Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Wistar Rats Hippocampal Neurons Response to Blood Low-Density Polyethylene Microplastics: A Pathway Analysis of SOD, CAT, MDA, 8-OHdG Expression in Hippocampal Neurons and Blood Serum Aβ42 Levels
Authors Sincihu Y , Lusno MFD , Mulyasari TM, Elias SM, Sudiana IK, Kusumastuti K, Sulistyorini L, Keman S
Received 7 November 2022
Accepted for publication 26 December 2022
Published 5 January 2023 Volume 2023:19 Pages 73—83
DOI https://doi.org/10.2147/NDT.S396556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yudhiakuari Sincihu,1,2,* Muhammad Farid Dimjadi Lusno,2 Tri Marthy Mulyasari,2 Saliza Mohd Elias,3,* I Ketut Sudiana,4,* Kurnia Kusumastuti,4,* Lilis Sulistyorini,5 Soedjajadi Keman5,*
1Faculty of Medicine, Widya Mandala Surabaya Catholic University, Surabaya, Indonesia; 2Doctoral Program of Public Health, Universitas Airlangga, Surabaya, Indonesia; 3Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia; 4Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia; 5Faculty of Public Health, Universitas Airlangga, Surabaya, Indonesia
*These authors contributed equally to this work
Correspondence: Soedjajadi Keman, Faculty of Public Health, Universitas Airlangga, Ir. Soekarno Street, Mulyorejo, Surabaya City, East Java, 60115, Indonesia, Tel +62 812 1729 908, Fax +62 31 5920948, Email [email protected]
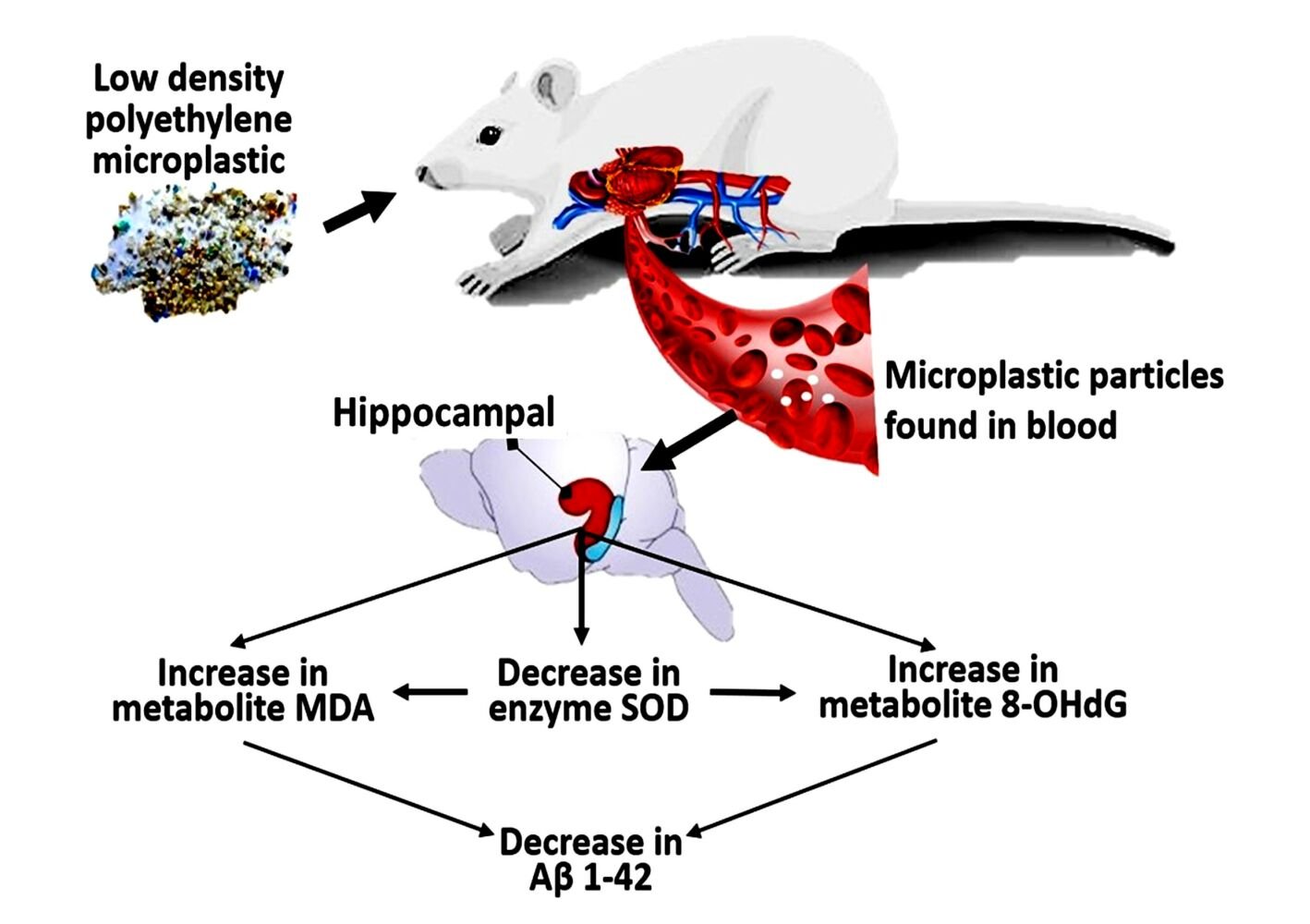
Purpose: Low-density polyethylene microplastics are ingested into the bloodstream and distributed to all the organ tissue, including the hippocampus, causing toxic effects. This research aimed to elucidate the responses of hippocampal neurons to microplastic in the blood based on the expressions of superoxide dismutase (SOD), catalase (CAT) enzymes, malondialdehyde (MDA), 8-oxo-7,8-dihydro-2-deoxyguanosine (8-OHdG) in hippocampal neurons, and blood serum amyloid beta 1– 42 (Aβ 42) levels using SMART PLS pathway analysis.
