Back to Journals » Infection and Drug Resistance » Volume 16
Voriconazole-Induced Hepatotoxicity in a Patient with Pulmonary Aspergillosis: A Case Report
Authors Gu L, Ai T, Pang L, Xu D, Wang H
Received 30 April 2023
Accepted for publication 1 August 2023
Published 18 August 2023 Volume 2023:16 Pages 5405—5411
DOI https://doi.org/10.2147/IDR.S419382
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Li Gu,1 Tao Ai,2 Ling Pang,1 Dong Xu,1 Han Wang3
1Department and Institute of Infectious Disease, Tongji Hospital, Tongji Medical College and State Key Laboratory for Diagnosis and Treatment of Severe Zoonostic Infectious Disease, Huazhong University of Science and Technology, Wuhan, Hubei Province, People’s Republic of China; 2Department of Radiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, People’s Republic of China; 3Department of Gastroenterology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, People’s Republic of China
Correspondence: Dong Xu, Department and Institute of Infectious Disease, Tongji Hospital, Tongji Medical College and State Key Laboratory for Diagnosis and Treatment of Severe Zoonostic Infectious Disease, Huazhong University of Science and Technology, Jiefang Avenue 1095, Wuhan, 430030, People’s Republic of China, Email [email protected] Han Wang, Department of Gastroenterology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Jiefang Avenue 1095, Wuhan, 430030, People’s Republic of China, Email [email protected]
Abstract: Voriconazole is the therapy of choice for aspergillosis. However, hepatotoxicity is the most common reason for the discontinuation of voriconazole. In contrast, posaconazole is well tolerated, with a low incidence of hepatotoxicity. In most cases, hepatotoxicity is associated with high voriconazole trough concentration influenced mainly by cytochrome P450 (CYP) 2C19 gene polymorphism. Compared with normal metabolizers, intermediate and poor metabolizers generally have higher voriconazole trough concentrations with an increased risk of hepatotoxicity. Here, we describe changes in hepatotoxicity throughout azole therapy in a patient with pulmonary aspergillosis (PA). Nevertheless, the patient with the normal metabolism genotype of CYP2C19 developed severe hepatotoxicity caused by voriconazole but tolerated posaconazole well, with a lack of direct cross-hepatotoxicity between the both. Interestingly, the patient had a high risk of hepatotoxicity at a low voriconazole trough concentration. Fortunately, elevated liver enzymes declined to the baselines with posaconazole treatment.
Keywords: hepatotoxicity, drug-induced liver injury, drug trough concentrations, voriconazole, posaconazole, pulmonary aspergillosis
Introduction
Triazoles, especially voriconazole, are preferred agents for treating and preventing aspergillosis in most patients.1 Despite its efficacy, clinical use of voriconazole appears complicated by its adverse events and narrow therapeutic range. Hepatotoxicity is a joint adverse event of azoles, but voriconazole appears to have a higher risk of liver injury than other modern antifungals.2 There was increasing evidence of a significant correlation between hepatotoxicity and voriconazole trough concentrations.3–5 But voriconazole plasma concentrations present wide interpatient variability, primarily due to gene polymorphism of cytochrome P450 (CYP) 2C19.6 High voriconazole trough concentrations are more likely to be observed in poor metabolizers than in normal metabolizers, with an increased risk of hepatotoxicity.6–9 Instead, we describe a normal metabolism patient with pulmonary aspergillosis (PA) who developed significant hepatotoxicity not associated with voriconazole plasma exposure.
Posaconazole, another triazole antifungal, is well-tolerated, with a lower incidence of treatment-related adverse events and discontinuation than voriconazole.10 Long-term posaconazole treatment does not increase the risk of any individual adverse events with a low incidence of hepatotoxicity.11 Even so, there remains concern that hepatotoxicity maintains or worsens when switching between a class of azoles. Here, we also reported that posaconazole successfully replaced voriconazole without the same adverse event, indicating a lack of direct cross-hepatotoxicity between the both.
