Back to Journals » Cancer Management and Research » Volume 11
Whole-brain helical tomotherapy with integrated boost for brain metastases in patients with malignant melanoma – final results of the BRAIN-RT trial
Authors Hauswald H , Bernhardt D , Krug D, Katayama S, Habl G, Lorenzo Bermejo J , Debus J , Sterzing F
Received 10 February 2019
Accepted for publication 10 April 2019
Published 24 May 2019 Volume 2019:11 Pages 4669—4676
DOI https://doi.org/10.2147/CMAR.S204729
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Henrik Hauswald,1–4 Denise Bernhardt,1–3 David Krug,1–3,5 Sonja Katayama,1,2 Gregor Habl,1–3,5 Justo Lorenzo Bermejo,6 Jürgen Debus,1–4,7–8 Florian Sterzing1–4,9
1Department of Radiation Oncology, Heidelberg University Hospital, 69120 Heidelberg, Germany; 2National Center for Radiation Research in Oncology (NCRO), Heidelberg Institute for Radiation Oncology (HIRO), 69120 Heidelberg, Germany; 3National Center for Tumor Diseases (NCT), Heidelberg, 69120, Germany; 4Clinical Cooperation Unit Radiation Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany; 5Radiologie München, 80331 München, Germany; 6Institute of Medical Biometry and Informatics, Heidelberg University Hospital, 69120 Heidelberg, Germany; 7Heidelberg Ion-Beam Therapy Center (HIT), Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, 69120, Germany; 8German Cancer Consortium (DKTK), Partner Site Heidelberg, Heidelberg, Germany; 9Department of Radiation Oncology, Hospital Kempten, 87439, Kempten, Germany
Background: Patients with multiple brain metastases (BMs) from malignant melanoma have a poor prognosis. Recent developments in radiation techniques allow simultaneous integrated boost (SIB) concepts while sparing organs at risk. Data on conventional versus dose-escalated radiation approaches in multiple BMs from malignant melanoma are warranted.
Methods: In this prospective, single-center, randomized two-armed study (trial ID: DRKS00005127), patients with multiple BMs from malignant melanoma were treated with either conventional whole-brain radiotherapy (WBRT) applying 30 Gy in 10 fractions (standard arm) or helical tomotherapy applying 30 Gy to the whole brain with an integrated boost to metastases of 50 Gy in 10 fractions and sparing of the hippocampus (HA-WBRT, experimental arm). The primary endpoint was treatment-related toxicity, while secondary endpoints were imaging response, intracerebral progression-free survival (PFS), overall survival (OS) and quality of life.
Results: The study was stopped early due to slow patient recruitment. A total number of 7 patients were enrolled (standard arm n=3, experimental arm n=4), and were followed-up for a median time of 5 months between August 2013 and July 2017. All patients were treated according to protocol. The median OS, intracerebral PFS and follow-up time were 5 months, 2 months and 5 months, respectively. The local control in every individual BM was significantly longer in the experimental versus the standard arm. No patient developed radiation-related high-grade toxicities.
Conclusion: HA-WBRT with SIB results in improved local control in the individual melanoma BMs without radiation-associated high-grade toxicities. Survival times were comparable to published data.