Methods: This was a pure experimental research on Wistar rats with a post-test control group design. Five experimental groups (X1, X2, X3, X4, X5) were given 0.0375 mg, 0.075 mg, 0.15 mg, 0.3 mg, and 0.6 mg of low-density polyethylene microplastics mixed in 2cc distilled water, respectively. Furthermore, except for control (C), the groups received microplastics an oral probe for 90 days.
Results: The molecular response of hippocampal neurons of Wistar rats to microplastics in the blood significantly decreased SOD enzyme expression, while CAT enzyme was unaffected. It considerably increased neuronal membrane damage (expression of MDA), increased considerably neuronal deoxyribonucleic acid damage (expression of 8-OHdG), and decreased blood serum Aβ 42 levels (pathway analysis, all t-value > 1.96).
Conclusion: The pathway analysis showed that hippocampal neurons were significantly affected by microplastic particles in the blood.
Keywords: amyloid beta 1-42, environmental pollution, hippocampal neuronal damage, microplastics, pathway analysis
Graphical Abstract:
Introduction
Microplastics (plastic particles <5 millimeters) have become a novel food contaminant for humans. Their consumption ranges from 106–113 and 126–142 particles per day in children and adults, respectively.1 The average content in food was 1.48 particles/g seafood, 0.44 particles/g sugar, 0.10 particles/g honey, 0.11 particles/g salt, 32.27 particles/L alcohol, and 94.37 particles/L water.1 This condition will worsen as plastic pollution in the environment continues to increase,2 which reached 350 million tons in 2015.3 It is estimated that this number will attain a hundred times by 2050.4 Meanwhile, only a 5–10% decrease has been achieved.1 Therefore, plastic reduction efforts have become a challenge to the United Nations Sustainable Development Goals.5
Microplastics being ingested by living things enter the digestive tract. Those up to 130 µm experience paracellular perception in intestinal epithelial cells, while sizes of <20 µm undergo phagocytosis to reach the blood circulation system.6,7 The microplastics will be circulated to all organ tissues, with those measuring <10 µm penetrating the cell membranes. They also cross the blood-brain barrier through passive transport, accumulating these particles and becoming toxic in the brain.7,8 Atamanalp et al discovered microplastic particles in the brain tissue of Mullus barbatus and Losa immaculata,9 but there are still no reports of their presence on human brain tissue. Furthermore, those not phagocytized into the circulatory system are excreted in the feces, including polyethylene.6,10,11
Microplastics in the blood and tissues will trigger oxidative stress due to free radicals.7,8,12 Research conducted using microplastics reported a decrease in antioxidant enzymes.12–15 Furthermore, disruption of the organ barrier due to reactive oxygen species (ROS) leads to the initiation of injury and cell death,16 including brain neurons.17 Continuous neuronal mortality will prevent the remaining neurons from maintaining normal brain function. This condition was the pathogenesis of Alzheimer’s disease.18 The first symptoms of hippocampal neuron damage are learning and memory impairment.17–19 However, microplastic toxicity requires the accumulation of adequate doses and time to cause cellular damage, which, when continued, will result in the development of disease symptoms.2,7,16 This research used a specific dose, time, particle size, and plastic polymer to explain the response of hippocampal neurons to microplastic ingestion as a novelty. There is still no treatment for Alzheimer’s, as an inadequate understanding of the molecular mechanisms involved in its pathogenesis is critical for successful therapy.20 This research will explain the molecular response in the hippocampus due to microplastics, specifically studying oxidative stress and damage in the neurons. Finally, oxidative stress due to free radicals is one of the hallmarks of neurodegenerative diseases.21
Microplastics in the blood induced free radicals derived from three components, namely plastic monomers, endogenous additives that are included during the production process such as phthalates, bisphenol A (BPA), nonylphenol, polybrominated diphenyl ether, and environmental pollutants that are absorbed in nature such as polychlorinated biphenyls, polycyclic aromatic hydrocarbon, 1,1,1-trichloro-2,2-bis(p-chlorophenyl) ethane, 1,1-dichloro-2,2-bis (chlorophenyl) ethylene, and heavy metal (aluminum, iron, manganese, copper, lead, zinc, silver).7,8,16,22,23 These toxic components are still found even in microplastics and contribute to negative health effects.7,24 At low levels of free radicals, intracellular antioxidants such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxide (GSH-Px) can reduce cellular damage caused by oxidative stress.25 However, continued exposure at adequate doses will damage neuronal membranes and deoxyribonucleic acid. Hydroxyl radicals cause this (OH*) and hydrogen peroxide (H2O2), resulting from reactive oxygen species. The cellular biomarker of neuronal phospholipid membrane and deoxyribonucleic acid damage, which reduce the ability to generate brain neurons, is malondialdehyde (MDA) and 8-oxo-7,8-dihydro-2-deoxyguanosine (8-OHdG), respectively.26,27
