJ, et al. Endoplasmic reticulum stress mediates withaferin A-induced apoptosis in human renal carcinoma cells. Toxicol In Vitro. 2011;25(3):692–698. doi:10.1016/j.tiv.2011.01.010
61. Xia S, Miao Y, Liu S. Withaferin A induces apoptosis by ROS-dependent mitochondrial dysfunction in human colorectal cancer cells. Biochem Biophys Res Commun. 2018;503(4):2363–2369. doi:10.1016/j.bbrc.2018.06.162
62. Choi BY, Kim B-W. Withaferin-A Inhibits Colon Cancer Cell Growth by Blocking STAT3 Transcriptional Activity. J Cancer Prevention. 2015;20(3):185–192. doi:10.15430/JCP.2015.20.3.185
63. Suman S, Das TP, Sirimulla S, et al. Withaferin-A suppress AKT induced tumor growth in colorectal cancer cells. Oncotarget. 2016;7(12):13854–13864. doi:10.18632/oncotarget.7351
64. Alnuqaydan AM, Rah B, Almutary AG, et al. Synergistic antitumor effect of 5-fluorouracil and withaferin-A induces endoplasmic reticulum stress-mediated autophagy and apoptosis in colorectal cancer cells. Am J Cancer Res. 2020;10(3):799–815.
65. Chung SS, Wu Y, Okobi Q, et al. Proinflammatory Cytokines IL-6 and TNF- α Increased Telomerase Activity through NF- κ B/STAT1/STAT3 Activation, and Withaferin A Inhibited the Signaling in Colorectal Cancer Cells. Mediators Inflamm. 2017;2017:5958429. doi:10.1155/2017/5958429
66. Das T, Roy KS, Chakrabarti T, et al. Withaferin A modulates the Spindle assembly checkpoint by degradation of Mad2-Cdc20 complex in colorectal cancer cell lines. Biochem Pharmacol. 2014;91(1):31–39. doi:10.1016/j.bcp.2014.06.022
67. Koduru S, Kumar R, Srinivasan S, et al. Notch-1 inhibition by Withaferin-A: a therapeutic target against colon carcinogenesis. Mol Cancer Ther. 2010;9(1):202–210. doi:10.1158/1535-7163.MCT-09-0771
68. Li X, Zhu F, Jiang J, et al. Simultaneous inhibition of the ubiquitin-proteasome system and autophagy enhances apoptosis induced by ER stress aggravators in human pancreatic cancer cells. Autophagy. 2016;12(9):1521–1537. doi:10.1080/15548627.2016.1191722
69. Yu Y, Hamza A, Zhang T, et al. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem Pharmacol. 2010;79(4):542–551. doi:10.1016/j.bcp.2009.09.017
70. Aliebrahimi S, Kouhsari SM, Arab SS, et al. Phytochemicals, withaferin A and carnosol, overcome pancreatic cancer stem cells as c-Met inhibitors. Biomed Pharmacother. 2018;106:1527–1536. doi:10.1016/j.biopha.2018.07.055
71. Liu X, Qi W, Cooke LS, et al. An analog of withaferin A activates the MAPK and glutathione “stress” pathways and inhibits pancreatic cancer cell proliferation. Cancer Invest. 2011;29(10):668–675. doi:10.3109/07357907.2011.626478
72. Gu M, Yu Y, Gunaherath GMKB, et al. Structure-activity relationship (SAR) of withanolides to inhibit Hsp90 for its activity in pancreatic cancer cells. Invest New Drugs. 2014;32(1):68–74. doi:10.1007/s10637-013-9987-y
73. Li X, Zhu F, Jiang J, et al. Synergistic antitumor activity of withaferin A combined with oxaliplatin triggers reactive oxygen species-mediated inactivation of the PI3K/AKT pathway in human pancreatic cancer cells. Cancer Lett. 2015;357(1):219–230. doi:10.1016/j.canlet.2014.11.026
74. Shiragannavar VD, Gowda NGS, Kumar DP, et al. Withaferin A Acts as a Novel Regulator of Liver X Receptor-α in HCC. Front Oncol. 2020;10:628506. doi:10.3389/fonc.2020.628506
75. Siddharth S, Muniraj N, Saxena N, et al. Concomitant Inhibition of Cytoprotective Autophagy Augments the Efficacy of Withaferin A in Hepatocellular Carcinoma. Cancers. 2019;11(4):453. doi:10.3390/cancers11040453