Keywords: melanoma, brain metastases, tomotherapy, hippocampal sparing, integrated boost
Introduction
The age-standardized incidence rate for malignant melanoma (MM) in Western Europe was 15.6651/100.000 in 2015.1 Patients with advanced tumor stages develop brain metastases (BM) in 15–55%,2,3 leading to a median survival of 2.1 months with best supportive care only.4 In patients with multiple BM from MM, treatment approaches include systemic therapy, whole-brain radiotherapy (WBRT), radiosurgery and eventually surgical resection, and might be used alone or in combination. Conventional WBRT for multiple BM results in a median survival of 3–4 months.4,5 In recent years, the combination of radiotherapy and immunotherapy was able to improve median overall survival (OS) from 6.2 months to 11.1 months.6 Furthermore, a recent trial on the anti-programmed cell death 1 protein (PD-1) checkpoint inhibitor pembrolizumab in melanoma BM showed a response rate of 26% and a median progression-free survival (PFS) and OS of 2 months and 17 months, respectively, while the combination of the anti-PD-1 checkpoint inhibitor nivolumab with the anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) monoclonal antibody ipilimumab showed a 6-month PFS of 64.2%.7,8 Likewise, data on targeted agents against B-Raf proto-oncogene (BRAF) or mitogen-activated protein kinase (MEK) mutations, for example, showed up to 39% objective response rates.9
Since advances in the management of melanoma BM have significantly improved prognosis, radiotherapy-related late adverse events (AEs), especially neurocognitive decline after conventional WBRT, come into focus.10,11 The incidence and severity of neurocognitive deficits have been linked to the radiation dose to the hippocampus, which plays a major role in memory function.12 Modern radiation techniques allow us to reduce the dose applied to the hippocampus while maintaining target coverage.13,14 In 2014, RTOG 0933 investigated hippocampus avoidance (HA)-WBRT and showed superior preservation of patients’ memory compared to historical controls.15 Recently, results from the NRG CC001 trial comparing HA-WBRT to conventional WBRT showed a significantly longer time to neurocognitive decline in patients treated with HA-WBRT.16 Furthermore, treatment plan comparisons have demonstrated HA-WBRT with simultaneous integrated boost (SIB) to be feasible.17,18 Another, yet not standard of care option for dose escalation and sparing of normal brain tissue in 10 or more BM is switching from WBRT to stereotactic radiosurgery.19 In addition, updated tools for prognostic assessment in BM from MM might help to better stratify patients for the different treatment approaches in the future.20
We have performed a worldwide first exploratory randomized controlled trial on HA-helical tomotherapy with SIB versus conventional WBRT to determine if the approach applying HA-WBRT with SIB is feasible and safe in BM of melanoma.
Patients and methods
Patients’ characteristics
Between August 2013 and July 2017, seven patients with BM from advanced MM were enrolled. Further patients’ characteristics are listed in Table 1.
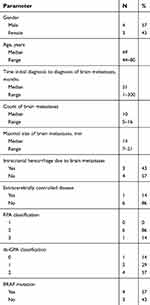 | Table 1 Patients’ characteristics |
Radiation treatment
Patients randomized to the standard treatment arm received conventional WBRT with opposing beams applying 30 Gy in 10 fractions. Treatment in the experimental arm consisted of helical tomotherapy applying 30 Gy in 10 fractions to the whole brain and 50 Gy SIB to all BM while sparing the hippocampus to a dose as low as reasonably achievable. None of the treatment courses had to be stopped early, but two patients had treatment interruptions of 1 day each (due to technical failure in one and poor patient’s condition in the other case). The median time interval between magnetic resonance imaging (MRI) of the brain and the start of radiotherapy was 17 days (range: 5–25 days).
Statistical design and classifications
We conducted a prospective, single-center, randomized two-arm trial. The primary endpoint was toxicity, while the secondary endpoints include imaging response, intracerebral PFS, OS and quality of life (QoL). QoL was evaluated using the European Organization for Research and Treatment of Cancer (EORTC)-QoL-Q C30 and BN-20 brain modules. All time estimates refer to the end of radiotherapy. Log-rank tests and Kaplan–Meier’s estimations were carried out using Microsoft Excel and I.B.M. SPSS 25. AEs were classified according to the common toxicity criteria forAEs version 4 (CTCAE V.4). The first follow-up examination including an MRI of the brain was scheduled 8 weeks after the end of radiotherapy. Thereafter, follow-ups were conducted every 2 months up to a total of 12 months. Intracerebral imaging treatment response was defined based on RECIST 1.1 criteria as either complete remission (CR, requiring no detectable disease), partial remission (PR, tumor mass reduction of at least 30%), stable disease (SD, <30% tumor mass reduction and <20% growth) or progressive disease (PD). Dose homogeneity was calculated as the maximum dose divided by the prescription dose. The conformity index (CI) was calculated according to RTOG (1993) as the prescription isodose volume divided by the target volume.
The study was sponsored by the Heidelberg University Hospital and approved by the local ethics committee (S-327/2012) as well as the federal authority (Bundesamt für Strahlenschutz; Z 5-22461/2-2013-001). The trial ID was DRKS00005127. All patients provided written informed consent. This trial was conducted in accordance with the Declaration of Helsinki.
Data sharing statement
Due to legal aspects of the trial protocol and patients’ informed consent, sharing of study data is not possible.