Amyloid beta 1–42 (Aβ42) was recognized as a marker for assessing cognitive abilities in Alzheimer’s disease.28 It is a protein resulting from the fragmentation of amyloid precursor by proteases. These proteins can penetrate neuronal membranes and play a role in the survival and repair of injured neurons.28,29 An increase in blood serum Aβ42 levels indicates that the fragmentation of amyloid precursor protein has occurred, which can lead to cognitive impairment. However, the fibrillation process decreased blood serum Aβ42 levels into amyloid plaques in brain neurons or reduced amyloid precursor protein synthesis due to neuron damage.18,29 Furthermore, high levels of reactive oxygen species in the body increase the deposition of amyloid plaques in brain neurons, significantly reducing cognitive abilities.28
This research aimed to elucidate the response of the hippocampal neurons of Wistar rats to low-density polyethylene microplastics based on the expression of SOD and CAT enzymes, membrane and deoxyribonucleic acid damage, and blood serum Aβ42 levels. The sample includes experimental animals that are genetically homologous to humans to model the occurrence of diseases caused by exposure to toxic material.
Materials and Methods
Research Design
This research, which was conducted at Widya Mandala Surabaya Catholic University from October 2021 to March 2022, applied pure experimental approach with a post-test control group design. Wistar rats were orally given low-density polyethylene microplastics of <20 µm diameter for 90 days. According to the Lemeshow formula (with α = 0.05 and β = 0.05), each group had seven rats randomly and equally distributed using a computerized number generator. The six research groups, namely X1, X2, X3, X4, and X5, were given microplastics of 0.0375 mg, 0.075 mg, 0.15 mg, 0.3 mg, and 0.6 mg, respectively, except for the control group (C). Wistar rats were then anesthetized using intraperitoneal injection of ketamine-xylazine, and 1cc of blood was drawn through the heart using a cardiac puncture technique. Subsequently, these experimental animals were euthanized by cervical dislocation technique. Brain organs were placed in a tissue tube containing NBF-10%, and the blood samples were taken to the Balai Besar Laboratorium Kesehatan at Surabaya to measure serum levels of Aβ42. The tissue was examined immunohistochemically based on SOD, CAT, MDA, 8-OHdG on hippocampal neurons at the Universitas Airlangga research center. The results were then statistically analyzed using the pathway analysis technique. Finally, the remaining animal parts were put in wooden boxes and cremated in order not to pollute nature.
Animal
Nine-week-old male Wistar rats weighing 140–170 grams were used in this research. The rats were purchased from the Farma Veterinary Center in Surabaya and certified. Subsequently, they were put in cages, given husks, covered with wire, received mineral water through a drinking bottle, and fed PUR594 ad libitum. The treatment environment was ensured to be clean, with stable air circulation, room temperature between 18°C and 26°C, and humidity of 40–70%.
Exposure Material
The exposure material is <20 µm diameter low-density polyethylene made from plastic bag fragmentation using Miller FCT-Z100® (Fomac, Indonesia). The plastic was milled until it turned into a fine powder, which was then filtered using an 800 mesh sieve (Anping Tianhao Wire Mesh Products Co., Ltd, China), resulting in particles measuring <20 µm. Furthermore, the exposure material was examined with a microscope to ascertain the particle size and Fourier-transform infrared spectroscopy to detect the polymer and identify the compounds contained. The plastic powder was weighed according to the dose, mixed with 2cc of distilled water, and shaken until turned into a suspension solution administered to each Wistar rats using an oral probe.
Examination of Microplastic Particles Levels in the Blood
The blood sample components were destroyed after 24 hours by adding 1cc of KOH-10% and HNO3-67% sequentially, and microplastic particles were isolated by centrifuging the solution for 2 minutes at 2300 rpm, followed by filtration with an S-PAK membrane filter 0.45 µm Millipore® mix cellulose sterile white gridded 47 mm. The filter paper was placed in a petri dish and dried at 40°C overnight. This procedure was previously used and described in detail by Monteleone.30 Finally, the microplastic particles were quantified using a binocular light microscope, and a clinical pathologist performed the examination procedure.