76. Hsu JH. Identification of Withaferin A as a Potential Candidate for Anti-Cancer Therapy in Non-Small Cell Lung Cancer. Cancers. 2019;11(7):1003. doi:10.3390/cancers11071003
77. Kyakulaga AH, Aqil F, Munagala R, et al. Withaferin A inhibits Epithelial to Mesenchymal Transition in Non-Small Cell Lung Cancer Cells. Sci Rep. 2018;8(1):15737. doi:10.1038/s41598-018-34018-1
78. Lin CC, Yang T-Y, Lu H-J, et al. Attenuating role of withaferin A in the proliferation and migration of lung cancer cells via a p53-miR-27a/miR-10b pathway. Oncol Lett. 2021;21(3):232. doi:10.3892/ol.2021.12493
79. Jan YH, Lai T-C, Yang C-J, et al. Adenylate kinase 4 modulates oxidative stress and stabilizes HIF-1α to drive lung adenocarcinoma metastasis. J Hematol Oncol. 2019;12(1):12. doi:10.1186/s13045-019-0698-5
80. Kyakulaga AH, Aqil F, Munagala R, et al. Synergistic combinations of paclitaxel and withaferin A against human non-small cell lung cancer cells. Oncotarget. 2020;11(16):1399–1416. doi:10.18632/oncotarget.27519
81. Cai Y, Sheng Z-Y, Chen Y, et al. Effect of Withaferin A on A549 cellular proliferation and apoptosis in non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15(4):1711–1714. doi:10.7314/APJCP.2014.15.4.1711
82. Malik V, Kumar V, Kaul SC, et al. Computational Insights into the Potential of Withaferin-A, Withanone and Caffeic Acid Phenethyl Ester for Treatment of Aberrant-EGFR Driven Lung Cancers. Biomolecules. 2021;11(2):160. doi:10.3390/biom11020160
83. Liu X, Chen L, Liang T, et al. Withaferin A induces mitochondrial-dependent apoptosis in non-small cell lung cancer cells via generation of reactive oxygen species. J buon. 2017;22(1):244–250.
84. Choudhary MI, Hussain S, Yousuf S, Dar A. Chlorinated and diepoxy withanolides from Withania somnifera and their cytotoxic effects against human lung cancer cell line. Phytochemistry. 2010;71(17–18):2205–2209. doi:10.1016/j.phytochem.2010.08.019
85. Rossato Viana A, Godoy Noro B, Lenz JC, et al. Cytotoxic screening and antibacterial activity of Withaferin A. J Toxicol Environ Health A. 2022;85(16):685–698. doi:10.1080/15287394.2022.2071787
86. Llanos GG, Araujo LM, Jiménez IA, et al. Withaferin A-related steroids from Withania aristata exhibit potent antiproliferative activity by inducing apoptosis in human tumor cells. Eur J Med Chem. 2012;54:499–511. doi:10.1016/j.ejmech.2012.05.032
87. Cohen SM, Mukerji R, Timmermann BN, et al. A novel combination of withaferin A and sorafenib shows synergistic efficacy against both papillary and anaplastic thyroid cancers. Am J Surg. 2012;204(6):895–900. doi:10.1016/j.amjsurg.2012.07.027
88. Shin JA, Kim L-H, Ryu MH, et al. Withaferin A mitigates metastatic traits in human oral squamous cell carcinoma caused by aberrant claudin-1 expression. Cell Biol Toxicol. 2022;38(1):147–165. doi:10.1007/s10565-021-09584-2
89. Panjamurthy K, Manoharan S, Nirmal MR, et al. Protective role of Withaferin-A on immunoexpression of p53 and bcl-2 in 7,12-dimethylbenz(a)anthracene-induced experimental oral carcinogenesis. Invest New Drugs. 2009;27(5):447–452. doi:10.1007/s10637-008-9199-z
90. Yu TJ, Tang J-Y, Ou-Yang F, et al. Low Concentration of Withaferin a Inhibits Oxidative Stress-Mediated Migration and Invasion in Oral Cancer Cells. Biomolecules. 2020;10(5):777. doi:10.3390/biom10050777
91. Peng SY, Wang YY, Lan TH, et al. Low Dose Combined Treatment with Ultraviolet-C and Withaferin a Enhances Selective Killing of Oral Cancer Cells. Antioxidants. 2020;9(11):1120.
