Back to Journals » OncoTargets and Therapy » Volume 9
Weekly nanoparticle albumin bound-paclitaxel in combination with cisplatin versus weekly solvent-based paclitaxel plus cisplatin as first-line therapy in Chinese patients with advanced esophageal squamous cell carcinoma
Authors Wang H, yao Z, tang H, zhao Y, zhang X, yao S, yang S, liu Y
Received 16 March 2016
Accepted for publication 6 July 2016
Published 23 September 2016 Volume 2016:9 Pages 5663—5669
DOI https://doi.org/10.2147/OTT.S108580
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Li
Hai-ying Wang, Zhi-hua Yao, Hong Tang, Yan Zhao, Xiao-san Zhang, Shu-na Yao, Shu-jun Yang, Yan-yan Liu
Department of Internal Medicine, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, Henan, People’s Republic of China
Objective: More effective regimens for advanced esophageal squamous cell carcinoma (ESCC) are urgently needed. Therefore, a retrospective study concerning the efficacy and safety of nanoparticle albumin-bound paclitaxel plus cisplatin (nab-TP) versus solvent-based paclitaxel plus cisplatin (sb-TP) as a first-line therapy was conducted in Chinese patients with advanced ESCC.
Methods: From June 2009 to June 2015, 32 patients were treated with nab-paclitaxel (125 mg/m2) on the first and eighth days (30 minutes infusion) and cisplatin (75 mg/m2) on the second day every 21 days (nab-TP arm). Also, 43 patients were treated with solvent-based paclitaxel (80 mg/m2) intravenously on the first and eighth days and the same dose of cisplatin (sb-TP arm). The two groups were compared in terms of objective response rate (ORR), disease control rate, progression-free survival (PFS), overall survival (OS), and safety profile. OS and PFS were estimated using Kaplan–Meier methods to determine associations between chemotherapy regimens and survival outcomes.
Results: Nab-TP demonstrated a higher ORR (50% vs 30%; P=0.082) and disease control rate (81% vs 65%; P=0.124) than sb-TP. Median OS was similar for nab-TP and sb-TP (12.5 vs 10.7 months; P=0.269). However, nab-TP resulted in a longer median PFS (6.1 months [95% confidence interval: 5.3–6.9]) than sb-TP (5.0 months [95% confidence interval: 4.4–5.6]) (P=0.029). The most common adverse events included anemia, leukopenia, neutropenia, febrile neutropenia, and thrombocytopenia in both the groups and no statistically significant differences were observed between the groups. With statistically significant differences, significantly less grade ≥3 peripheral neuropathy, arthralgia, and myalgia occurred in the nab-TP arm (all P<0.05). Dose reduction, treatment delays, and second-line therapy were similar between the two regimens. There were no treatment-related deaths in either group.
Conclusion: Nab-paclitaxel plus cisplatin is found to be an effective and tolerable option for advanced ESCC in the People’s Republic of China.
Keywords: paclitaxel, advanced esophageal squamous cell carcinoma, nanoparticle albumin-bound paclitaxel, chemotherapy
Introduction
As a highly aggressive neoplasm, esophageal cancer is the ninth most common malignancy and the sixth most common cause of cancer death throughout the world.1 Adenocarcinoma and squamous cell carcinoma are the principal histological types of esophageal cancer.2 Over the past three decades, the incidence of adenocarcinoma has increased in the US and Europe. Nevertheless, esophageal squamous cell carcinoma (ESCC) is still the dominant histological type around the world, which accounts for >95% of esophageal cancers in the People’s Republic of China.3 Esophageal cancer is often diagnosed at a very advanced stage and approximately half of all patients present with unresectable, locally advanced, or metastatic disease.4 With a median survival of only 6–8 months, the prognosis for advanced esophageal cancer is extremely poor.5 Cytotoxic chemotherapy has been used to control tumor growth, improve life quality, and prolong survival of these patients.6 A large number of clinical trials have demonstrated that platinum-, fluoropyrimidine-, and taxane-based regimens are standard and effective chemotherapies.6–8 However, the treatment outcomes of these regimens in advanced ESCC were not satisfactory in terms of efficacy or long-term outcome and therapeutic advances significantly lag behind those for other solid tumors such as non-small cell lung carcinoma. Therefore, developing more effective and less cytotoxic chemotherapy regimens has been an urgent task in advanced esophageal cancer.