Immunohistochemical Staining Procedure
The brain tissue was dehydrated with alcohol for 30 minutes and cleaned with xylol 3:1 for 60 minutes, and then paraffin was used to block infiltration. The hippocampus region was reached using a 6 µm coronal rotation microtome, fixed to a slide, and then heated in an incubator at 460°C to 520°C for 24 hours. Furthermore, the object glass was deparaffinized twice with xylol and H2O, while 3% H2O2, 0.025% Trypsin, and Ultra V Block were dropped sequentially, each rinsed three times with Phosphate Buffered Saline. Monoclonal antibody (1:100) was added and incubated for 30 minutes at room temperature. This staining procedure refers to the manufactory protocol, namely SOD1/Cu-Zn SOD Antibody (JF1005) NBP2-67158 (Novus Biologicals, LLC® USA), catalase antibody [JM11-12] (GeneTex, Inc® North America), JAI-MMD-030N anti-Malondialdehyde [MDA], mAb (1F83) (AdipoGen Life Sciences, Inc® San Diego), 8-Hydroxy-2’-deoxyguanosine Antibody, and Monoclonal (Immundiagnostik AG® Germany). Biotinylated and HRP (Goat anti-Rabbit IgG Secondary Antibody for SOD and CAT, as well as Goat anti-Rabbit IgG Secondary Antibody for MDA and 8-OHdG) were also added after monoclonal. Lastly, DAB chromogen was included and incubated in a dark place for 15 minutes while washed slides were stained with Mayer’s Hematoxylin. This protocol was previously used and described by Li et al, Sakamoto et al, Topal et al, and Yang et al.31–34
Assessment of SOD, CAT, MDA, and 8-OHdG Expression
Immunohistochemical slide assessment of SOD, CAT, MDA, and 8-OHdG expression in the neuron hippocampus of Wistar rats was conducted using a binocular light microscope with 400x magnification. Furthermore, the mean number of hippocampal neurons expressing SOD, CAT, MDA, and 8-OHdG was calculated sequentially in each of the nine visual fields and recorded on the examination sheet. This expression was shown in dark brown neuron cells due to the use of DAB chromogen.
Examination of Aβ42 Levels in Blood Serum
Aβ42 in blood serum was examined with Sandwich-ELISA following the Rat Aβ1-42 ELISA Kit protocol (Elabscience Biotechnology Inc®, USA). A total of 1cc of Wistar rats whole blood was put in a plain vacutainer and left for 24 hours at 8°C. Subsequently, 100 μL of each standard, blank, and blood sample dilution was added into the appropriate wells, then covered with sealer and incubated for 90 minutes at 37°C. Immediately, 100 μL Biotinylated Detection Ab working solution was included in each well, covered with a new sealer, and incubated for 60 minutes. A total of 100 μL HRP conjugate working solution was added, followed by 90 μL substrate reagent, and they were incubated for 15–30 minutes. Finally, 50 μL of stop solution was added. The optical density of each well was determined with a microplate reader set to 450 nm, and the protein complex Aβ42 will fluoresce; hence, the number can be deduced. A clinical pathologist performed this examination procedure.
Statistical Test
Statistical analysis of examination results was performed at least three times independently using SPSS for Windows version 18.0. The table shows the results as mean ± standard deviation (SD). Pathway analysis was conducted using SMART PLS to elucidate the response of Wistar rats hippocampal neurons to low-density polyethylene microplastics. This is a multiple regression analysis that correlates between variables to determine the pathogenesis model of disease. It is used to determine the pathogenesis of changes in Aβ42 levels due to microplastic intake. The significance of pathway analysis was set at a t-value >1.96 (95% confidence interval). The direction and strength of the relationship were based on its coefficient value. Furthermore, the research data were not normally distributed according to the Shapiro–Wilk test (p-value <0.05).
Ethics Statement
This research has followed the Institutional Animal Care and Use Committee (IACUC) guidelines on the Welfare of Laboratory Animals and received an ethical certificate from the Health Research Ethics Committee (HREC) of Widya Mandala University in August 2021 (Reference number 209/WM12/KEPK/DOSEN/T/2021).
Results
Description of Exposure Material
Fourier-transform infrared spectroscopy of the exposed material showed peaks of 2848–2915 cm−1, 1396–1463 cm−1, and 875 cm−1 at the end of the waves, which are probably the C-H Alkene functional groups, a characteristic of low-density polyethylene. In addition, there are 875 cm−1 waves of aromatic rings, 1050–1300 cm−1 waves of C-O Alcohol/Ether/Carboxylic Acid/Esters functional groups, and 1180–1360 cm−1 waves which are C-N Amine. Other compounds identified are NO2 and OH, as shown in Figure 1A. According to Figure 1B, observations on a microscope with 400x magnification and 10 µm scale showed that the low-density polyethylene microplastic particles used in this research were <18 µm in diameter with sharp and irregular edges.
 |
Figure 1 (A) Fourier-transform infrared spectroscopy examination. (B) Microscope observation of low-density polyethylene microplastic particles. |
Description of Microplastic Particles Levels in the Blood
Table 1 shows that the number of microplastic particles found in the blood of Wistar rats increased with higher doses of exposure. The group exposed to 0.15 mg daily showed the highest increase compared to the other groups.
