Back to Journals » Infection and Drug Resistance » Volume 14
Whole Genome Sequencing of First Janibacter indicus Isolate in China Revealed Three Unique Genomic Islands Compared with Saprophytic Strains
Authors Zhang M, Wang Z, Liang Z, Hu N
Received 11 October 2021
Accepted for publication 2 December 2021
Published 14 December 2021 Volume 2021:14 Pages 5351—5361
DOI https://doi.org/10.2147/IDR.S341591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Min Zhang,1,2,* Ziwen Wang,1,3,* Zhenshan Liang,4 Niya Hu2
1Department of Blood Transfusion, The Third Affiliated Hospital of Nanchang University, Nanchang City, 330006, People’s Republic of China; 2Department of Laboratory Medicine, The First Affiliated Hospital of Nanchang, Nanchang City, 330006, People’s Republic of China; 3Department of Laboratory Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, 330006, People’s Republic of China; 4Department of Laboratory Medicine, The Affiliated Children’s Hospital of Nanchang University, Nanchang City, 330006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Min Zhang; Niya Hu Email [email protected]; [email protected]
Introduction: Janibacter caused bacteriemia is one of the rare infections.
Methods: In the present study, we report the first isolation of Janibacter, a rare bacterial infection, from a bacteremia patient in China. Its 16S rDNA was amplified and designated as Janibacter YFY001, which belongs to J. indicus. In addition, its genome was sequenced through combined second- and third-generation genome sequencing methods.
Results: Based on its genome, we identified many virulence factors, such as catalase, gelatinase, FbpABC systems, and resistant genes, among others. Interestingly, three genomic islands were found in YFY001 by comparing its genome to environmental Janibacter strains.
Discussion: Our study not only provides the necessary genomic information for in-depth study of Janibacter, but also provides a novel methodology for studying future cases of rare bacterial infection.
Keywords: bacteriemia, Janibacter, genomic sequencing
Introduction
Rare bacterial infection represents a diverse group of bacterial species with ≤10 clinical reports in the PubMed database.1 Patients infected with rare bacteria likely acquire infection through environmental exposure, including medical exposure, as most of them commonly reside in the natural environment; nonetheless, a small number of reports indicate that these bacteria infrequently cause human infections. Indeed, some Janibacter species cause rare bacterial infections, with three clinical cases reported to date. In 2005, two different groups isolated two distinct species of Janibacter from patients at the same time.2–4 In 2015, a research group in Spain reported their first finding of J. terrae-infected human blood.4 Janibacter is difficult to identify using morphological characteristics and biochemical reactions, instead requiring rRNA sequencing and phylogenetic analysis of positive culture samples. Meanwhile, it is important to acquire an initial diagnostic report as Janibacter is resistant to many antibiotics.
The genus Janibacter contains gram-positive aerobic cocci to rod-like bacteria that are non-spore-forming, non-motile, and lack mycelia. Additionally, these bacteria are catalase-positive, urease-negative, and oxidase-variable. Its colonies are smooth, circular, and usually yellow.5 Optimal growth occurs at 30 degrees Celsius. Janibacter has a unique complex fatty acid profile and lacks mycolic acids. The G+C content of its DNA is approximately 70 mol%. Until now, Janibacter could be divided into nine strain types: J. limosus, J. terrae, J. indicus, J. melonis, J. anophelis, J. hoylei, J. corallicola, J. alkaliphilus, and J. cremeus.5–13 J. indicus was first isolated by Zhang et al in the Indian Ocean. The phylogenetic tree of J. indicus and other Janibacter strains was constructed by 16s rRNA, and revealed that J. indicus is related to J. terrae, J. cremeus, J. anophelis, J. hoylei, J. limosus, J. corallicola, J. melonis, and J. alkaliphilus (Zhang et al.). Most of these strains exist only in the natural environment, except for J. terrae and J. melonis, which have previously been isolated from patients. Nonetheless, we isolated J. indicus from a bacteremia patient in the present study and thereby demonstrated that this strain could also cause host infection.