The trial protocol with details on the study design, study population and the inclusion and exclusion criteria has been published previously.21 The inclusion and exclusion criteria are shown in Table 2. The trial was designed to enroll 25 patients in each of the treatment arms and terminated early due to slow patient recruitment.
 | Table 2 Inclusion and exclusion criteria |
Results
Between August 2013 and July 2017, three patients were randomized into the standard arm and four patients into the experimental arm. The median follow-up time was 5 months (range: 1–10 months). Unfortunately, due to the small patient number, QoL measures during follow-up were limited and not analyzable. Additionally, comparative analyses between the two treatment arms were not valid for the same reason.
Dosimetric characteristics
Details on the dosimetric characteristics in the experimental arm are shown in Table 3. Mean homogeneity of the SIB was 1.05 (range, 1.03–1.08) and the mean CI was 0.991 (range, 0.969–0.996).
 | Table 3 Dosimetric characteristics for 4 patients within the experimental arm applying hippocampal avoidance helical tomotherapy with simultaneous integrated boost |
Adverse events
None of the patients were diagnosed with CTCAE grade 3 or 4 toxicities related to the study treatment during treatment or follow-up. One patient had fatigue CTCAE grade 2, an increase of preexisting headache from CTCAE grade 1 to 2 at the end of treatment as well as neurological deterioration including vomiting, cranial nerve and motor deficits most likely caused by his preexisting leptomeningeal spread. Other patients in the experimental arm reported the following symptoms at the end of treatment: nausea CTCAE grade 1 (n=1), fatigue CTCAE grade 1 (n=2), alopecia CTCAE grade 2 (n=1) and dermatitis CTCAE grade 2 (n=1). In the standard arm, one patient reported nausea CTCAE grade 1 and vomiting CTCAE grade 1. Of 3 patients reporting their clinical situation at the first follow-up, all in the experimental arm, 1 patient had stable headache CTCAE grade 1 and improved dermatitis CTCAE grade 1. One other patient reported dermatitis CTCAE grade 2 and mild concentration deficits. One other patient diagnosed with intracranial progression reported a new onset of nausea, vomiting, epilepsy and paresis, CTCAE grade 1 each.
Survival
The median OS was 5 months (range: 1–10 months, Figure 1). The 6-month OS rate for the entire cohort was 43%. The median OS for men was 5 months and 4 months for women. The median OS for patients with recursive partitioning analysis (RPA) class 2 and 3 was 4 and 5 months, respectively. For diagnosis, specific graded prognostic assessment (ds-GPA) scores 0, 1 and 2 median OS were 5, 1 and 7 months, respectively. The median OS for radiotherapy within the experimental arm was 5 months versus 4 months within the standard arm. The median OS of patients with intracerebral progression at the first follow-up was 4 months and in the two cases with at least intracerebral SD at the first follow-up, median OS was 5 months. Cause of death was pulmonary embolism in 1 case and tumor progression in all other cases. One of the patients dying 1 month after finishing radiotherapy within the experimental arm developed leptomeningeal spread and systemic tumor progression with a steady decline of his general condition. One other patient with a 1-month survival was diagnosed with intracerebral tumor progression 3 weeks after ending WBRT in the standard arm. All except one patient received different systemic therapies for PD after the trial (dacarbazine n=2, dabrafenib/trametinib n=3, pembrolizumab n=1, nivolumab/ipilimumab n=1, carboplatin/paclitaxel n=1, benzamide n=1).
 | Figure 1 Overall survival of all 7 patients treated within the trial protocol. |
At the first follow-up, one patient had intracerebral PR, one other patient showed intracerebral SD, all others intracerebral PD. The two patients with at least an intracerebral SD after the study treatment were both treated in the experimental arm. All but one patient had systemic progression. The median intracerebral PFS time in the whole cohort was 2 months (range, 0–5 months) and 2 months in each of the two study arms, respectively. The local control in every individual melanoma BM was significantly longer in the experimental versus the standard arm (median control: 2.1 months versus 1.7 months; p=0.002; log-rank; Figure 2). Further details on the treatment outcome are shown in Table 4.
 | Table 4 Treatment outcome of all 7 patients enrolled into the study protocol |
Discussion
Our study’s intention was to evaluate the safety and feasibility of HA-WBRT with SIB in a prospective, single-institution, randomized Phase II trial. The trial was terminated early due to slow patient recruitment caused by competing pharmaceutical trials as well as a trend toward upfront radiosurgery even in patients with multiple BM. Unfortunately, due to the small patient number, comparative analyses were not possible.