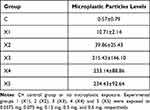 |
Table 1 Mean ±SD Microplastic Particles Levels in Blood (in Particle/mL) |
Description of SOD, CAT, MDA, and 8-OHdG Expression in Hippocampal Neurons
Table 2 shows that the mean expression of SOD enzymes in hippocampal neurons of Wistar rats increased in small doses of microplastics by 0.0375 mg and 0.075 mg compared to the control group. The number expression was lower in the group with higher doses. Meanwhile, the mean expression of the CAT enzyme in hippocampal neurons increased at 0.0375 mg, 0.075 mg, and 0.15 mg. There was a decreased in the group exposed to high doses of microplastics but not below the control. The mean expression of MDA and 8-OHdG metabolites were higher in the microplastic exposed group. However, it was observed that the numerical expression of the 8-OHdG metabolite was higher than the number expression of MDA. Figure 2 shows the microscopic images for immunohistochemical staining (expression of SOD, CAT, MDA, and 8-OHdG).
 |
Table 2 Mean ±SD Expression of SOD, CAT, MDA, and 8-OHdG in Hippocampal Neurons (in Cells/Field of View) |
Description of Aβ42 Levels in Blood Serum
In Table 3, the mean blood serum Aβ42 level of Wistar rats in all groups exposed to microplastics was lower than the control. Meanwhile, it increased in the experimental group when exposed to small doses of 0.075 mg and 0.15 mg, then decreased at higher doses. The lowest Aβ42 levels were observed in X5, which received the highest microplastic exposure dose of 0.6 mg.
 |
Table 3 Mean ±SD Aβ42 Levels in Blood Serum (in Pg/mL) |
Pathway Analysis of Hippocampal Neuron Response to Blood Microplastics
The path analysis in Figure 3 described that hippocampal neurons of Wistar rats responded to microplastics by decreasing the synthesis of the intracellular antioxidant enzyme. This was due to the ingestion in Wistar rats, which significantly increased the number of particles in their blood. Statistically, the large number of microplastics in the blood resulted in lower SOD enzyme expression (t-value >1.96). However, this result did not occur in the expression of the CAT enzyme. The low levels of SOD expression caused membrane (seen from the expression of MDA metabolites) and deoxyribonucleic acid damages (seen from the expression of 8-OHdG metabolites) in the hippocampal neurons (t-value >1.96). The increase in MDA and 8-OHdG metabolites correlated with decreased blood Aβ42 levels in Wistar rats. However, the expression of MDA significantly lowered Aβ42 levels in blood serum based on path coefficients.
 |
Figure 3 Pathway analysis of hippocampal neuron response to microplastic in blood. |
Discussion
The microplastic particles have been found in the blood of Wistar rats (Table 1). Oxygen-dependent and independent mechanisms will destroy microplastic particles in the blood of Wistar rats through oxidative processes and inflammatory mediators, respectively.26 However, this mechanism cannot destroy the dense particles; hence, the process which initiates the formation of reactive oxygen species continues.14,16 Plastic particles dense, sharp, and rigid edges will cause injury to the biological components they touch. Hydrophobic microplastics associated with organic chemicals (HOCs), such as aromatic compounds, carbon chains in the alcohol/ether/carboxylic acid/ester groups, and carbon amine chains (Figure 1A) discovered in the exposed material also play a role in the cytotoxicity mechanism of these particles. These conditions will trigger a cellular response, namely the synthesis of antioxidant enzymes to compensate for the free radical compounds formed.
SOD and CAT enzymes will reduce free radicals in hippocampal neurons exposed to microplastics. SOD is a metalloenzyme that plays a role in catalyzing the radical reduction reaction of superoxide anion (O2*) to hydrogen peroxide (H2O2) and oxygen (O2). Furthermore, O2* was an unpaired electron from toxic microplastic material. One molecule of it is reduced through the phosphorylation process in the mitochondria of neuronal cells by 4 electrons and H+ to form 2 molecules of H2O. The process is stated to be complete, and O2* becomes a free radical when the number of reduction electrons is less than 4. The H2O2 produced from the above reaction was converted into H2O and O2 by the CAT, together with the GSH-Px enzymes. This explains that the expression of SOD and CAT enzymes was increased in the experimental group exposed to small doses of microplastics and decreased due to higher doses. In experimental group 5 (X5), the SOD enzyme expression level was lower than that of the control group, whereas its CAT counterpart was not lower (Table 2). This is because the SOD enzyme first performs the role of the reduction reaction against free radical compounds.