Second-generation sequencing (SGS) technologies have promoted the characterization of microbial genomes; however, drawbacks in SGS methods have also hindered this process. SGS produces a huge amount of short length reads which can be difficult to assemble into a complete genome. Therefore, many genomes are incomplete and are instead submitted as draft genomes—sometimes with hundreds of contigs which could provide limited information. Alternatively, third-generation sequencing methods—such as the Pacific Biosciences (PacBio) single-molecule real-time (SMRT) sequencing platform—can provide reads more than 3 kb long. These longer reads can contain the repeat region, and thus facilitate the generation of complete genomes without the need for additional sequencing. Subsequently, many of these genomes could help to carry out comparative analysis, functional, virulence, and antibiotic resistance studies.
Recently, we isolated and identified the first Janibacter strain in China from the blood of a patient who suffered from a viral central nervous system (CNS) infection with secondary sepsis. In order to identify the virulence factors underlying disease, a greater understanding of this bacteria at the genomic level is required. Therefore, we attempted to complete the genome of this strain; however, the high GC content of Janibacter prevented complete genome assembly using SGS methods. Thus, we combined SGS (Hi-seq) and third-generation sequencing (Pacific Bio method) techniques to assemble the completed genome. Finally, we compared the genomes of our isolate and two other environmental strains to identify the resistance genes and virulence factors of Janibacter.
Materials and Methods
Strain
Janibacter YFY001 was isolated from the blood of a patient who was diagnosed as having an unknown viral CNS infection with secondary sepsis. Written informed consent for the publication of their details was obtained from the study participant. A single colony was recovered from blood agar (5% sheep blood with Colombia agar, Oxoid) and maintained in nutrient broth (Oxoid). This study was approved by the First Affiliated Hospital of Nanchang University (approval no. 2014036).
Bacterial Identification and in vitro Drug Susceptibility Tests
The patient’s blood was inoculated into aerobic and anaerobic bottles and cultured using the BACTEC FX system. After positive alarm, the media was transferred onto blood agar, China blue agar, and chocolate agar with 0.2% (10μg/mL) Vancomycin. After being cultured for 48 to 96 hours at 35°C, bacterial isolate was identified using the Vitek 2 Compact System (BioMérieux Clinical Diagnostics) and the MicroScan WalkAway 96 System. Antibiotic susceptibility tests were performed following the standard S. aureus procedure provided by the CLSI in 2020.14
Bacterial DNA Extraction, 16S rDNA Sequencing, and Phylogenetic Analysis
Janibacter YFY001 was grown in nutrient broth (Co. Oxford) for 96 hours at 37 degrees Celsius before genomic DNA was extracted using a QIAamp DNA Mini Kit. Subsequently, 16S rDNA amplification was carried out using the universal 16S rDNA primers 27f (AGAGTTTGATCMTGGCTCAG) and 1492r (GGTTACCTTGTTACGACTT).15 The amplification product was sequenced using the Applied Biosystems 3730 platform. After sequencing, contigs were assembled and blasted using the NCBI website (http://www.ncbi.nlm.nih.gov/) using the 16S ribosome RNA database.
To elucidate the phylogeny between our isolate and remaining strains, 16S rDNA sequences were collected and MEGA6 was used for the phylogenetic analysis.
Genome Sequencing
Janibacter was isolated from the patient’s blood and stored in our hospital. Genomic DNA for Janibacter was extracted using a Qiagen MagAttract HMW DNA Kit (QIAGEN 67563). For PacBio RS sequencing (Menlo Park, CA), a total of 5 µg of genomic DNA was sheared by g-TUBE (Covaris, US) and a library of 10 kb sequences was constructed using the standard PacBio RS sample preparation instructions for sequencing on Pacific Biosciences RS II (Menlo Park, CA) platforms. In addition, a 300 bp paired end library was prepared according to the Illumina TruSeq DNA sample preparation recommendations and was sequenced on HiSeq 2500 platforms with read lengths of 150 bp.