As a solvent-free formulation of paclitaxel, nanoparticle albumin-bound paclitaxel (nab-PTX, nab-paclitaxel) is developed to avoid the toxicities of polyethoxylated castor oil vehicle used in solvent-based PTX (sb-PTX).9 Preclinical models suggest that nab-PTX reaches a tenfold higher peak concentration of free PTX, delivers over 33% drug to tumors, and crosses endothelial cells more efficiently when compared with sb-PTX.10,11 Based on preclinical evidence, numerous clinical studies have confirmed that nab-PTX has higher tumor retention, lower toxicity, and more potent antitumor effects on breast cancer,12 non-small cell lung carcinoma,13 pancreatic cancer,14 melanoma,15 and ovarian cancer, when compared with solvent-based PTX.16 Especially, in a study of advanced ESCC patients, the optimal safety and efficacy profile were determined through the application of 250 mg/m2 nab-PTX plus cisplatin (DDP) every 3 weeks, which showed an objective response rate (ORR) of 60.6% and a median survival of >15.5 months when compared with other traditional regimens.17 Many clinical studies have shown that weekly administration of PTX is better than a 3-weekly administration, even though the treatment effects are comparable because the incidences of side effects are clearly lower for the weekly administration.18–20 In addition, weekly nab-PTX in combination with DDP has been used for locally advanced ESCC, which showed a pathological complete response (CR) rate of 13.3% and a near pathological CR rate of 6.7% in a Phase II study.21 Moreover, a Phase II study has shown that weekly PTX plus DDP is an active regimen with excellent tolerability for advanced gastric and gastroesophageal cancer.22
Based on the promising results of the study, the efficacy and safety of weekly nab-PTX plus DDP (nab-TP) administered every 3 weeks were compared with those of weekly sb-PTX plus DDP (sb-TP) administered every 3 weeks in advanced ESCC in this trial.
Materials and methods
Patient characteristics
From June 2009 to June 2015, 32 (two recurrent) patients with advanced ESCC were treated with the nab-TP regimen as first-line chemotherapy. Another 43 (three recurrent) patients were treated with the sb-TP regimen. Retrieved clinical data included patient’s age at diagnosis, disease stage at diagnosis, tumor histology, tumor grade, ethnicity, patient and family cancer history, comorbidities, surgical management, first-line chemotherapy, treatment toxicity, progression-free survival (PFS), overall survival (OS), and status at the most recent follow-up. For patients (aged 18 years or older) with histologically/cytologically confirmed ESCC and unresectable metastatic disease, an Eastern Cooperative Oncology Group performance status of 0–2 and a life expectancy of >3 months were applied. Adequate hematological (absolute neutrophil count ≥1.5×109/L, platelet count ≥100×109/L, hemoglobin ≥9 g/dL), renal (serum creatinine ≤1.5× the upper limit of normal), and hepatic (total bilirubin ≤2.0 mg/dL and serum transaminase level ≤3× the upper limit of normal) parameters were also required. Prior chemotherapy for advanced disease was not permitted; however, adjuvant or neoadjuvant chemotherapy was allowed on the condition that it was completed at least 6 months before starting first-line therapy. Other key exclusion criteria included: clinically significant cardiovascular disease; concomitant cancers; clinically detectable ascites; neuropathy; parallel radiation therapy; brain or leptomeningeal involvement; and uncontrolled significant comorbid conditions. Before the treatment, written informed consent for chemotherapy drugs, treatment schedule, and toxicity was obtained from all patients. As this was a retrospective study, ethical approval and patient consent were not required, as stated by the medical ethics committee of the Henan Cancer Hospital.