This result was comparable to that of Wang et al35 which showed a decrease and increase in SOD and CAT enzyme levels, respectively, in the body of Eisenia fetida using low-density polyethylene of about 20×104 mg/Kg with a diameter of 300 µm. According to Yu et al,36 the expression of SOD enzymes in the digestive tissue of Eriocheir sinensis increased in the low-dose group (40 g/L and 400 g/L), but decreased in the high-dose group (4.000 g/L and 40.000 g/L) after exposure to polystyrene with a diameter of 0.5 µm. Revel et al37 using polyethylene with a diameter of 0.4 µm for 10 days, reported an increase in the expression of SOD and CAT in digestive tissue at 0.008 g/L and 10 g/L doses. On exposure to 100 g/L, there was a decrease in the expression of SOD, while CAT still increased. The research by Magni et al38 on Dreissena polymorpha exposed to polystyrene of about 1×106 particle/L, with a diameter of 10 µm, also showed an increase in CAT in the body. According to Rodriguez-Saijo et al,39 using low-density polyethylene with a diameter of 250–1000 µm at about 62.5–1000 mg/Kg indicated a decrease in CAT. The dose and application are huge compared to those employed in this research and other above. The neurotoxic effect of microplastics in the brain tissue was size and concentration-dependent.40 Long-term exposure results in a decrease in cell viability, and it also changes gene expression and neural tissue patterns.40
Li et al15 stated that lower antioxidant enzymes correlated with higher cellular damage triggered by oxidative stress. In human cerebral cells, oxidative stress is one of the cytotoxic mechanisms that explain the effects of microplastics.41 This research indicates that membrane and deoxyribonucleic acid damage in the hippocampus neurons increased with higher dose of exposure, as shown in Table 2. There is an increase in antioxidant enzymes at X1, X2, and X3, but damage to the membranes and deoxyribonucleic acid of hippocampal neurons still occurred. This is because of the imbalanced ratio between antioxidants and oxidant levels. The number of oxidants continues to increase due to the microplastic administration for 90 days, while the amount of synthesized antioxidants is insufficient to compensate. Furthermore, the number of damaged neurons also reduces the amount of antioxidant enzyme synthesis. Figure 2 is a representative microscopic image of the expression of neuronal damage and cellular antioxidants.
Oxidative stress occurs after cellular antioxidants decrease and can no longer perform the reduction process. It is one of the body’s biological responses to microplastic exposure due to high levels of free radicals.7,8,27,42 Furthermore, oxidative stress causes neurodegeneration in the brain.43 Deng et al12 stated that exposure to microplastics will decrease cellular antioxidant enzymes and increase the expression of MDA and 8-OHdG metabolites as indicators of free radical reactions in the body of rats. Another research by Chang et al44 showed an increase in oxidative stress with decreased SOD and CAT gene expression and an increase in MDA and 8-OHdG metabolites. Perez et al45 reviewed that the compounds in plastic trigger oxidative stress in mammals with biomarkers of increased expression of MDA and 8-OHdG in the body.
There is an attempt to repair the injured neurons during the process of membrane and deoxyribonucleic acid damage in hippocampal neurons. Amyloid precursor protein plays a role in protecting and restoring these neurons.46 Aβ42 is a fragmentation of the amyloid precursor protein by proteases,29,47 and its absence renders neuronal injury irreversible. Elevated levels of Aβ42 in the blood explain the extent of neuronal damage. Furthermore, it will undergo fibrillation into amyloid plaques in brain neurons. A decrease in its levels in the blood indicates the formation of amyloid plaques.18 Damaged neurons also decrease the synthesis of the amyloid precursor protein. The level of Aβ42 in the blood increased and decreased in the small and high dose groups, respectively, as shown in Table 3. A fibrillation process allegedly converted Aβ42 in the blood into amyloid plaques in brain neurons at 0.6 mg group. Further evidence is needed by examining amyloid plaques in hippocampal neurons. Statistically, these results showed an effect between increased expression of MDA and 8-OHdG metabolites, which decreased levels of Aβ42 in the blood serum of Wistar rats (t-value >1.96), as presented in Figure 3.
This research was limited by the inability to determine when the decrease in Aβ42 is due to its fibrillation into amyloid plaques in the neurons of the brain or damage resulting in low amyloid precursor protein synthesis. Furthermore, it did not show the presence of microplastic particles in hippocampal neurons. The plaques in the neuron’s hippocampus should be identified to clarify the pathogenesis of amyloid accumulation changes due to blood microplastic. The particles in the hippocampus need to be examined to explain the accumulation rate and particle size.
Conclusion
The pathway analysis showed that hippocampal neurons were significantly affected by microplastic particles in the blood. The molecular response indicates a decrease in the neuronal superoxide dismutase enzyme, damage to the neuronal membrane, and deoxyribonucleic acid, followed by a decrease in Aβ42 levels in blood serum.
Acknowledgments
The authors are grateful to the Universitas Airlangga for the support provided under grant number 777/UN3.15/PT/2021. The authors are also grateful to all staff at the Biomedical Laboratory of Widya Mandala Surabaya Catholic University, Universitas Airlangga research center, and Balai Besar Laboratorium Kesehatan Surabaya as a place for conducting research, and to Adi Pramono, Ellyza Setya Maryiantari, and Anang Subagio who are involved in this research.
Disclosure
There are no conflicts of interest in this research.