Genome Assembly and Annotation
The PacBio data (one single-molecule real-time SMRT cell, 3975 bp average read length, approximately 53× coverage) were assembled using the Hierarchical Genome Assembly Process (HGAP) software,16 resulting solely in one-contig assembly. The HiSeq data (approximately 300× coverage) were mapped against the assembly to proofread the PacBio data using Bowtie217 and SAMtools.18 Finally, a whole genome assembly without redundancy was obtained.
Gene prediction was performed using Glimmer 3.02.19 tRNA and rRNA genes were predicted by tRNAscan-SE20 and RNAmmer,21 respectively. Predicted genes were blasted in a non-redundancy (nr) database on NCBI and annotated. Pathways involved in the genes were constructed using the Kyoto Encyclopedia of Genes and Genomes (KEGG).22 Gene COG were classified according to a conserved domain database.23
Comparative Genomics
Mauve 2.3.1 (http://darlinglab.org/mauve/user-guide/introduction.html), Blast-2.2.30+, PATRIC, and Perl Scripts were used for comparative genomic studies.
Nucleotide Sequence Accession Numbers
SubmissionID: SUB1159744
BioProject ID: PRJNA300616
Results
Case Report
A 61-year-old woman presented to the Emergency Department at the First Affiliated Hospital of Nanchang University on 7 July 2014. Her family members explained that she had developed a fever with headaches, chills, dizziness, and body aches after a rainstorm and had lost consciousness two day later, resulting in hospitalization. Upon physical examination, she was found to be afebrile with a heart rate of 88 bpm, blood pressure of 124/77 mm Hg, and a respiratory rate of 22 breaths per minute. Subsequent examination of the patient’s head, heart, lungs, and abdomen yielded normal results. Kernig’s sign was positive on both sides of the body. Babinski and Brudzinski signs were both negative. On 8 July, a computed tomography (CT) scan of the head and abdomen also returned normal results. Chest CT scans revealed pleural effusion on both sides and mild lung infection. Laboratory results—including routine complete blood count, hepatic function, coagulation function, and cardiograph—were all normal. On 9 July, a CSF chemical study indicated normal glucose and chloride levels and a protein level of 1913 mg/L. On 10 July, ESR was 46 mm/h. The ER physicians concluded that she had a central nervous system infection, viral meningitis, and lung infection. No improvement was seen after four days of anti-infective, mannitol, and fluid infusion treatment, and the patient was transferred to the Department of Infectious Diseases (DID) on 11 July. At the DID, she was treated with mannitol, dexamethasone, and Rocephin, as in the ER, but with the addition of acyclovir. On 14 July, the patient had a fever with a body temperature of 39 degrees Celsius. Laboratory studies indicated a leukocyte concentration of 10.18E9/L and a neutrophil concentration of 6.92E9/L. Blood culture was sent to the Department of Laboratory Medicine (DLM) and the patient’s antibiotic was changed to Tazocin. The patient then became well the next day. On 18 July, the DLM reported that they had cultured gram-positive bacteria. Dermacoccus nishinomiyaensis and susceptibility results were reported on 24 July before the patient was finally discharged on 4 August (Figure 1).
Bacterial Isolation and Identification and Antibiotic Susceptibility Test
Aerobic and anaerobic blood cultures were directed to the DLM. After almost five days of culture, the equipment presented a positive alarm for the aerobic bottle. After another 48 hours of culture, wet yellow colonies could be seen only on blood agar. Gram staining indicated a gram-positive coccoid bacteria. An identification report obtained with the VITEK 2 Compact System indicated that the isolate was Dermacoccus. In addition, we analyzed the isolate using the MicroScan WalkAway 96 system, where it was identified as “Micrococcus”, a different result to that of the VITEK 2 Compact System report. Subsequently, we confirmed the catalase and oxidase activity of the isolate. However, the isolate exhibited catalase-positive and oxidase-negative reactions, which differs from Dermacoccus. Therefore, partial 16S rDNA sequencing and Blast were carried out on our isolate, revealing a 99.8% match to Janibacter.