Case Presentation
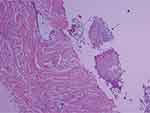
A 44-year-old man patient was admitted to another hospital for hemoptysis and diagnosed with pulmonary aspergillosis based on the pathological result (Figure 1). The patient had a history of old tuberculosis and chronic gastritis, not currently treated with any medication. Subsequently, the patient was admitted to Tongji Hospital for fungal infection and started antifungal treatment with a standard dose (200 mg twice daily) of intravenous voriconazole for one week without liver enzyme abnormalities (Figure 2). After discharge, he was prescribed oral voriconazole at the same dose.
After six weeks, severe hepatotoxicity with significant liver enzyme elevations was found at the first liver function test, including alanine transaminase (ALT) [899U/L (≤41 U/L)], aspartate transaminase (AST) [821 U/L (≤40 U/L)], alkaline phosphatase (ALP) [256 U/L (40~130U/L)], and gamma-glutamyl transpeptidase (γ-GGT) [451 U/L (10~71U/L)]. It was worth noting that the patient has not received any other medications recently, including herbal products or dietary supplements, besides voriconazole. Therefore, he was advised to discontinue voriconazole due to consideration of voriconazole-induced hepatotoxicity and elevations of ALT and AST more than five times the upper limit of normal (ULN). After five days, liver enzymes slightly declined, including ALT (826 U/L), AST (598 U/L), ALP (227 U/L), and γ-GGT (427 U/L). As a result, the patient was re-admitted to our hospital with hepatic insufficiency.
After admission, laboratory tests revealed increased levels of ALT (879 U/L), AST (529 U/L), ALP (219 U/L), γ-GGT (410U/L), direct bilirubin [16.7 μmol/L (≤8.0 μmol/L)], lactate dehydrogenase [409 U/L (135~225 U/L)], 5’-nucleotidase [11.2 U/L (≤10 U/L)], α-L-fucosidase [52 IU/L (5~40 IU/L)], and blood ammonia [91 μmol/L (16~60 μmol/L)]. The patient was then given hepatoprotective medications for injection (Polyene Phosphatidylcholine, Glutathione, Compound Ammonium Glycyrrhetate S) and Micafungin Sodium for injection (150 mg once daily). Some examinations, including ultrasound and tests for viral hepatitis and autoimmune liver diseases, revealed no abnormalities except for small cysts. Serum ceruloplasmin was within the normal range [0.49 g/L (0.22~0.58 g/L)]. After eight days of discontinuation and three days of treatment, the patient was discharged with a significant decline of liver enzymes, including ALT (350 U/L), AST (100 U/L), ALP (145 U/L) and γ-GGT (309 U/L). The blood ammonia also returned to normal (58 μmol/L).
Based on the above information and the Roussel-Ucalaf Causality Assessment Method (RUCAM), the patient scored six points, indicating a “probable” drug-induced liver injury (DILI). According to the judgment criteria of the Council for International Organizations of Medical Sciences (CIOMS), the type of liver injury in the patient was characterized as a mixed hepatocellular-cholestatic injury due to ALT ≥ 3 ULN, ALP ≥ 2 ULN, and 2 < R < 5 (the R-value was obtained by dividing the ratio of the measured ALT value to the ULN of ALT by the ratio of the measured ALP value to the ULN of ALP).12
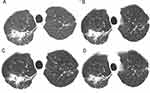
After discharge, the patient was advised to continue hepatoprotective therapy and to follow up regularly. One week later, liver enzymes declined further, including ALT (123 U/L), AST (69 U/L), APL (116 U/L), and γ-GGT (225 U/L). Then the patient was prescribed posaconazole suspension at 100 mg once daily due to concerns about the same adverse event. Fortunately, liver enzymes remained progressively lower after two weeks. But posaconazole trough concentrations were below the therapeutic range (≥1.0 μg/mL) according to weekly therapeutic drug monitoring (TDM) (Figure 2). Hence, he was prescribed posaconazole suspension at 100 mg twice daily starting the third week. The trough concentration reached 1.17 μg/mL, within the therapeutic range. All liver enzymes returned to normal after three weeks and to the baselines after five weeks. During antifungal therapy, the patient’s symptoms gradually resolved, and chest computed tomography (CT) showed gradual resorption of the lesion in the upper lobe of the right lung (Figure 3).