92. Chang HW, Li R-N, Wang H-R, et al. Withaferin A Induces Oxidative Stress-Mediated Apoptosis and DNA Damage in Oral Cancer Cells. Front Physiol. 2017;8:634. doi:10.3389/fphys.2017.00634
93. Samadi AK, Cohen SM, Mukerji R, et al. Natural withanolide withaferin A induces apoptosis in uveal melanoma cells by suppression of Akt and c-MET activation. Tumour Biol. 2012;33(4):1179–1189. doi:10.1007/s13277-012-0363-x
94. Xu K, Zhang C, Li Y, et al. Withaferin A suppresses skin tumor promotion by inhibiting proteasome-dependent isocitrate dehydrogenase 1 degradation. Transl Cancer Res. 2019;8(6):2449–2460. doi:10.21037/tcr.2019.09.57
95. Li W, Zhao Y. Withaferin A suppresses tumor promoter 12-O-tetradecanoylphorbol 13-acetate-induced decreases in isocitrate dehydrogenase 1 activity and mitochondrial function in skin epidermal JB6 cells. Cancer Sci. 2013;104(2):143–148. doi:10.1111/cas.12051
96. McKenna MK, Gachuki BW, Alhakeem SS, et al. Anti-cancer activity of withaferin A in B-cell lymphoma. Cancer Biol Ther. 2015;16(7):1088–1098. doi:10.1080/15384047.2015.1046651
97. Clesham K, Walf-Vorderwülbecke V, Gasparoli L, et al. Identification of a c-MYB-directed therapeutic for acute myeloid leukemia. Leukemia. 2022;36(6):1541–1549. doi:10.1038/s41375-022-01554-9
98. Yang ES, Choi MJ, Kim JH, et al. Combination of withaferin A and X-ray irradiation enhances apoptosis in U937 cells. Toxicol In Vitro. 2011;25(8):1803–1810. doi:10.1016/j.tiv.2011.09.016
99. Okamoto S, Tsujioka T, Suemori S-I, et al. Withaferin A suppresses the growth of myelodysplasia and leukemia cell lines by inhibiting cell cycle progression. Cancer Sci. 2016;107(9):1302–1314. doi:10.1111/cas.12988
100. Malik F, Kumar A, Bhushan S, et al. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis. 2007;12(11):2115–2133. doi:10.1007/s10495-007-0129-x
101. Hassannia B, Wiernicki B, Ingold I, et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest. 2018;128(8):3341–3355. doi:10.1172/JCI99032
102. Yco LP, Mocz G, Opoku-Ansah J, et al. Withaferin A Inhibits STAT3 and Induces Tumor Cell Death in Neuroblastoma and Multiple Myeloma. Biochem Insights. 2014;7:1–13. doi:10.4137/BCI.S18863
103. Tang Q, Ren L, Liu J, et al. Withaferin A triggers G2/M arrest and intrinsic apoptosis in glioblastoma cells via ATF4-ATF3-CHOP axis. Cell Prolif. 2020;53(1):e12706. doi:10.1111/cpr.12706
104. Shah N, Kataria H, Kaul SC, et al. Effect of the alcoholic extract of Ashwagandha leaves and its components on proliferation, migration, and differentiation of glioblastoma cells: combinational approach for enhanced differentiation. Cancer Sci. 2009;100(9):1740–1747. doi:10.1111/j.1349-7006.2009.01236.x
105. Grogan PT, Sleder KD, Samadi AK, et al. Cytotoxicity of withaferin A in glioblastomas involves induction of an oxidative stress-mediated heat shock response while altering Akt/mTOR and MAPK signaling pathways. Invest New Drugs. 2013;31(3):545–557. doi:10.1007/s10637-012-9888-5
106. Chang E, Pohling C, Beygui N, et al. Synergistic inhibition of glioma cell proliferation by Withaferin A and tumor treating fields. J Neurooncol. 2017;134(2):259–268. doi:10.1007/s11060-017-2534-5
107. Grogan PT, Sarkaria JN, Timmermann BN, et al. Oxidative cytotoxic agent withaferin A resensitizes temozolomide-resistant glioblastomas via MGMT depletion and induces apoptosis through Akt/mTOR pathway inhibitory modulation. Invest New Drugs. 2014;32(4):604–617. doi:10.1007/s10637-014-0084-7
108. Chang E, Pohling C, Natarajan A, et al. AshwaMAX and Withaferin A inhibits gliomas in cellular and murine orthotopic models. J Neurooncol. 2016;126(2):253–264. doi:10.1007/s11060-015-1972-1
109. Shohat B, Shaltiel A, Ben-Bassat M, et al. The effect of withaferin A, a natural steroidal lactone, on the fine structure of S-180 tumor cells. Cancer Lett. 1976;2(2):71–77. doi:10.1016/S0304-3835(76)80014-6
110. Li AX, Sun M, Li X. Withaferin-A induces apoptosis in osteosarcoma U2OS cell line via generation of ROS and disruption of mitochondrial membrane potential. Eur Rev Med Pharmacol Sci. 2017;21(6):1368–1374.