Advances in systemic therapy like immunotherapy and targeted therapies promise improved outcomes in patients with BM from MM.22 In a recent trial on nivolumab combined with ipilimumab, an intracranial clinical benefit rate of 57% was reported, while 55% developed grade 3 and 4 AE and 1 patient showed grade 5 AE. The reported 6-month PFS and OS were promising with 64.2% and 92.3%, respectively.8 In addition, a recent trial on pembrolizumab in melanoma BM showed a response rate of 26% and a PFS of 2 months (median OS 17 months),7 which is comparable to the PFS in both of the study arms in this trial. Recent trials suggest that the combination of immunotherapy and radiotherapy improves the prognosis in melanoma BM. In the analysis by Gabani et al on radiotherapy and immunotherapy in melanoma BM, for example, the combination of immunotherapy and WBRT improved the OS from 4.4 months in WBRT only to 8.5 months in the combined approach.6
Recent developments in radiotherapy techniques might be helpful to further improve the outcome of patients diagnosed with BM from MM. In 2012, Dana Greene-Schloesser et al reviewed data on radiation-induced brain injury and concluded in their summary that up to 90% of brain tumor patients surviving more than 6 months after fractionated WBRT develop radiation-induced neurocognitive impairment.23 In addition, recent reports on radiation effects in the brain suggest an increased vulnerability not only of the hippocampus, but also of the corpus callosum and the white matter in general.24,25 In case of WBRT sparing of the hippocampus to doses as low as reasonably achievable might improve neurocognitive outcome as seen in RTOG 0933.15 In conventional WBRT, the hippocampi receive the same dose as the surrounding brain tissue – for example, in the case of a homogeneous dose distribution of median 30 Gy in 10 fractions. In our experimental arm, the median doses to the left and right hippocampi were 8.77 Gy and 8.52 Gy, respectively. The minimum doses to the left and right hippocampi were 7.04 and 6.97 Gy and the maximum doses 18.53 and 18.91 Gy, respectively. This dose reduction is comparable to previous reports, for example, by Awad et al, where the authors reported a significantly lower minimum (8.4 Gy), maximum (32.2 Gy) and mean dose (20.4 Gy) to the hippocampus in hippocampus-sparing volumetric modulated arc therapy (VMAT) applying 30 Gy in 15 fractions with a SIB of 50 Gy.26 Furthermore, advances in stereotactic radiosurgery allowing multitarget treatments might further improve clinical and oncological outcome in patients with multiple BM.19,27 In the analysis by Fiorention et al on radiosurgery in less than 5 BM the ipsilateral hippocampus dose was as low as 1.54 Gy, and the contralateral dose 0.7 Gy while applying total doses of 15–30 Gy to the BM in 1–5 fractions.28
In our trial, the oncological outcome was poor with a median OS of 5 months. However, this is in line with previously published data. In the cohort of Ostheimer et al, the reported median OS times for patients with multiple BM from MM were around 2.5 months despite systemic and local treatment and as short as 1.5 months with best supportive care only.29 In a recent analysis by Frinton et al, OS was 4.8 months from the diagnosis of BM and 2.2 months in patients receiving WBRT only.30 Our own previously published data had shown a median OS of 3.5 months in patients treated with WBRT and a relatively improved outcome with a higher irradiation total dose.5 Known prognostic parameters include the number of BM and extracerebral metastases. In our trial, 6 of the enrolled patients had unfavorable disease with uncontrolled extracerebral metastases and all 7 patients had multiple BM. In comparison, with a median OS of 5 months in both arms, this trial’s results compare favorably to prior publications despite an unfavorable prognostic situation. However, they might be biased by advances in systemic therapies in the last years. In addition, the time interval of median 17 days between brain MRI and start of radiotherapy might negatively bias the outcome in our cohort in terms of tumor progression prior to study treatment.