However, the patient strongly requested antifungal therapy with the remaining previous voriconazole for his family economy. Considering its safety, the patient was advised to take oral voriconazole at 50 mg twice daily. One week later, liver enzyme elevations, including ALT (111 U/L) and AST (60 U/L), were found again, suggesting intolerance to voriconazole. Therefore, according to the RUCAM scoring scale, the patient scored an additional three points for nine points, indicating a “high probable” DILI. Meanwhile, the TDM of voriconazole revealed a trough concentration of 0.32 μg/mL (reference range: 1~5.5 μg/mL). The pharmacogenetic test showed that the patient was a normal metabolizer with a genotype of CYP2C19*1/*1 and could be treated with the standard dose of voriconazole. Finally, the patient discontinued voriconazole and was prescribed posaconazole suspension at 100 mg twice daily for continuous antifungal treatment. Liver enzymes returned to the baselines after two weeks.
Discussion
With the increase in immunosuppressed patients, invasive aspergillosis with high mortality has increased dramatically,13 and so has the demand for antifungal drugs. Voriconazole serves as the primary treatment for invasive PA and as the first-line treatment for chronic PA.1,14 However, hepatotoxicity is the most frequent adverse effect of voriconazole and the common reason for dose adjustments.15 A meta-analysis involving more than 8000 patients showed that 19.7% of voriconazole users developed serum elevations of liver enzymes.16 According to the Common Terminology Criteria for Adverse Events (CTCAE, v.5.0), the severity of hepatotoxicity can display Grades 1 through 5, ranging from mild, middle, severe, to life-threatening consequences and even death. Although abnormalities in liver enzymes are common during voriconazole treatment, severe adverse event in the liver does not always occur. Hepatotoxicity in our patient was classified as Grade 4 due to an ALT over 20 times the ULN, indicating life-threatening consequences or urgent intervention. There are still no specific biomarkers for diagnosing DILI in the clinical practice, relying mostly on excluding other causes of liver disease and diagnostic scales. Some scales are available to assess the causality of adverse drug reactions, such as the Naranjo’s Scale, the WHO-UMC causality assessment system, and the RUCAM scoring scale, an assessment tool for DILI. Based on the patient information above, all three scales suggested that liver injury was highly associated with voriconazole.
Hepatotoxicity may be associated with drug exposure, particularly voriconazole.17 The safety administration of voriconazole presents challenges due to the narrow therapeutic range and wide variability in plasma concentrations. Most recent studies reported hepatotoxicity correlated with high voriconazole trough concentrations.3–6,18,19 Some studies reported that voriconazole trough concentrations >3.0~6.0 μg/mL were associated with increased hepatotoxicity.5,15,19,20 In contrast, no correlation between the both was also reported in other studies.21,22 Overall, no specific voriconazole trough concentration thresholds can clearly predict hepatotoxicity. Liver metabolism plays a crucial role in voriconazole plasma exposure that varies wildly due to CYP2C19 gene polymorphism, co-medications, liver function, and age.23 Voriconazole is primarily metabolized by CYP2C19 in the liver, whose gene polymorphism can affect the voriconazole plasma concentrations.6,24 Based on the ability of CYP2C19 to metabolize substrates, individuals can be classified into five categories: ultrarapid metabolizers (UM, CYP2C19*17/*17), rapid metabolizers (RM, CYP2C19*1/*17), normal metabolizers (NM, CYP2C19*1/*1), intermediate metabolizers (IM, CYP2C19*1/*2, CYP2C19*1/*3, CYP2C19*2/*17) and poor metabolizers (PM, CYP2C19*2/*2, CYP2C19*2/*3, CYP2C19*3/*3).25 Compared with NMs, IMs and PMs are easier to present higher trough concentrations with an increased risk of hepatotoxicity, especially in Asians.6,8,24
However, the relationship between hepatotoxicity and voriconazole exposure and the effect of CYP2C19 genotype on the relationship cannot be applied to all populations due to individual differences. Our patient with a CYP2C19 genotype of 1*1 and normal liver function could be given a standard dose of voriconazole.25 But he developed severe hepatotoxicity without co-medications, herbal products, or dietary supplements. Furthermore, we further found by TDM that liver enzyme elevations still occurred at a low trough concentration of 0.32 μg/mL. Consequently, the hepatotoxicity experienced by our patient may not be associated with the CYP2C19 genotype and the voriconazole plasma levels. Other factors may contribute to voriconazole-induced hepatotoxicity, except for some factors influencing drug exposure.