Treatment
For the patients who were assigned to receive nab-TP, nab-PTX was intravenously infused at a dosage of 125 mg/m2 on the first and eighth days and DDP was intravenously administered at 75 mg/m2 with adequate hydration on the first day of every 21-day cycle. For the patients who were assigned to receive sb-TP, PTX was intravenously infused at a dosage of 80 mg/m2 on the first and eighth days (3-hour infusion) and DDP was administered in the same manner on the first day of every 21-day cycle. Approximately 30 minutes before the administration of PTX, patients were premedicated with 20 mg of dexamethasone, 45.5 mg of pheniramine maleate, and 20 mg of famotidine. The efficacy was evaluated for every two cycles. Until disease progression or the occurrence of unacceptable toxicity, patients received chemotherapy for up to six or eight cycles.
Efficacy and toxicity evaluation
Pretreatment evaluation included a full medical history, physical examination, tumor-related symptoms, complete blood cell count, blood chemistry analysis, creatinine clearance, upper gastrointestinal endoscopies, electrocardiograph, and thoracic and abdominal computed tomographic scans. In addition, toxicities were graded according to the National Cancer Institute of Canada Common Toxicity Criteria (version 2.0).23 In accordance with the Response Evaluation Criteria In Solid Tumors ( RECIST) guidelines, tumor assessments were carried out by employing chest and abdomen computed tomographic scans every two cycles of therapy.24 Responses were confirmed through repeated assessments carried out <4 weeks apart. After six or eight chemotherapy cycles, patients were monitored for every 12 weeks until disease progression.
Statistical analysis
Statistical Package for Social Sciences version 14.0 software (SPSS Inc., Chicago, IL, USA) was adopted to analyze all data. Descriptive variables of patient characteristics and toxicities were directly calculated from the database. In order to compare toxicities and response in the two groups, χ2 test and Fisher’s exact test were employed. Meanwhile, PFS was calculated from the first day of treatment to the date of progressive disease or the date of death from any causes, whereas OS was measured from the initiation of chemotherapy to the date of the last follow-up or death. PFS and OS were calculated and plotted by making use of Kaplan–Meier methods and compared by means of the log-rank test. A P-value of <0.05 (two-sided) was considered to be statistically significant.
Results
Patient characteristics
A total of 32 patients were treated with nab-TP regimen and 43 were treated with sb-TP. The baseline characteristics of the patients treated with nab-TP were similar to those treated with sb-TP (Table 1). The median age of these patients was 54 years (range: 29–73) and they consisted of 46 males and 29 females. Also, 88% of patients had an Eastern Cooperative Oncology Group performance status of 0–1.
  | Table 1 Baseline characteristics of the patients |
Treatment
The median number of cycles administered was four in the nab-TP arm group (range: 3–8) and five in the sb-TP group (range: 2–8). In total, 14 (43.8%) of the 32 nab-TP treated patients and 23 (53.5%) of the 43 TP-treated patients completed ≥5 cycles of chemotherapy. A total of 149 and 168 cycles were separately administered in the nab-TP and sb-TP arms, respectively. Besides, dose reduction occurred in seven nab-TP patients (22%) and ten sb-TP patients (23%); treatment delay of >7 days occurred in 12 nab-TP patients (37.5%) and 17 sb-TP patients (40%). A similar proportion of patients received second-line chemotherapy (nab-TP =66%, sb-TP =70%) (Table 2).