Additionally, antimicrobial susceptibility to 20 antimicrobials was determined using MicroScan plates which were incubated in air at 35 degrees Celsius and read after 72 hours. The susceptibility results were performed according to CLSI M100 standards.14 Based on the anti-drug susceptibility test results, J. indicus may be resistant to rifampin, clindamycin, and ceftazidime. In addition, compared with J. terrae, J. indicus was sensitive to piperacillin (Table 1).
 |
Table 1 Comparison of Biochemical Reactions and AST Results Between YFY001 and Other Janibacter |
De Novo Assembly of Janibacter YFY001 Using the PacBio RS Platform and Illumina HiSeq
To identify any possible virulence and antibiotic resistant factors at the genetic level, the whole genome of Janibacter YFY001 was sequenced. We combined second- and third-generation sequencing methods to complete the genome using the Illumina and PacBio platforms, respectively. Using one single-molecule real-time sequencing (SMRT) cell, 64,983 PacBio subreads totaling 180,137,073 nucleotides were obtained with a mean length of 3772 bp. Meanwhile, using the Illumina HiSeq platform, 3,969,587 paired reads totaling 1,198,815,274 nucleotides were recovered with a mean length of 300 bp after trimming and quality filtering.
One single contig was produced for Janibacter YFY001 with the automated gap closing tool FGAP using only PacBio subreads (Figure 2A). A whole genome of 3,401,190 bp was produced, comprising 3455 predicted open reading frames (ORFs), with a G+C content of 71.24%. The genome has two predicted copies of 5S, 16S, and 23S rRNA genes and 47 predicted tRNAs. There are putative genes for complete glycolysis, the tricarboxylic acid cycle, pentose–phosphate pathways, and predicted genes for the utilization of fructose, sucrose, and galactose. Of the annotated ORFs, 772 hypothetical proteins, 3326 proteins with COG assignments (Figure 2B), and 913 proteins with pathway assignments were identified. There are 65 predicted ABC transporters (or ABC transporter-related proteins). The genome contains Sec-dependent pathway proteins, including SecY, Sec D/F, YidC, SecA, Ffh, and FtsY. Signal peptidase I and II could also be found in the genome. All the essential amino acid genes could be predicted in the genome. In addition, there are two predicted phage-related proteins: gp37 and gp43.
Identification of Possible Virulence Factors Using Genomic Information
Gelatinase Gene (+orf2875)
Gelatin hydrolysis tests showed that Janibacter could degrade gelatin and a putative gelatinase was identified in the genome. As gelatinase (GelE) has been mostly studied in Enterococcus,24 we blasted the GelE sequence of Enterococcus from NCBI against theYFY001 genome and found a homologous protein of GelE, designated as orf02875 (JaniGelE). Using a conserved domain search engine, we found that orf02875 has an M4 neutral protease domain, and a LasB (also known as Zn-dependent metalloprotease) domain, which is similar to the structure of gelatinase. GelE belongs to the M4 family of bacterial zinc metalloendopeptidases.25 Metalloproteases are the most diverse of the four main types of protease, with more than 50 families identified to date. In these enzymes, a divalent cation, usually zinc, activates the water molecule. It always contains an “abXHEbbHbc” structure, where “a” is most often valine or threonine and forms part of the S1’ subsite in thermolysin and neprilysin, “b” is an uncharged residue, and “c” is a hydrophobic residue. JaniGelE has a similar structure to GelE.