References
1. Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. Human consumption of microplastics. Environ Sci Technol. 2019;53(12):7068–7074. doi:10.1021/acs.est.9b01517
2. Rhodes CJ. Plastic pollution and potential solutions. Sci Prog. 2018;101(3):207–260. doi:10.3184/003685018X15294876706211
3. Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3:1–5. doi:10.1126/sciadv.1700782
4. Seltenrich N. New link in the food chain? Marine plastic pollution and seafood safety. Environ Health Perspect. 2015;123(2):34–42. doi:10.1289/ehp.123-A34
5. Borrelle SB, Ringma J, Law KL, et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Sceince. 2020;369:1515–1518. doi:10.1126/science.aba3656
6. Leslie HA, Velzen MJ, Van, Brandsma SH, Vethaak AD, Vellejo JJG, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;107199. doi:10.1016/j.envint.2022.107199
7. Wright SL, Kelly FJ. Plastic and human health: a micro issue? Environ Sci Technol. 2017;51(12):6634–6647. doi:10.1021/acs.est.7b00423
8. Campanale C, Massarelli C, Savino I, Locaputo V, Uricchio VF. A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health. 2020;17(4):1212. doi:10.3390/ijerph17041212
9. Atamanalp M, Köktürk M, Uçar A, et al. Microplastics in tissues (brain, gill, muscle and gastrointestinal) of Mullus barbatus and Alosa immaculata. Arch Environ Contam Toxicol. 2021;81:460–469. doi:10.1007/s00244-021-00885-5
10. Schwabl P, Koppel S, Konigshofer P, et al. Detection of various microplastics in human stool. Ann Intern Med. 2019;171:453–458. doi:10.7326/M19-0618
11. Yan Z, Zhao H, Zhao Y, et al. An efficient method for extracting microplastics from feces of different species. J Hazard Mater. 2019;121489:1–39. doi:10.1016/j.jhazmat.2019.121489
12. Deng Y, Zhang Y, Qiao R, et al. Evidence that microplastics aggravate the toxicity of organophosphorus flame retardants in mice (Mus musculus). J Hazard Mater. 2018;357:348–354. doi:10.1016/j.jhazmat.2018.06.017
13. Karbalaei S, Hanachi P, Walker TR, Cole M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ Sci Pollut Res. 2018;25(36):36046–36063. doi:10.1007/s11356-018-3508-7
14. Shengchen W, Jing L, Yujie Y, Yue W, Shiwen X. Polystyrene microplastics-induced ROS overproduction disrupts the skeletal muscle regeneration by converting myoblasts into adipocytes. J Hazard Mater. 2021;417:(April):1–12. doi:10.1016/j.jhazmat.2021.125962
15. Li Z, Zhu S, Liu Q, et al. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/β-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ Pollut. 2020;265:115025. doi:10.1016/j.envpol.2020.115025
16. Hwang J, Choi D, Han S, Choi J, Hong J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci Total Environ. 2019;684:657–669. doi:10.1016/j.scitotenv.2019.05.071
17. Guan R, Wen X, Liang Y, Xu D, He B, Feng X. Trends in Alzheimer’s disease research based upon machine learning analysis. Int J Biol Sci. 2019;15(10):2065–2074. doi:10.7150/ijbs.35743
18. Rasmussen J, Langerman H. Alzheimer’s Disease - why we need early diagnosis. Degenrative Neurol Neuromuscul Dis. 2019;9:123–130.
19. Alzheimer Association. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019;15:321–387. doi:10.1016/j.jalz.2019.01.010
20. Ghosh S, Durgvanshi S, Agarwal S, Raghunath M, Sinha JK. Current status of drug targets and emerging therapeutic strategies in the management of Alzheimer’s Disease. Curr Neuropharmacol. 2020;18(9):883–903. doi:10.2174/1570159x18666200429011823
21. Mishra P, Mittal AK, Kalonia H, et al. SIRT1 promotes neuronal fortification in neurodegenerative diseases through attenuation of pathological hallmarks and enhancement of cellular lifespan. Curr Neuropharmacol. 2021;19(7):1019–1037. doi:10.2174/1570159X18666200729111744
22. Brennecke D, Duarte B, Paiva F, Caçador I, Canning-clode J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci. 2016;178:189–195. doi:10.1016/j.ecss.2015.12.003
23. Prata JC. Airborne Microplastics: consequences to Human Health? Environ Pollut. 2018;234:115–126. doi:10.1016/j.envpol.2017.11.043
24. Ragusa A, Svelato A, Santacroce C, et al. Plasticenta: first evidence of microplastics in human placenta. Environ Int. 2021;146:106274. doi:10.1016/j.envint.2020.106274
25. Prokić MD, Radovanović TB, Gavrić JP, Faggio C. Ecotoxicological effects of microplastics: examination of biomarkers, current state and future perspectives. Trends Anal Chem. 2019;111:37–46. doi:10.1016/j.trac.2018.12.001
26. Sincihu Y, Sudiana IK, Keman S, et al. Membranes and deoxyribonucleic acid of hippocampal neurons damage due to low- density polyethylene microplastics in blood of Wistar rats. Int J Health Sci. 2022;6(S7):3490–3503.