  | Table 2 Overall treatment summary |
Overall response and survival
All patients were evaluated for drug efficacy. The ORR and disease control rate (DCR) were 50% and 81% (1 CR, 15 partial response [PR], and 10 stable disease [SD]), respectively, in the nab-TP group and 30% and 65% (1 CR, 12 PR, and 15 SD), respectively, in the sb-TP group (Table 3). The nab-TP arm demonstrated a higher ORR (50% vs 30%; P=0.082) and DCR (81% vs 65%; P=0.124) than the sb-TP group (Table 3). The median PFS was 6.1 months (95% confidence interval [CI]: 5.5–7.1 months) in the nab-TP arm and 5.0 months (95% CI: 4.1–6.1 months) in the sb-TP arm (P=0.029) (Figure 1A; Table 4). Furthermore, the median OS was 12.5 months (95% CI: 9.4–15.6 months) for the nab-TP group and 10.7 months (95% CI: 8.1–13.3 months) for the sb-TP group (P=0.269) (Figure 1B; Table 4).
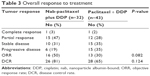  | Table 3 Overall response to treatment |
  | Table 4 Kaplan–Meier analysis |
In the nab-TP arm, there was an improvement in PFS (median, 6.1 vs 5.0; P=0.029). In spite of the favorable OS trend in the nab-TP arm, statistical significance was not reached. Regarding the application of second-line therapy, there were no major imbalances between the treatment groups (nab-TP arm, 66% and sb-TP arm, 70%).
Safety and tolerability
All patients were assessable for toxicity. Grade 3 or higher major treatment-related adverse events are shown in Table 5. Although the occurrence of the most common grades 3 and 4 leukopenia, neutropenia, febrile neutropenia, and anemia was more frequent in nab-TP, no significant difference was observed between the two groups (all P>0.05). During the entire treatment period, thrombocytopenia was similar in both arms. In general, nonhematologic toxicities were mild and manageable. Significantly less common grade ≥3 peripheral neuropathy, arthralgia, and myalgia occurred in the nab-PC arm (all P<0.05). In either treatment group, no treatment-related deaths occurred.
Discussion
Because of its extremely aggressive nature and poor survival rate, advanced ESCC is one of the least studied and deadliest cancers worldwide.25 To date, the National Comprehensive Cancer Network (NCCN) guidelines concerning ESCC are based on the results of various clinical trials that have included a number of patients with gastroesophageal junction or gastric adenocarcinoma due to a relative rarity of enough clinical evidence from randomized Phase III trials for ESCC.26,27 However, our study is based on a homogenous cohort of advanced ESCC patients, so as to find effective regimens for them.
As far as we know, this is the first comparative study of nab-PTX plus platinum doublet with PTX-DDP. Our findings revealed that the combined therapy of nab-TP regimen was associated with a higher response rate (50% vs 30%) and DCR (81% vs 65%) when compared with sb-TP and no significant difference was observed between them. Nab-TP possessed a longer median PFS (6.1 vs 5.0 months; P=0.029) than sb-TP. According to the results of Kaplan–Meier curve analysis, the endpoint tended to be better in nab-TP, although the difference in OS was not statistically significant. Studies demonstrated that the ability to deliver a higher dose of PTX, enhanced tissue distribution, and tumor uptake of nab-PTX versus sb-PTX likely contributed to more favorable efficacy and safety profile of the albumin-bound formulation of PTX.10 In general, these findings of sb-TP were comparable with other previously reported studies.17,28 These findings suggested that nab-TP might become a promising treatment option for ESCC.