Previously published reports suggest that dose-escalated radiotherapy might improve patient outcomes. In the Australian cohort reported by Awad et al using VMAT to apply hippocampal avoidance WBRT to 30 Gy in 15 fractions and a SIB to 50 Gy to median two BM, the median OS was 9.4 months.26 In addition, in a recent analysis on radiation techniques in BM from various tumors, Dobi et al reported dose-escalated radiotherapy to be able to improve survival in patients with BM from MM from 3.2 months after WBRT only to 6.5 months after dose-escalated treatment.31 In accordance to those prior publications, the analysis of the imaging response in every individual BM in the standard versus experimental arm in our trial revealed the SIB in the experimental arm to result in a significantly improved local control of the individual BM. However, the favorable outcome achieved in the Australian cohort might be the result of a different patient selection (considering median 2 BM versus median 10 BM in our cohort).
Overall, the treatment was tolerated well. None of our patients was diagnosed with radiation-associated AE CTCAE grade 3 or 4, which is in accordance with the data by Awad et al26.
Conclusion
Hippocampus-sparing WBRT with simultaneous integrated boost is feasible and results in improved local control in the individual melanoma brain metastases. In our study, no radiation associated high-grade toxicities were documented and the treatment was tolerated well. The survival was comparable to prior published data.
Disclosure
Dr Henrik Hauswald reports support from TomoTherapy Inc., during the conduct of the study. Dr David Krug reports personal fees from MSD International GmbH, outside the submitted work. Prof. Dr. Jürgen Debus report grants from Viewray Inc, CRI The CLinical Research Institute GmbH, Accuray International Sari, RaySearch Laboratories AB, Vision RT Limited, Merck Serono GmbH, Astellas Pharma GmbH, Astra Zeneca GmbH, Siemens Healthcare GmbH, Solution Akademie GmbH, Egomed PLC Surrey Research Park, Quintiles GmbH, Pharmaceutical Research Associates GmbH, Boehringer Ingelheim Pharma GmbH&CoKG, PTW Freiburg Dr. Pychlau GmbH, and Nanobiotix S.A., outside the submitted work. Prof. Dr. Florian Sterzing reports non-financial support from Accuray, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Karimkhani C, Green AC, Nijsten T, et al. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol. 2017;177(1):134–140.
2. Samlowski WE, Moon J, Witter M, et al. High frequency of brain metastases after adjuvant therapy for high-risk melanoma. Cancer Med. 2017;6(11):2576–2585. doi:10.1002/cam4.1223
3. Patel JK, Didolkar MS, Prickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135(6):807–810. doi:10.1016/0002-9610(78)90171-X
4. Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293–1300. doi:10.1200/JCO.2004.08.140
5. Hauswald H, Dittmar JO, Habermehl D, et al. Efficacy and toxicity of whole brain radiotherapy in patients with multiple cerebral metastases from malignant melanoma. Radiat Oncol. 2012;7:130. doi:10.1186/1748-717X-7-130
6. Gabani P, Fischer-Valuck BW, Johanns TM, et al. Stereotactic radiosurgery and immunotherapy in melanoma brain metastases: patterns of care and treatment outcomes. Radiother Oncol. 2018;128(2):266–273. doi:10.1016/j.radonc.2018.06.017
7. Kluger HM, Chiang V, Mahajan A, et al. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a Phase II trial. J Clin Oncol. 2019;37(1):52–60. doi:10.1200/JCO.18.01616
8. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730. doi:10.1056/NEJMoa1805453
9. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. doi:10.1016/S1470-2045(12)70140-7
10. Gondi V, Paulus R, Bruner DW, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys. 2013;86(4):656–664. doi:10.1016/j.ijrobp.2013.02.033
11. Sun A, Bae K, Gore EM, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29(3):279–286. doi:10.1200/JCO.2010.29.6053
12. Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35(6):659–663. doi:10.3109/02841869609083995
13. Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(4):1244–1252. doi:10.1016/j.ijrobp.2010.01.039
14. Marsh JC, Godbole RH, Herskovic AM, Gielda BT, Turian JV. Sparing of the neural stem cell compartment during whole-brain radiation therapy: a dosimetric study using helical tomotherapy. Int J Radiat Oncol Biol Phy. 2010;78(3):946–954. doi:10.1016/j.ijrobp.2009.12.012
15. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. doi:10.1200/JCO.2013.54.6911
16. Gondi V, Deshmukh S, Brown PD, et al. Preservation of Neurocognitive Function (NCF) with conformal avoidance of the hippocampus during whole-brain radiotherapy (HA-WBRT) for brain metastases: preliminary results of phase III trial NRG oncology CC001. Int J Radiat Oncol Biol Phys. 2018;102(5):1607. doi:10.1016/j.ijrobp.2018.08.056
17. Hsu F, Carolan H, Nichol A, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for 1-3 brain metastases: a feasibility study using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2010;76(5):1480–1485. doi:10.1016/j.ijrobp.2009.03.032
18. Lagerwaard FJ, van der Hoorn EA, Verbakel WF, Haasbeek CJ, Slotman BJ, Senan S. Whole-brain radiotherapy with simultaneous integrated boost to multiple brain metastases using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2009;75(1):253–259. doi:10.1016/j.ijrobp.2009.03.029
19. Yamamoto M, Kawabe T, Sato Y, et al. Stereotactic radiosurgery for patients with multiple brain metastases: a case-matched study comparing treatment results for patients with 2–9 versus 10 or more tumors. J Neurosurg. 2014;121 Suppl:16–25.