The exact mechanism of hepatotoxicity induced by voriconazole remains unclear. It was reported that oxidative stress was the leading cause of voriconazole-induced hepatotoxicity.26,27 Damaged hepatocytes, caused by cumulative oxidative stress, release damage-associated molecular patterns that activate innate immune cells via pattern recognition receptors and induce an inflammatory response. The severe inflammatory response promotes oxidative stress, so the positive feedback effect between both further leads to hepatocyte dysfunction and liver injury. Moreover, the immune response may contribute to voriconazole-induced hepatotoxicity. It was reported that adaptive immune responses mediate the process of hepatocyte injury in most idiosyncratic drug-induced liver injuries.28 Reactive metabolites of the drugs bind to modified proteins to form neoantigens, which are taken up and presented by the antigen-presenting cell, contributing to the full activation of adaptive immune responses, notably CD8+ T cell-mediated cytotoxicity. Therefore, we propose the hypothesis that immune responses probably play a role in voriconazole-induced hepatotoxicity and that the infectious or inflammatory state of the liver potentially contributes to its development, which may explain our patient had a high risk of hepatotoxicity even though he was a normal metabolizer with a low trough concentration. However, few studies have discussed the role of immune responses triggered by voriconazole in hepatotoxicity. More extensive studies are expected to confirm the impact of immune mechanisms on voriconazole-associated hepatotoxicity.
In general, there is no perfect positive correlation between voriconazole exposure and hepatotoxicity. It remains challenging to establish an appropriate therapeutic range to balance the efficacy and safety of voriconazole. Although hepatotoxicity occurs at higher voriconazole trough concentrations in most cases, careful monitoring of liver enzymes seems more critical than TDM to prevent severe hepatotoxicity in non-typical patients, such as those with hepatotoxicity not associated with voriconazole exposure. Hence, careful follow-up with close liver function tests and TDM in the early course of therapy is necessary for all patients receiving long-term antifungal treatment to detect potential hepatotoxicity and to avoid subtherapeutic concentrations.
If liver enzymes elevate ≥ 5 × ULN, switching to another agent is reasonable in clinical practice.17 However, a cross-reactivity concerning hepatotoxicity needs to be taken into account when switching between azoles. Our case confirmed the possibility of replacing voriconazole with posaconazole without a class of hepatotoxicity, as has been reported in other cases.29,30 A retrospective study supported the feasibility of posaconazole for salvage therapy in patients with prior administration of another triazole.31 A clinical trial investigation found that posaconazole was not inferior to voriconazole in the treatment of invasive aspergillosis and also was better tolerated.10 Consequently, posaconazole may be an effective alternative to voriconazole after intolerant hepatotoxicity, especially in outpatients receiving long-term antifungal treatment. Additionally, patients with PA may be treated with antifungals and intravenous hepatoprotective medications for transition in the acute phase of significant hepatotoxicity. In our case, micafungin supplemented with hepatoprotective therapy decreased liver enzymes significantly within three days. Further research is expected to provide more effective and safe strategies for selecting and switching a class of antifungal agents in treating aspergillosis.