Catalase Gene (orf01884)
During clinical diagnosis, YFY001 was found to be catalase positive. Previous studies revealed that almost all of the Janibacter strains exhibit a positive reaction in catalase tests. Consistent with these findings, we were able to identify a catalase gene (orf01884) in the YFY001 genome.
Virulence Factors
In order to determine the possible virulence factors in Janibacter YFY001, we blasted the genome using the VF database26 with E-value 1e-5, identity 20%, and coverage 50%. The results indicated 82 possible virulence factors for YFY001 (Table S1).
Antibiotic Resistant Genes
According to the antibiotic susceptibility test, YFY001 demonstrated resistance to certain drugs. Therefore, Resistance Gene Identifier VERSION 2 was used for the selection of resistant genes, and 16 possible resistant genes were found in the YFY001 genome.27 They belong to five different COG categories, including carbohydrate transport and metabolism; cell envelope outer-membrane biogenesis; defense mechanisms; DNA replication, recombination, and repair; and signal transduction mechanisms.
Previous studies indicated that most Janibacter were resistant to Rifampin, but they did not elucidate the mechanism of resistance. Rifampin was previously shown to be active in vitro against most Micrococcus.28 Until now, studies which focused on Rifampin resistance mechanisms demonstrated that it was related to rpoB mutations.29 A RIF-resistance-determining region (RRDR), which indicates that mutations occur in an 81 bp region of cluster I, was found across all bacterial species.29 Here, we compared the sequence of rpoB among YFY001, E. coli, and Mtb and determined that there was an amino acid mutation in this RRDR region (S443N). This novel mutation has not been reported before and might be responsible for the RIF resistance seen in Janibacter.
Genomic Comparison Indicates That YFY001 Has Three Possible Genomic Islands
In addition to YFY001, two other Janibacter genomes have been sequenced previously. Information on these three strains is presented in Table 2. Comparative genomic analysis was carried out using BlastP with E-value 1e-5, BBH identity 20%, and coverage 50%, and 1009 core orthologs could be found in all three strains (Figure 3A). Additionally, 985 specific orthologs could only be found in YFY001, which could be mapped to carbohydrate metabolism; energy metabolism; lipid metabolism; nucleotide metabolism; amino acid metabolism; metabolism of other amino acids; glycan biosynthesis and metabolism; metabolism of cofactors and vitamins; metabolism of terpenoids and polyketides; biosynthesis of other secondary metabolites; biodegradation and metabolism of xenobiotics; translation, replication, and repair; membrane transport; and signal transduction pathways (Figure 3B).
 |
Table 2 Basic Statistics of the Janibacter YFY001 Genome Sequenced in This Study, Compared with Those of Janibacter PVAS, and HTCC2649 |
 |
Figure 3 Comparative genomic analysis using Janibacter YFY001, PVAS, and HTCC 2649 genomes. (A) Venn figure of the comparative genomic results. (B) COG analysis of the comparison results. |
Interestingly, three specific regions—located in the 255,480–287,000 bp; 2,997,051–3,023,851 bp; and 3,238,801–3,302,701 bp regions—were found in genomic comparison results. Most of the genes in these areas are absent from environmental strains and thus may be inserted into the genome by horizontal gene transfer (HGT). In order to identify the genomic islands in YFY001, IslandViewer 3 and NCBI-Blast were both used. Finally, three probable genomic islands were found and designated as JaniIsland 1 (255,480–287,000 bp), JaniIsland 2 (2,997,051–3,023,851 bp), and JaniIsland 3 (3,238,801–3,302,701 bp). The structures of these three islands are presented in Figure 2C. In addition, we used VFDB, ARDB, and COG to predict the functions of the genes in these islands.26,27 Subsequently, we considered that JaniIsland 1 may be a pathogenic island as it exhibits their classical structure, containing tRNA, virulence factors, etc.