20. Sperduto PW, Jiang W, Brown PD, et al. Estimating survival in melanoma patients with brain metastases: an update of the graded prognostic assessment for melanoma using molecular markers (Melanoma-molGPA). Int J Radiat Oncol Biol Phys. 2017;99(4):812–816. doi:10.1016/j.ijrobp.2017.06.2454
21. Hauswald H, Habl G, Krug D, et al. Whole brain helical Tomotherapy with integrated boost for brain metastases in patients with malignant melanoma-a randomized trial. Radiat Oncol. 2013;8:234. doi:10.1186/1748-717X-8-234
22. Glitza Oliva IC, Schvartsman G, Tawbi H. Advances in the systemic treatment of melanoma brain metastases. Ann Oncol. 2018;29(7):1509–1520. doi:10.1093/annonc/mdx807
23. Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: A review. Front Oncol. 2012;2:73. doi:10.3389/fonc.2012.00073
24. Connor M, Karunamuni R, McDonald C, et al. Regional susceptibility to dose-dependent white matter damage after brain radiotherapy. Radiother Oncol. 2017;123(2):209–217. doi:10.1016/j.radonc.2017.04.006
25. Redmond KJ, Hildreth M, Sair HI, et al. Association of neuronal injury in the genu and body of corpus callosum after cranial irradiation in children with impaired cognitive control: a prospective study. Int J Radiat Oncol Biol Phys. 2018;101(5):1234–1242. doi:10.1016/j.ijrobp.2018.04.037
26. Awad R, Fogarty G, Hong A, et al. Hippocampal avoidance with volumetric modulated arc therapy in melanoma brain metastases - the first Australian experience. Radiat Oncol. 2013;8:62. doi:10.1186/1748-717X-8-62
27. El Shafie RA, Paul A, Bernhardt D, et al. Robotic radiosurgery for brain metastases diagnosed with either SPACE or MPRAGE sequence (CYBER-SPACE)-A single-center prospective randomized trial. Neurosurgery. 2019;84(1):253–260. doi:10.1093/neuros/nyy137
28. Fiorentino A, Tebano U, Sicignano G, et al. Hippocampal dose during Linac-based stereotactic radiotherapy for brain metastases: an observational study. Phys Med. 2018;49:135–138. doi:10.1016/j.ejmp.2017.09.129
29. Ostheimer C, Bormann C, Fiedler E, Marsch W, Vordermark D. Malignant melanoma brain metastases: treatment results and prognostic factors–a single-center retrospective study. Int J Oncol. 2015;46(6):2439–2448. doi:10.3892/ijo.2015.2970
30. Frinton E, Tong D, Tan J, et al. Metastatic melanoma: prognostic factors and survival in patients with brain metastases. J Neurooncol. 2017;135(3):507–512. doi:10.1007/s11060-017-2588-4
31. Dobi A, Fodor E, Maraz A, et al. Boost irradiation integrated to whole brain radiotherapy in the management of brain metastases. Pathol Oncol Res. 2018. doi:10.1007/s12253-018-0383-y
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