27. Trestrail C, Nugegoda D, Shimeta J. Invertebrate responses to microplastic ingestion: reviewing the role of the antioxidant system. Sci Total Environ. 2020;734(138559):1–18. doi:10.1016/j.scitotenv.2020.138559
28. Tse K, Herrup K. Re-imagining Alzheimer’s disease – the diminishing importance of amyloid and a glimpse of what lies ahead. J Neurochem. 2016;143(4):432–444. doi:10.1111/ijlh.12426
29. Ma T, Klann E. Amyloid beta: linking synaptic plasticity failure to memory disruption in Alzheimer’s Disease. J Neurochem. 2012;120:(Suppl.1):140–148. doi:10.1111/j.1471-4159.2011.07506.x
30. Monteleone A, Schary W, Fath A, Wenzel F. Validation of an extraction method for microplastics from human materials. Clin Hemorheol Microcirc. 2019;73(1):203–217. doi:10.3233/CH-199209
31. Sakamoto H, Akamatsu M, Hirano M, Kusunoki S, Nakamura Y. Multiple system involvement in a Japanese patient with a V31A mutation in the SOD1 gene. Inf Healthc. 2014;15:312–314. doi:10.3109/21678421.2013.873051
32. Yang C, Hsu S, Chen K, Chien C. Effect of Adenoviral Catalase Gene Transfer on Renal Ischemia / Reperfusion Injury in Rats. Chin J Physiol. 2015;58(6):420–430. doi:10.4077/CJP.2015.BAD324
33. Li G, Feng Y, Cheng TS, Yin J, Zhang C. Edaravone, a novel free radical scavenger, prevents steroid-induced osteonecrosis in Rabbits. Rheumatology. 2013;52:438–447. doi:10.1093/rheumatology/kes313
34. Topal A, Alak G, Ozkaraca M, et al. Neurotoxic responses in brain tissues of rainbow trout exposed to imidacloprid pesticide: assessment of 8-hydroxy-2-deoxyguanosine activity, oxidative stress and acetylcholinesterase activity. Chemosphere. 2017;175:186–191. doi:10.1016/j.chemosphere.2017.02.047
35. Wang YL, Lee YH, Chiu IJ, Lin YF, Chiu HW. Potent impact of plastic nanomaterials and micromaterials on the food chain and human health. Int J Mol Sci. 2020;21:5. doi:10.3390/ijms21051727
36. Yu P, Liu Z, Wu D, Chen M, Lv W, Zhao Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat Toxicol. 2018;200:28–36. doi:10.1016/j.aquatox.2018.04.015
37. Revel M, Lagarde F, Perrein-ettajani H, Bruneau M. Tissue-specific biomarker responses in the Blue mussel mytilus spp. exposed to a mixture of microplastics at environmentally relevant concentrations. Front Environ Sci. 2019;7(33):1–14. doi:10.3389/fenvs.2019.00033
38. Magni S, Gagné F, André C, et al. Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: bivalvia). Sci Total Environ. 2018;631:778–788. doi:10.1016/j.scitotenv.2018.03.075
39. Rodríguez-seijo A. Oxidative stress, energy metabolism and molecular responses of earthworms (Eisenia fetida) exposed to low-density polyethylene microplastics. Environ Sci Pollut Res. 2018;25(33):33599–33610. doi:10.1007/s11356-018-3317-z
40. Hua T, Kiran S, Li Y, Sang QXA. Microplastics exposure affects neural development of human pluripotent stem cell- derived cortical spheroids. J Hazard Mater. 2022. doi:10.1016/j.jhazmat.2022.128884
41. Schirinzi GF, Pérez-pomeda I, Sanchís J, Rossini C, Farre M, Barcelo D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ Res. 2017;159:(June):579–587. doi:10.1016/j.envres.2017.08.043
42. Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep. 2017;7:1–10. doi:10.1038/srep46687
43. Dehaut A, Cassone AL, Frère L, et al. Microplastics in Seafood: benchmark protocol for their extraction and characterization. Environ Pollut. 2016;215:223–233. doi:10.1016/j.envpol.2016.05.018
44. Cheng Y, Zhu L, Song W, et al. Combined effects of mulch film-derived microplastics and atrazine on oxidative stress and gene expression in earthworm (Eisenia fetida). Sci Total Environ. 2020;746(141280):1–11. doi:10.1016/j.scitotenv.2020.141280
45. Perez EB, Camacho CJH, Martagon VL, Medina JPV, Robles RG, Savin TZ. Review article “oxidative stress induced by phthalates in mammals: state of the art and potential biomarkers”. Environ Res. 2022;206:112636. doi:10.1016/j.envres.2021.112636
46. Dejakaisaya H, Harutyunyan A, Kwan P, Jones NC. Altered metabolic pathways in a transgenic mouse model suggest mechanistic role of amyloid precursor protein overexpression in Alzheimer’s disease. Metabolomics. 2021;17(42):1–12. doi:10.1007/s11306-021-01793-4
47. Supraja P, Tripathy S, Singh R, Singh V. Towards point-of-care diagnosis of Alzheimer’s disease: multi-analyte based portable chemiresistive platform for simultaneous detection of. Biosens Bioelectron. 2021;186:113294. doi:10.1016/j.bios.2021.113294
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