Discussion
Identification and Taxonomy
Janibacter YFY001 was the first Janibacter strain isolated from a patient’s blood in China. However, in our opinion, a number of Janibacter strains might be classified as other kinds of bacteria due to numerous complications which may arise during diagnosis. First of all, most hospitals use automatic chemical identification systems such as VITEK, MicroScan, and others. In the present study, both systems were used for detection. Using the VITEK-2 system, the isolate was initially identified as Dermococcus using a GP card, which can only identify 115 taxa of the most significant non-spore-forming, gram-positive bacteria. Janibacter is not on the list of identifiable bacteria for the VITEK 2 or the MicroScan 96 system, and therefore Janibacter infection is not considered. In addition, both Dermacoccus/Kytococcus and Janibacter belong to Actinomycetales, Micrococcineae, which may show similar biochemical reactions and lead to problems in identification. This indicates that many Janibacter infections may be misidentified as Dermacoccus/Kytococcus or Micrococcus due to the use of automatic identification systems. Therefore, we suggest that clinical isolates classified as Dermacoccus/Kytococcus or Micrococcus should undergo further 16S rDNA sequencing prior to final confirmation, which will provide physicians with more accurate results. Similar to most of Janibacter spp., Janibacter YFY001 belongs to J. indicus and demonstrated catalase (+), oxidase (-), and gelatin hydrolysis (+) characteristics. The a-glucosidase activity was negative for Janibacter YFY001, which was different from J. terrae and J. brevis (Table 1).
Antibiotic Resistance-Related Genes
Antibiotic resistance is one of the key hazards of rare bacterial infections. At present, four different studies have indicated that Janibacter could be resistant to Ceftazidime, Clindamycin, and Rifampin. However, they did not elucidate the possible genes responsible for this resistance. In this study, we identified 16 genes that might be related to Janibacter resistance against these antibiotics, including many drug-resistant transporters, which would facilitate resistance to antibiotics (Table 3). In addition, the molecular mechanism of YFY001 resistance to RIF was found to most likely be caused by a mutation in the RRDR gene.
 |
Table 3 Predicted Antibiotic Resistance Genes |
Pathogenicity and Genomic Islands
As very few studies have reported on the virulence factors of Janibacter, we were only able to predict possible virulence factors using bioinformatics-based methods. Initially, we identified Janibacter gelatinase, which might be related to Janibacter virulence. Through combined VFDB and genomic comparison results, we concluded that only 17 probable virulence factors existed in YFY001.
Iron acquisition plays an important role in the survival of pathogenic bacteria within the host. Neisseria gonorrhoeae, Neisseria meningitidis, and Haemophilus influenza can employ the FbpABC system (also called HitABC in Haemophilus influenza) for iron acquisition from hosts. Interestingly, we found a similar structure in YFY001 but not in the other two environmental strains. A hitABC-like operon was found in the YFY001 genome and was designated as jfuABC. The HitABC operon was confirmed to be an important factor for iron acquisition from hosts, which indicated that this system is essential for YFY001 survival in vivo. In addition, we compared the similarity and identity among these strains, as shown in Table 4.
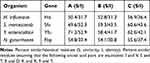 |
Table 4 Similarities and Identities Between Different Bacteria with FbpABC Operon |
Gene acquisition, gene loss, and other genomic alterations are important in the adaptive evolution of prokaryotes and are achieved by horizontal gene transfer and homologous recombination.30 Over the past few decades, studies have shown that genomic islands (GEIs) are a new kind of rapid microbial gene exchange element.30 Genomic islands are considered to be a way for microbes to adapt to the changing external environment, carrying pathogenic elements necessary for microbes and special structures of metabolism-related components. When compared with other parts of microbial genomes, GEIs have an obvious difference in terms of their GC content. In this study, we predicted three GEIs within the genome of YFY001 by comparing it with two existing environmental Janibacter genomes. We found that most of the genes in the GEI components of YFY001 strains were absent from the environmental strains; however, the function of most of these genes remain unclear (Figure 2C). According to COG, most of the proteins in Island 1 contribute to metabolism while those of Island 3 plays a role in information storage and processing, implying that the islands would provide different functions during pathogenesis.