111. Yang H, Wang Y, Cheryan VT, et al. Withaferin A inhibits the proteasome activity in mesothelioma in vitro and in vivo. PLoS One. 2012;7(8):e41214. doi:10.1371/journal.pone.0041214
112. Nagy Z, Cheung BB, Tsang W, et al. Withaferin A activates TRIM16 for its anti-cancer activity in melanoma. Sci Rep. 2020;10(1):19724. doi:10.1038/s41598-020-76722-x
113. Mayola E, Gallerne C, Esposti DD, et al. Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis. 2011;16(10):1014–1027. doi:10.1007/s10495-011-0625-x
114. Kalthur G, Pathirissery UD. Enhancement of the response of B16F1 melanoma to fractionated radiotherapy and prolongation of survival by withaferin A and/or hyperthermia. Integr Cancer Ther. 2010;9(4):370–377. doi:10.1177/1534735410378664
115. Kalthur G, Mutalik S, Pathirissery UD. Effect of Withaferin A on the development and decay of thermotolerance in B16F1 melanoma: a preliminary study. Integr Cancer Ther. 2009;8(1):93–97. doi:10.1177/1534735408330715
116. Subramanian C, Zhang H, Gallagher R, et al. Withanolides are potent novel targeted therapeutic agents against adrenocortical carcinomas. World J Surg. 2014;38(6):1343–1352. doi:10.1007/s00268-014-2532-0
117. Samadi AK, Tong X, Mukerji R, et al. Withaferin A, a Cytotoxic Steroid from Vassobia breviflora, Induces Apoptosis in Human Head and Neck Squamous Cell Carcinoma. J Nat Prod. 2010;73(9):1476–1481. doi:10.1021/np100112p
118. Kim G, Kim T-H, Hwang E-H, et al. Withaferin A inhibits the proliferation of gastric cancer cells by inducing G2/M cell cycle arrest and apoptosis. Oncol Lett. 2017;14(1):416–422. doi:10.3892/ol.2017.6169
119. Xu K, Shi H, Du Y, Ou J. Withaferin A inhibits proliferation of human endometrial cancer cells via transforming growth factor-β (TGF-β) signalling. Biotech. 2021;11(7):323.
120. Liu X, Li Y, Ma Q, et al. Withaferin-A Inhibits Growth of Drug-Resistant Breast Carcinoma by Inducing Apoptosis and Autophagy, Endogenous Reactive Oxygen Species (ROS) Production, and Inhibition of Cell Migration and Nuclear Factor kappa B (Nf-κB)/Mammalian Target of Rapamycin (m-TOR) Signalling Pathway. Med Sci Monit. 2019;25:6855–6863. doi:10.12659/MSM.916931
121. Kim SH, Hahm E-R, Arlotti JA, et al. Withaferin A inhibits in vivo growth of breast cancer cells accelerated by Notch2 knockdown. Breast Cancer Res Treat. 2016;157(1):41–54. doi:10.1007/s10549-016-3795-y
122. Ur Rasool R, Rah B, Amin H, et al. Dual modulation of Ras-Mnk and PI3K-AKT-mTOR pathways: a Novel c-FLIP inhibitory mechanism of 3-AWA mediated translational attenuation through dephosphorylation of eIF4E. Sci Rep. 2016;6(1):18800. doi:10.1038/srep18800
123. Yang Z, Garcia A, Xu S, et al. Withania somnifera root extract inhibits mammary cancer metastasis and epithelial to mesenchymal transition. PLoS One. 2013;8(9):e75069. doi:10.1371/journal.pone.0075069
124. Samanta SK, Sehrawat A, Kim S-H, et al. Disease Subtype-Independent Biomarkers of Breast Cancer Chemoprevention by the Ayurvedic Medicine Phytochemical Withaferin A. J Natl Cancer Inst. 2017;109(6):djw293. doi:10.1093/jnci/djw293
125. Yang H, Shi G, Dou QP. The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from “Indian winter cherry”. Mol Pharmacol. 2007;71(2):426–437. doi:10.1124/mol.106.030015
126. Suman S, Das TP, Moselhy J, et al. Oral administration of withaferin A inhibits carcinogenesis of prostate in TRAMP model. Oncotarget. 2016;7(33):53751–53761. doi:10.18632/oncotarget.10733
127. Chandrasekaran B, Pal D, Kolluru V, et al. The chemopreventive effect of withaferin A on spontaneous and inflammation-associated colon carcinogenesis models. Carcinogenesis. 2018;39(12):1537–1547. doi:10.1093/carcin/bgy109
128. Kuppusamy P, Nagalingam A, Muniraj N, et al. Concomitant activation of ETS-like transcription factor-1 and Death Receptor-5 via extracellular signal-regulated kinase in withaferin A-mediated inhibition of hepatocarcinogenesis in mice. Sci Rep. 2017;7(1):17943. doi:10.1038/s41598-017-18190-4
129. Kunimasa K, Nagano T, Shimono Y, et al. Glucose metabolism-targeted therapy and withaferin A are effective for epidermal growth factor receptor tyrosine kinase inhibitor-induced drug-tolerant persisters. Cancer Sci. 2017;108(7):1368–1377. doi:10.1111/cas.13266
130. Samadi AK, Mukerji R, Shah A, et al. A novel RET inhibitor with potent efficacy against medullary thyroid cancer in vivo. Surgery. 2010;148(6):1228–1236. doi:10.1016/j.surg.2010.09.026
131. Manoharan S, Panjamurthy K, Balakrishnan S, et al. Circadian time-dependent chemopreventive potential of withaferin-A in 7,12-dimethylbenz[a]anthracene-induced oral carcinogenesis. Pharmacol Rep. 2009;61(4):719–726. doi:10.1016/S1734-1140(09)70125-2
132. Manoharan S, Panjamurthy K, Menon VP, et al. Protective effect of Withaferin-A on tumour formation in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis in hamsters. Indian J Exp Biol. 2009;47(1):16–23.