In Shi et al’s study, a sample of 33 patients without control arm showed a clinically significant response rate (60.6%) and DCR (87.9%), as well as a median PFS of 6.2 months and a longer median OS of 15.5 months, when compared with other traditional taxane-based regimens.17 Approximately 87.9% patients who received subsequent treatment after progression may have contributed to better OS.17 Although the differences were not statistically significant, another study showed that in advanced esophageal cancer patients, there was a tendency of higher ORR and DCR with nab-PTX-based regimens than with PTX/ docetaxel (DTX)-based regimens (25% vs 19.7% and 81.3% vs 59.1%, respectively), in which nab-PTX, PTX, DDP, and 5-FU (TPF), and TP groups were included. Nab-PTX had a tendency to result in longer PFS and OS than TP regimen. Among nab-PTX, TPF, and TP groups, there were no significant differences in ORR, PFS, and OS.28 With an ORR of 37.0%, a DCR of 44.4%, and a median PFS of 6.6 months, the outcome of second-line chemotherapy with nab-PTX was reported to show promising efficacy in advanced ESCC by Yuan et al.29
With lower rates of grades 3 and 4 neuropathy, arthralgia, and myalgia, nab-TP was generally better tolerated (all P<0.05). The relief of neuropathy-associated symptom burden may be considered when making decisions on chemotherapy options.30 Neuropathy is irreversible with PTX in some patients, which has been attributed to the excipient cremophor.31,32 Although a higher percentage of patients in the nab-TP arm developed anemia, febrile neutropenia, leukopenia, and neutropenia, this difference was not statistically significant. Dose reduction (22% vs 23%; P=0.88) and treatment delays (37.5% vs 40%; P=0.86) were similar in the two regimens. The safety profile for both regimens was consistent with that in previous reports.17,28,29 When compared with sb-TP, the rate of serious life-threatening adverse events was not increased with the application of nab-TP. Adverse events were generally grade 3 or lower and resolved without specific treatment. But the occurrence of neutropenia, febrile neutropenia, and thrombocytopenia was greater in this study than with 3-weekly nab-TP with DDP as reported by Shi et al.17 Two additional cycles may have contributed to increased bone marrow suppression in our study (six to eight chemotherapy cycles) when compared to Shi et al’s trial (four to six chemotherapy cycles).
Limitations
There are some limitations in this study. First of all, it was a retrospective study performed at a single institution. Second, the follow-up schedule for the two treatment regimens was not identical because nab-TP-treated patients were followed more closely and evaluated weekly for toxicity. Therefore, there was a possible underestimation of bone marrow toxicity in the sb-TP group since midcycle bone marrow toxicity might not have been reported in the sb-TP group. Another limitation of the study was that life quality was not measured.
Conclusion
Advanced ESCC patients had a higher ORR and DCR with the administration of nab-TP versus sb-TP as a first-line therapy. Significant improvement was observed in favor of the nab-TP arm for PFS. When compared with sb-TP, nab-TP produced less severe neuropathy, myalgia, and arthralgia. Taken together, the nab-TP regimen has a more favorable risk–benefit profile than that of sb-TP as a first-line therapy for all advanced ESCC patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Siersema PD. Esophageal cancer. Gastroenterol Clin North Am. 2008;37(4):943–964. | ||
Blot WJ, McLaughlin JK. The changing epidemiology of esophageal cancer. Semin Oncol. 1999;26(5 Suppl 15):2–8. | ||
Ekman S, Dreilich M, Lennartsson J, et al. Esophageal cancer: current and emerging therapy modalities. Expert Rev Anticancer Ther. 2008;8(9):1433–1448. | ||
Enzinger PC, Ilson DH, Kelsen DP. Chemotherapy in esophageal cancer. Semin Oncol. 1999;26(5 Suppl 15):12–20. | ||
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. | ||
Albertsson M, Johansson B, Friesland S, et al. Phase II studies on docetaxel alone every third week, or weekly in combination with gemcitabine in patients with primary locally advanced, metastatic, or recurrent esophageal cancer. Med Oncol. 2007;24(4):407–412. | ||