Furthermore, VFDB Blast results indicated that a virulence factor existed in Island 1, and that the structure of its components conformed to the basic structure of virulent islands. According to the genomic information obtained, we predict that Island 1 is a pathogenic island as it shows identical characteristics to classical pathogenic islands. In future studies, we intend to focus on the function of this pathogenic island.
Conclusion
In this study, we used genomic sequencing methods to elucidate the virulence mechanisms and possible resistance genes of Janibacter YFY001—a rare bacterial infection in humans. Our study provides a practical method for the study of rare clinical isolates and a way in which to obtain sufficient information to study the pathogenic mechanisms of rare pathogens. These techniques are expected to contribute to our understanding of rare pathogens and enable physicians to better treat patients affected by rare bacterial infections.
Ethics Statement
This study was approved by the First Affiliated Hospital of Nanchang University (approval no. 2014036).
Acknowledgment
This study was supported by the Science and Technology Plan Project of the Health Commission of Jiangxi Province (20203655).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Seng P, Abat C, Rolain JM, et al. Identification of rare pathogenic bacteria in a clinical microbiology laboratory: impact of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2013;51(7):2182–2194. doi:10.1128/JCM.00492-13
2. Elsayed S, Zhang K. Bacteremia caused by Janibacter melonis. J Clin Microbiol. 2005;43(7):3537–3539. doi:10.1128/JCM.43.7.3537-3539.2005
3. Loubinoux J, Rio B, Mihaila L, et al. Bacteremia caused by an undescribed species of Janibacter. J Clin Microbiol. 2005;43(7):3564–3566. doi:10.1128/JCM.43.7.3564-3566.2005
4. Fernandez-Natal MI, Saez-Nieto JA, Medina-Pascual MJ, et al. First report of bacteremia by Janibacter terrae in humans. Infection. 2015;43(1):103–106. doi:10.1007/s15010-014-0672-7
5. Martin K, Schumann P, Rainey FA, Schuetze B, Groth I. Janibacter limosus gen. nov., sp. nov., a new actinomycete with meso-diaminopimelic acid in the cell wall. Int J Syst Bacteriol. 1997;47(2):529–534. doi:10.1099/00207713-47-2-529
6. Yoon JH, Lee KC, Kang SS, Kho YH, Kang KH, Park YH. Janibacter terrae sp. nov., a bacterium isolated from soil around a wastewater treatment plant. Int J Syst Evol Microbiol. 2000;50(Pt 5):1821–1827. doi:10.1099/00207713-50-5-1821
7. Yoon JH, Lee HB, Yeo SH, Choi JE. Janibacter melonis sp. nov., isolated from abnormally spoiled oriental melon in Korea. Int J Syst Evol Microbiol. 2004;54(Pt 6):1975–1980. doi:10.1099/ijs.0.63167-0
8. Kampfer P, Terenius O, Lindh JM, Faye I. Janibacter anophelis sp. nov., isolated from the midgut of anopheles arabiensis. Int J Syst Evol Microbiol. 2006;56(Pt 2):389–392. doi:10.1099/ijs.0.63905-0
9. Shivaji S, Chaturvedi P, Begum Z, et al. Janibacter hoylei sp. nov., Bacillus isronensis sp. nov. and Bacillus aryabhattai sp. nov., isolated from cryotubes used for collecting air from the upper atmosphere. Int J Syst Evol Microbiol. 2009;59(Pt 12):2977–2986. doi:10.1099/ijs.0.002527-0
10. Kageyama A, Takahashi Y, Yasumoto-Hirose M, Kasai H, Shizuri Y, Omura S. Janibacter corallicola sp. nov., isolated from coral in Palau. J Gen Appl Microbiol. 2007;53(3):185–189. doi:10.2323/jgam.53.185
11. Li J, Long LJ, Yang LL, et al. Janibacter alkaliphilus sp. nov., isolated from coral Anthogorgia sp. Antonie van Leeuwenhoek. 2012;102(1):157–162. doi:10.1007/s10482-012-9723-1
12. Hamada M, Shibata C, Tamura T, Yamamura H, Hayakawa M, Suzuki K. Janibacter cremeus sp. nov., an actinobacterium isolated from sea sediment. Int J Syst Evol Microbiol. 2013;63(Pt 10):3687–3690. doi:10.1099/ijs.0.051532-0
13. Zhang G, Ren H, Wang S, et al. Janibacter indicus sp. nov., isolated from hydrothermal sediment of the Indian Ocean. Int J Syst Evol Microbiol. 2014;64(Pt 7):2353–2357. doi:10.1099/ijs.0.059527-0