133. Li W, Zhang C, Du H, et al. Withaferin A suppresses the up-regulation of acetyl-coA carboxylase 1 and skin tumor formation in a skin carcinogenesis mouse model. Mol Carcinog. 2016;55(11):1739–1746. doi:10.1002/mc.22423
134. Sanchez-Martin M, Ambesi-Impiombato A, Qin Y, et al. Synergistic antileukemic therapies in NOTCH1 -induced T-ALL. Proc Natl Acad Sci U S A. 2017;114(8):2006–2011. doi:10.1073/pnas.1611831114
135. Amin H, Nayak D, Ur Rasool R, et al. Par-4 dependent modulation of cellular β-catenin by medicinal plant natural product derivative 3-azido Withaferin A. Mol Carcinog. 2016;55(5):864–881. doi:10.1002/mc.22328
136. Agarwalla P, Mukherjee S, Sreedhar B, et al. Glucocorticoid receptor-mediated delivery of nano gold-withaferin conjugates for reversal of epithelial-to-mesenchymal transition and tumor regression. Nanomedicine. 2016;11(19):2529–2546. doi:10.2217/nnm-2016-0224
137. Uma Devi P, Kamath R. Radiosensitizing effect of withaferin A combined with hyperthermia on mouse fibrosarcoma and melanoma. J Radiat Res. 2003;44(1):1–6. doi:10.1269/jrr.44.1
138. Sharada AC, Solomon FE, Devi PU, et al. Antitumor and radiosensitizing effects of withaferin A on mouse Ehrlich ascites carcinoma in vivo. Acta Oncol. 1996;35(1):95–100. doi:10.3109/02841869609098486
139. Devi PU, Sharada AC, Solomon FE. In vivo growth inhibitory and radiosensitizing effects of withaferin A on mouse Ehrlich ascites carcinoma. Cancer Lett. 1995;95(1–2):189–193. doi:10.1016/0304-3835(95)03892-Z
140. Shohat B, Joshua H. Effect of withaferin A on Ehrlich ascites tumor cells. II. Target tumor cell destruction in vivo by immune activation. Int J Cancer. 1971;8(3):487–496. doi:10.1002/ijc.2910080317
141. Gupta RC, Bansal SS, Aqil F, et al. Controlled-release systemic delivery - A new concept in cancer chemoprevention. Carcinogenesis. 2012;33(8):1608–1615. doi:10.1093/carcin/bgs209
142. Pires N, Gota V, Gulia A, et al. Safety and pharmacokinetics of Withaferin-A in advanced stage high grade osteosarcoma: a phase I trial. J Ayurveda Integr Med. 2020;11(1):68–72. doi:10.1016/j.jaim.2018.12.008
143. Malik V, Radhakrishnan N, Kaul SC, et al. Computational Identification of BCR-ABL Oncogenic Signaling as a Candidate Target of Withaferin A and Withanone. Biomolecules. 2022;12(2):212. doi:10.3390/biom12020212
144. Kalra RS, Kumar V, Dhanjal JK, et al. COVID19-inhibitory activity of withanolides involves targeting of the host cell surface receptor ACE2: insights from computational and biochemical assays. J Biomol Struct Dyn. 2022;40(17):7885–7898. doi:10.1080/07391102.2021.1902858
145. Surya Ulhas R, Malaviya A. In-silico validation of novel therapeutic activities of withaferin a using molecular docking and dynamics studies. J Biomol Struct Dyn. 2022;1–12. doi:10.1080/07391102.2022.2078410
146. Bargagna-Mohan P, Hamza A, Kim Y-E, et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem Biol. 2007;14(6):623–634. doi:10.1016/j.chembiol.2007.04.010
147. Falsey RR, Marron MT, Gunaherath GMKB, et al. Actin microfilament aggregation induced by withaferin A is mediated by annexin II. Nat Chem Biol. 2006;2(1):33–38. doi:10.1038/nchembio755
148. Ozorowski G, Ryan CM, Whitelegge JP, et al. Withaferin A binds covalently to the N-terminal domain of annexin A2. Biol Chem. 2012;393(10):1151–1163. doi:10.1515/hsz-2012-0184
149. Stewart JA, Bhagwat AS. A redox-sensitive iron-sulfur cluster in murine FAM72A controls its ability to degrade the nuclear form of uracil-DNA glycosylase. DNA Repair (Amst). 2022;118:103381. doi:10.1016/j.dnarep.2022.103381
150. Vanden Berghe W, Sabbe L, Kaileh M, et al. Molecular insight in the multifunctional activities of Withaferin A. Biochem Pharmacol. 2012;84(10):1282–1291. doi:10.1016/j.bcp.2012.08.027
151. Lee I-C, Choi BY. Withaferin-A--A Natural Anticancer Agent with Pleitropic Mechanisms of Action. Int J Mol Sci. 2016;17(3):290. doi:10.3390/ijms17030290
152. Chirumamilla CS, Pérez-Novo C, Van Ostade X, et al. Molecular insights into cancer therapeutic effects of the dietary medicinal phytochemical withaferin A. Proc Nutr Soc. 2017;76(2):96–105. doi:10.1017/S0029665116002937
153. Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer. 2008;60(Suppl 1):51–60. doi:10.1080/01635580802381477
154. Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102(1):1–4. doi:10.1016/S0092-8674(00)00003-9
155. Anichini A, Mortarini R, Sensi M, et al. APAF-1 signaling in human melanoma. Cancer Lett. 2006;238(2):168–179. doi:10.1016/j.canlet.2005.06.034
156. Mandal C, Dutta A, Mallick A, et al. Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade. Apoptosis. 2008;13(12):1450–1464. doi:10.1007/s10495-008-0271-0
157. Ichikawa H, Takada Y, Shishodia S, et al. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol Cancer Ther. 2006;5(6):1434–1445. doi:10.1158/1535-7163.MCT-06-0096
158. Zhang X, Zhang L, Yang H, et al. c-Fos as a proapoptotic agent in TRAIL-induced apoptosis in prostate cancer cells. Cancer Res. 2007;67(19):9425–9434. doi:10.1158/0008-5472.CAN-07-1310
159. Krueger A, Schmitz I, Baumann S, et al. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276(23):20633–20640. doi:10.1074/jbc.M101780200
160. Fukazawa T, Fujiwara T, Uno F, et al. Accelerated degradation of cellular FLIP protein through the ubiquitin-proteasome pathway in p53-mediated apoptosis of human cancer cells. Oncogene. 2001;20(37):5225–5231. doi:10.1038/sj.onc.1204673
161. Woo SM, Min K-J, Kim S, et al. Axl is a novel target of withaferin A in the induction of apoptosis and the suppression of invasion. Biochem Biophys Res Commun. 2014;451(3):455–460. doi:10.1016/j.bbrc.2014.08.018
162. Li L, Niu B, Zhang W, et al. Withaferin A inhibits cell proliferation of U266B1 and IM-9 human myeloma cells by inducing intrinsic apoptosis. Acta Biochim Pol. 2022;69(1):197–203. doi:10.18388/abp.2020_5938
163. Su M, Mei Y, Sinha S. Role of the Crosstalk between Autophagy and Apoptosis in Cancer. J Oncol. 2013;2013:102735. doi:10.1155/2013/102735
164. Bommareddy A, Hahm E-R, Xiao D, et al. Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells. Cancer Res. 2009;69(8):3704–3712. doi:10.1158/0008-5472.CAN-08-4344
165. Jung YY, Um J-Y, Chinnathambi A, et al. Withanolide modulates the potential crosstalk between apoptosis and autophagy in different colorectal cancer cell lines. Eur J Pharmacol. 2022;928:175113. doi:10.1016/j.ejphar.2022.175113
166. Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell. 2019;35(6):830–849. doi:10.1016/j.ccell.2019.04.002
167. Dixon SJ, Lemberg K, Lamprecht M, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi:10.1016/j.cell.2012.03.042
168. Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830(5):3289–3303. doi:10.1016/j.bbagen.2012.11.020
169. Logie E, Novo CP, Driesen A, et al. Phosphocatalytic Kinome Activity Profiling of Apoptotic and Ferroptotic Agents in Multiple Myeloma Cells. Int J Mol Sci. 2021;22(23):12731. doi:10.3390/ijms222312731
170. Nishi M, Akutsu H, Kudoh A, et al. Induced cancer stem-like cells as a model for biological screening and discovery of agents targeting phenotypic traits of cancer stem cell. Oncotarget. 2014;5(18):8665–8680. doi:10.18632/oncotarget.2356
171. Bolós V, Peinado H, Perez-Moreno MA, et al. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116(Pt 3):499–511. doi:10.1242/jcs.00224
172. Winter M, Meignan S, Völkel P, et al. Vimentin Promotes the Aggressiveness of Triple Negative Breast Cancer Cells Surviving Chemotherapeutic Treatment. Cells. 2021;10(6):1504. doi:10.3390/cells10061504
173. Chaudhary A, Kalra RS, Malik V, et al. 2, 3-Dihydro-3β-methoxy Withaferin-A Lacks Anti-Metastasis Potency: bioinformatics and Experimental Evidences. Sci Rep. 2019;9(1):17344. doi:10.1038/s41598-019-53568-6
174. Mohan R, Hammers H, Bargagna-mohan P, et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7(2):115–122. doi:10.1007/s10456-004-1026-3
175. Prasanna KS, Shilpa P, Salimath BP. Withaferin A suppresses the expression of vascular endothelial growth factor in Ehrlich ascites tumor cells via Sp1 transcription factor. Curr Trends Biotechnol Pharm. 2009;3(2):138–148.