Conclusion
Our case report highlights the importance of the management of azoles during the antifungal treatment of aspergillosis, especially voriconazole. Because normal metabolism patients with the genotype of CYP2C19*1/*1 have a potential risk of severe hepatotoxicity with the standard dose of voriconazole and without co-medications, and liver enzymes still elevated at a low voriconazole trough concentration of 0.32 μg/mL. It indicated that other factors may contribute to voriconazole-induced hepatotoxicity, except for some factors influencing drug exposure. Furthermore, our case may provide an experience of safe and effective alternative treatment of PA after intolerant hepatotoxicity to voriconazole.
Abbreviations
CYP29C19, cytochrome P450 (CYP) 2C19; PA, pulmonary aspergillosis; IFD, invasive fungal diseases; ALT, alanine transaminase; AST, aspartate transaminase; APL, alkaline phosphatase; γ-GGT, gamma-glutamyl transpeptidase; ULN, the upper limit of normal; DILI, drug-induced liver injury; TDM, therapeutic drug monitoring; UM, ultrarapid metabolizers; RM, rapid metabolizers; NM, normal metabolizers; IM, intermediate metabolizers; PM, poor metabolizers.
Patient Consent and Ethics Statement
The patient provided informed consent for the publication of the case report. The ethical committee approval was not required for the case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study did not receive any funding.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63(4):e1–e60. doi:10.1093/cid/ciw326
2. Wang J-L, Chang C-H, Young-Xu Y, Chan KA. Systematic review and meta-analysis of the tolerability and hepatotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob Agents Chemother. 2010;54(6):2409–2419. doi:10.1128/AAC.01657-09
3. Hamada Y, Ueda T, Miyazaki Y, et al. Effects of antifungal stewardship using therapeutic drug monitoring in voriconazole therapy on the prevention and control of hepatotoxicity and visual symptoms: a multicentre study conducted in Japan. Mycoses. 2020;63(8):779–786. doi:10.1111/myc.13129
4. Wang T, Miao L, Shao H, et al. Voriconazole therapeutic drug monitoring and hepatotoxicity in critically ill patients: a nationwide multi-centre retrospective study. Int J Antimicrob Agents. 2022;60(5–6):106692. doi:10.1016/j.ijantimicag.2022.106692
5. Jin H, Wang T, Falcione BA, et al. Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J Antimicrob Chemother. 2016;71(7):1772–1785. doi:10.1093/jac/dkw045
6. Wang T, Zhu H, Sun J, et al. Efficacy and safety of voriconazole and CYP2C19 polymorphism for optimised dosage regimens in patients with invasive fungal infections. Int J Antimicrob Agents. 2014;44(5):436–442. doi:10.1016/j.ijantimicag.2014.07.013
7. Lin X-B, Z-W L, Yan M, et al. Population pharmacokinetics of voriconazole and CYP2C19 polymorphisms for optimizing dosing regimens in renal transplant recipients. Br J Clin Pharmacol. 2018;84(7):1587–1597. doi:10.1111/bcp.13595
8. Lee S, Kim B-H, Nam W-S, et al. Effect of CYP2C19 polymorphism on the pharmacokinetics of voriconazole after single and multiple doses in healthy volunteers. J Clin Pharmacol. 2012;52(2):195–203. doi:10.1177/0091270010395510
9. Scholz I, Oberwittler H, Riedel K-D, et al. Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. Br J Clin Pharmacol. 2009;68(6):906–915. doi:10.1111/j.1365-2125.2009.03534.x
10. Maertens JA, Rahav G, Lee D-G, et al. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: a Phase 3, randomised, controlled, non-inferiority trial. Lancet. 2021;397(10273):499–509. doi:10.1016/S0140-6736(21)00219-1
11. Raad II, Graybill JR, Bustamante AB, et al. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis. 2006;42(12):1726–1734. doi:10.1086/504328
12. Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34(2):134–144. doi:10.1055/s-0034-1375955
13. Latgé J-P, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev. 2019;33(1). doi:10.1128/CMR.00140-18
14. Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the infectious diseases society of America. Clin Infect Dis. 2008;46(3):327–360. doi:10.1086/525258
15. Shen K, Gu Y, Wang Y, et al. Therapeutic drug monitoring and safety evaluation of voriconazole in the treatment of pulmonary fungal diseases. Ther Adv Drug Saf. 2022;13:20420986221127503. doi:10.1177/20420986221127503
16. Troke PF, Hockey HP, Hope WW. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob Agents Chemother. 2011;55(10):4782–4788. doi:10.1128/AAC.01083-10
17. Tverdek FP, Kofteridis D, Kontoyiannis DP. Antifungal agents and liver toxicity: a complex interaction. Expert Rev Anti Infect Ther. 2016;14(8):765–776. doi:10.1080/14787210.2016.1199272
18. Tang D, Yan M, Song B-L, et al. Population pharmacokinetics, safety and dosing optimization of voriconazole in patients with liver dysfunction: a prospective observational study. Br J Clin Pharmacol. 2021;87(4):1890–1902. doi:10.1111/bcp.14578
19. Luong M-L, Al-Dabbagh M, Groll AH, et al. Utility of voriconazole therapeutic drug monitoring: a meta-analysis. J Antimicrob Chemother. 2016;71(7):1786–1799. doi:10.1093/jac/dkw099
20. Ueda K, Nannya Y, Kumano K, et al. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int J Hematol. 2009;89(5):592–599. doi:10.1007/s12185-009-0296-3
21. Zonios D, Yamazaki H, Murayama N, et al. Voriconazole metabolism, toxicity, and the effect of cytochrome P450 2C19 genotype. J Infect Dis. 2014;209(12):1941–1948. doi:10.1093/infdis/jiu017
22. Chu HY, Jain R, Xie H, Pottinger P, Fredricks DN. Voriconazole therapeutic drug monitoring: retrospective cohort study of the relationship to clinical outcomes and adverse events. BMC Infect Dis. 2013;13:105. doi:10.1186/1471-2334-13-105
23. Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45(7):649–663. doi:10.2165/00003088-200645070-00002
24. Zhang Y, Hou K, Liu F, et al. The influence of CYP2C19 polymorphisms on voriconazole trough concentrations: systematic review and meta-analysis. Mycoses. 2021;64(8):860–873. doi:10.1111/myc.13293
25. Moriyama B, Obeng AO, Barbarino J, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin Pharmacol Ther. 2017;102(1):45–51. doi:10.1002/cpt.583
26. S-L W, Cheng C-N, Wang -C-C, Lin S-W, Kuo C-H. Metabolomics analysis of plasma reveals voriconazole-induced hepatotoxicity is associated with oxidative stress. Toxicol Appl Pharmacol. 2020;403:115157. doi:10.1016/j.taap.2020.115157
27. S-L W, Wei T-Y, Lin S-W, K-Y S, Kuo C-H. Metabolomics investigation of voriconazole-induced hepatotoxicity in mice. Chem Res Toxicol. 2019;32(9):1840–1849. doi:10.1021/acs.chemrestox.9b00176
28. Jee A, Sernoskie SC, Uetrecht J. Idiosyncratic drug-induced liver injury: mechanistic and clinical challenges. Int J Mol Sci. 2021;22(6). doi:10.3390/ijms22062954
29. Foo H, Gottlieb T. Lack of cross-hepatotoxicity between voriconazole and posaconazole. Clin Infect Dis. 2007;45(6):803–805. doi:10.1086/521174
30. Martínez-Casanova J, Carballo N, Luque S, Sorli L, Grau S. Posaconazole achieves prompt recovery of voriconazole-induced liver injury in a case of invasive aspergillosis. Infect Drug Resist. 2018;11:317–321. doi:10.2147/IDR.S154457
31. Heinz WJ, Egerer G, Lellek H, Boehme A, Greiner J. Posaconazole after previous antifungal therapy with voriconazole for therapy of invasive aspergillus disease, a retrospective analysis. Mycoses. 2013;56(3):304–310. doi:10.1111/myc.12023
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.