14. CLSI M100. Performance Standards for Antimicrobial Susceptibility Testing.
15. Drancourt M, Bollet C, Carlioz A, Martelin R, Gayral JP, Raoult D. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J Clin Microbiol. 2000;38(10):3623–3630. doi:10.1128/JCM.38.10.3623-3630.2000
16. Chin CS, Alexander DH, Marks P, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10(6):563–569. doi:10.1038/nmeth.2474
17. Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012;9(4):357–359. doi:10.1038/nmeth.1923
18. Li H, Handsaker B, Wysoker A, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi:10.1093/bioinformatics/btp352
19. Delcher AL, Bratke KA, Powers EC, Salzberg SL. Identifying bacterial genes and endosymbiont DNA with glimmer. Bioinformatics. 2007;23(6):673–679. doi:10.1093/bioinformatics/btm009
20. Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005;33(WebServer issue):W686–W689. doi:10.1093/nar/gki366
21. Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35(9):3100–3108. doi:10.1093/nar/gkm160
22. Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi:10.1093/nar/28.1.27
23. Marchler-Bauer A, Derbyshire MK, Gonzales NR, et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015;43(Databaseissue):D222–D226. doi:10.1093/nar/gku1221
24. Del Papa MF, Hancock LE, Thomas VC, Perego M. Full activation of Enterococcus faecalis gelatinase by a C-terminal proteolytic cleavage. J Bacteriol. 2007;189(24):8835–8843. doi:10.1128/JB.01311-07
25. Makinen PL, Clewell DB, An F, Makinen KK. Purification and substrate specificity of a strongly hydrophobic extracellular metalloendopeptidase (“gelatinase”) from Streptococcus faecalis (strain 0G1-10). J Biol Chem. 1989;264(6):3325–3334. doi:10.1016/S0021-9258(18)94069-X
26. Chen L, Zheng D, Liu B, Yang J, Jin Q. VFDB 2016: hierarchical and refined dataset for big data analysis–10 years on. Nucleic Acids Res. 2016;44(D1):D694–D697. doi:10.1093/nar/gkv1239
27. McArthur AG, Waglechner N, Nizam F, et al. The comprehensive antibiotic resistance database. Antimicrob Agents Chemother. 2013;57(7):3348–3357. doi:10.1128/AAC.00419-13
28. von Eiff C, Herrmann M, Peters G. Antimicrobial susceptibilities of Stomatococcus mucilaginosus and of Micrococcus spp. Antimicrob Agents Chemother. 1995;39(1):268–270. doi:10.1128/AAC.39.1.268
29. Campbell EA, Korzheva N, Mustaev A, et al. Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell. 2001;104(6):901–912. doi:10.1016/S0092-8674(01)00286-0
30. Dobrindt U, Hochhut B, Hentschel U, Hacker J. Genomic islands in pathogenic and environmental microorganisms. Nat Rev Microbiol. 2004;2(5):414–424. doi:10.1038/nrmicro884
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.