176. Saha S, Islam MK, Shilpi JA, et al. Inhibition of VEGF: a novel mechanism to control angiogenesis by Withania somnifera’s key metabolite Withaferin A. Silico Pharmacol. 2013;1(1):11. doi:10.1186/2193-9616-1-11
177. Wang Y-X, Ding W-B, Dong C-W. Withaferin A Suppresses Liver Tumor Growth in a Nude Mouse Model by Downregulation of Cell Signaling Pathway Leading to Invasion and Angiogenesis. Trop J Pharm Res. 2015;14(6):1005–1011. doi:10.4314/tjpr.v14i6.10
178. Gao R, Shah N, Lee J-S, et al. Withanone-rich combination of Ashwagandha withanolides restricts metastasis and angiogenesis through hnRNP-K. Mol Cancer Ther. 2014;13(12):2930–2940. doi:10.1158/1535-7163.MCT-14-0324
179. Rah B, Amin H, Yousuf K, et al. A novel MMP-2 inhibitor 3-azidowithaferin A (3-azidoWA) abrogates cancer cell invasion and angiogenesis by modulating extracellular Par-4. PLoS One. 2012;7(9):e44039. doi:10.1371/journal.pone.0044039
180. Royston KJ, Udayakumar N, Lewis K, et al. A Novel Combination of Withaferin A and Sulforaphane Inhibits Epigenetic Machinery, Cellular Viability and Induces Apoptosis of Breast Cancer Cells. Int J Mol Sci. 2017;18(5):1092. doi:10.3390/ijms18051092
181. Zhang Y, Tan Y, Liu S. Implications of Withaferin A for the metastatic potential and drug resistance in hepatocellular carcinoma cells via Nrf2-mediated EMT and ferroptosis. Toxicol Mech Methods. 2022;1–9.
182. Makol A, Kaur H, Sharma S, et al. Vimentin as a potential therapeutic target in sorafenib resistant HepG2, a HCC model cell line. Clin Mol Hepatol. 2020;26(1):45–53. doi:10.3350/cmh.2019.0031
183. Maxwell SA, Cherry EM, Bayless KJ. Akt, 14-3-3ζ, and vimentin mediate a drug-resistant invasive phenotype in diffuse large B-cell lymphoma. Leuk Lymphoma. 2011;52(5):849–864. doi:10.3109/10428194.2010.551793
184. Mandlik Ingawale DS, Namdeo AG. Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. J Diet Suppl. 2021;18(2):183–226. doi:10.1080/19390211.2020.1741484
185. Devi PU, Sharada AC, Solomon FE, et al. In vivo growth inhibitory effect of Withania somnifera (Ashwagandha) on a transplantable mouse tumor, Sarcoma 180. Indian J Exp Biol. 1992;30(3):169–172.
186. Gupta SK, Jadhav S, Gohil D, et al. Safety, toxicity and pharmacokinetic assessment of oral Withaferin-A in mice. Toxicol Rep. 2022;9:1204–1212. doi:10.1016/j.toxrep.2022.05.012
187. Shohat B, Gitter S, Abraham A, et al. Antitumor activity of withaferin A (NSC-101088). Cancer Chemother Rep. 1967;51(5):271–276.