Iizuka T, Kakegawa T, Ide H, et al. Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese Esophageal Oncology Group trial. Jpn J Clin Oncol. 1992;22(3):172–176. | ||
Lee J, Im YH, Cho EY, et al. A phase II study of capecitabine and cisplatin (XP) as first-line chemotherapy in patients with advanced esophageal squamous cell carcinoma. Cancer Chemother Pharmacol. 2008;62(1):77–84. | ||
Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. | ||
Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–1324. | ||
Gardner ER, Dahut WL, Scripture CD, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14(13):4200–4205. | ||
Volk LD, Flister MJ, Chihade D, Desai N, Trieu V, Ran S. Synergy of nab-paclitaxel and bevacizumab in eradicating large orthotopic breast tumors and preexisting metastases. Neoplasia. 2011;13(4):327–338. | ||
Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–2062. | ||
Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548–4554. | ||
Kottschade LA, Suman VJ, Amatruda T 3rd, et al. A phase II trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group Study, N057E(1). Cancer. 2011;117(8):1704–1710. | ||
Coleman RL, Brady WE, McMeekin DS, et al. A phase II evaluation of nanoparticle, albumin-bound (nab) paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;122(1):111–115. | ||
Shi Y, Qin R, Wang ZK, Dai GH. Nanoparticle albumin-bound paclitaxel combined with cisplatin as the first-line treatment for metastatic esophageal squamous cell carcinoma. OncoTargets Ther. 2013;6:585–591. | ||
Rosenberg P, Andersson H, Boman K, et al. Randomized trial of single agent paclitaxel given weekly versus every three weeks and with peroral versus intravenous steroid premedication to patients with ovarian cancer previously treated with platinum. Acta Oncol. 2002;41(5):418–424. | ||
Osman MA, Elkady MS, Nasr KE. Weekly paclitaxel versus three-weekly paclitaxel in recurrent platinum-resistant epithelial ovarian and peritoneal cancers: a phase III study. Clin Med Insights Oncol. 2016;10:35–41. | ||
Dalton HJ, Yu X, Hu L, et al. An economic analysis of dose dense weekly paclitaxel plus carboplatin versus every-3-week paclitaxel plus carboplatin in the treatment of advanced ovarian cancer. Gynecol Oncol. 2012;124(2):199–204. | ||
Fan Y, Jiang Y, Zhou X, et al. Phase II study of neoadjuvant therapy with nab-paclitaxel and cisplatin followed by surgery in patients with locally advanced esophageal squamous cell carcinoma. Oncotarget. Epub 2016 May 23. | ||
Sun Q, Liu C, Zhong H, et al. Multi-center phase II trial of weekly paclitaxel plus cisplatin combination chemotherapy in patients with advanced gastric and gastro-esophageal cancer. Jpn J Clin Oncol. 2009;39(4):237–243. | ||
Trotti A, Byhardt R, Stetz J, et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47(1):13–47. | ||
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. | ||
Kono K, Mimura K, Fujii H, Shabbir A, Yong WP, Yan So JB. Potential therapeutic significance of HER-family in esophageal squamous cell carcinoma. Ann Thorac Cardiovasc Surg. 2012;18(6):506–513. | ||
Ross P, Nicolson M, Cunningham D, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002;20(8):1996–2004. | ||
Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24(31):4991–4997. | ||
Jiang C, Liao FX, Rong YM, et al. Efficacy of taxane-based regimens in a first-line setting for recurrent and/or metastatic Chinese patients with esophageal cancer. Asian Pac J Cancer Prev. 2014;15(13):5493–5498. | ||
Yuan Y, Zhang Y, Shi L, Mei JF, Feng JE, Shen B. Clinical research on albumin-bound paclitaxel-based chemotherapy for advanced esophageal cancer. Asian Pac J Cancer Prev. 2015;16(12):4993–4996. | ||
Cella D, Peterman A, Hudgens S, Webster K, Socinski MA. Measuring the side effects of taxane therapy in oncology: the functional assesment of cancer therapy-taxane (FACT-taxane). Cancer. 2003;98(4):822–831. | ||
Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. Assessment of neurotoxicity following repeated cremophor/ethanol injections in rats. Neurotox Res. 2001;3(3):301–306. | ||
Scripture CD, Figg WD, Sparreboom A. Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol. 2006;4(2):165–172. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