188. Shohat B, Gitter S, Lavie D. Effect of withaferin A on Ehrlich ascites tumor cells--cytological observations. Int J Cancer. 1970;5(2):244–252. doi:10.1002/ijc.2910050212
189. Tyagi A, Kolluru V, Chandrasekaran B, et al. ASR488, a novel small molecule, activates an mRNA binding protein, CPEB1, and inhibits the growth of bladder cancer. Oncol Lett. 2020;20(1):850–860. doi:10.3892/ol.2020.11593
190. Zhang H, Samadi AK, Cohen MS, et al. Anti-proliferative withanolides from the Solanaceae: a structure-activity study. Pure Appl Chem. 2012;84(6):1353–1367. doi:10.1351/PAC-CON-11-10-08
191. Tyagi A, Chandrasekaran B, Kolluru V, et al. ASR490, a Small Molecule, Overrides Aberrant Expression of Notch1 in Colorectal Cancer. Mol Cancer Ther. 2020;19(12):2422–2431. doi:10.1158/1535-7163.MCT-19-0949
192. Dai T, Jiang W, Guo Z, et al. Studies on oral bioavailability and first-pass metabolism of withaferin A in rats using LC-MS/MS and Q-TRAP. Biomed Chromatogr. 2019;33(9):e4573. doi:10.1002/bmc.4573
193. Aqil F, Jeyabalan J, Kausar H, et al. Multi-layer polymeric implants for sustained release of chemopreventives. Cancer Lett. 2012;326(1):33–40. doi:10.1016/j.canlet.2012.07.017
194. Akram M, Shah SA. Monograph. Withania somnifera. Altern Med Rev. 2004;9(2):211–214.
195. Sharada M, Ahuja A, Vij SP. Application of Biotechnology in Indian Ginseng (Ashwagandha): progress and prospects. Recent Adv Plant Tissue Culture Appl. 2008;1:67.
196. Ankad GM, Pai SR, Hiremath J, et al. Traditional Horticulture Practices Increase the Production of Selected Withanolides in Withania Somnifera (L.) Dunal-A RP-UFLC Analysis. J Chromatogr Sci. 2020;58(10):899–906. doi:10.1093/chromsci/bmaa057
197. Singh M, Agrawal S, Afzal O, et al. Optimization of Elicitation Conditions to Enhance the Production of Potent Metabolite Withanolide from Withania somnifera (L.). Metabolites. 2022;12(9):854. doi:10.3390/metabo12090854
198. Tripathi D, Meena RP, Pandey-Rai S. Short term UV-B radiation mediated modulation of physiological traits and withanolides production in Withania coagulans (L.) Dunal under in-vitro condition. Physiol Mol Biol Plants. 2021;27(8):1823–1835. doi:10.1007/s12298-021-01046-7
199. Tripathi D, Rai KK, Pandey-Rai S. Impact of green synthesized WcAgNPs on in-vitro plant regeneration and withanolides production by inducing key biosynthetic genes in Withania coagulans. Plant Cell Rep. 2021;40(2):283–299. doi:10.1007/s00299-020-02630-z
200. Pandey SS, Singh S, Pandey H, et al. Endophytes of Withania somnifera modulate in planta content and the site of withanolide biosynthesis. Sci Rep. 2018;8(1):5450. doi:10.1038/s41598-018-23716-5
201. Grover A, Samuel G, Bisaria VS, et al. Enhanced withanolide production by overexpression of squalene synthase in Withania somnifera. J Biosci Bioeng. 2013;115(6):680–685. doi:10.1016/j.jbiosc.2012.12.011
202. Mishra S, Bansal S, Mishra B, et al. RNAi and Homologous Over-Expression Based Functional Approaches Reveal Triterpenoid Synthase Gene-Cycloartenol Synthase Is Involved in Downstream Withanolide Biosynthesis in Withania somnifera. PLoS One. 2016;11(2):e0149691. doi:10.1371/journal.pone.0149691
203. Sivanandhan G, Kapil Dev G, Theboral J, et al. Sonication, Vacuum Infiltration and Thiol Compounds Enhance the Agrobacterium-Mediated Transformation Frequency of Withania somnifera (L.) Dunal. PLoS One. 2015;10(4):e0124693. doi:10.1371/journal.pone.0124693
204. Ray S, Jha S. Production of withaferin A in shoot cultures of Withania somnifera. Planta Med. 2001;67(5):432–436. doi:10.1055/s-2001-15811
205. Sivanandhan G, Arun M, Mayavan S, et al. Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind Crops Prod. 2012;37(1):124–129. doi:10.1016/j.indcrop.2011.11.022
206. Sivanandhan G, Kapil Dev G, Jeyaraj M, et al. Increased production of withanolide A, withanone, and withaferin A in hairy root cultures of Withania somnifera (L.) Dunal elicited with methyl jasmonate and salicylic acid. Plant Cell Tissue Organ Cult. 2013;114(1):121–129. doi:10.1007/s11240-013-0297-z
207. Sivanandhan G, Kapil Dev G, Jeyaraj M, et al. A promising approach on biomass accumulation and withanolides production in cell suspension culture of Withania somnifera (L.) Dunal. Protoplasma. 2013;250(4):885–898. doi:10.1007/s00709-012-0471-x
208. Ciddi VJ. Withaferin A from cell cultures of Withania somnifera. Indian J Pharm Sci. 2006;68(4).
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
