Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Vitamin B12, Folate, Homocysteine, Inflammatory Mediators (Interleukin-6, Tumor Necrosis Factor-α and C-Reactive Protein) Levels in Adolescents with Anxiety or Depressive Symptoms
Authors Tan Y , Zhou L, Huang J, Chen X, Wu Y, Song X, Wang J, Hu H, Yang Q
Received 2 December 2022
Accepted for publication 15 March 2023
Published 7 April 2023 Volume 2023:19 Pages 785—800
DOI https://doi.org/10.2147/NDT.S399378
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Richard J Porter
Yongjun Tan,1 Li Zhou,1 Jiagui Huang,1 Xia Chen,1 Youlin Wu,1 Xiaosong Song,1 Jiani Wang,1 Hua Hu,2 Qin Yang1
1Department of Neurology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Qin Yang, Department of Neurology, The First Affiliated Hospital of Chongqing Medical University, 1 Youyi Road, Yuzhong District, Chongqing, 400016, People’s Republic of China, Tel +86-23-89012008, Fax +86-23-68811487, Email [email protected] Hua Hu, Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, 1 Youyi Road, Yuzhong District, Chongqing, 400016, People’s Republic of China, Tel +86-23-89012008, Fax +86-23-68811487, Email [email protected]
Purpose: To evaluate the prevalence of abnormal vitamin B12, folate, total homocysteine (tHcy), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), C-reactive protein (CRP) levels, to analyze the relationship between these parameters and the severity of anxiety or depressive symptoms, and to explore the possible factors associated with abnormal levels of these parameters in adolescents with anxiety or depressive symptoms.
Methods: Adolescent (aged 12– 18 years) outpatients with anxiety or depressive symptoms were recruited. The patient health questionnaire-9 and generalized anxiety disorder scale-7 were used to measure the severity of depression and anxiety. Serum vitamin B12, folate, tHcy, IL-6, TNF-α, and CRP levels were determined.
Results: 128 subjects were recruited. The prevalence of vitamin B12 and folate deficiency, tHcy, TNF-α, IL-6, and CRP elevation was 8.6%, 10.2%, 25.8%, 14.8%, 21.9%, and 10.2%, respectively, in adolescents with anxiety or depressive symptoms. Lower vitamin B12 levels were correlated with a higher risk of severe anxiety and depressive symptoms. The severity of some symptoms of anxiety or depression were weakly correlated with vitamin B12, folate, tHcy, IL-6, and CRP levels. Vitamin B12, folate, and tHcy levels were not associated with inflammatory mediators. Vitamin B12 deficiency was associated with older age and higher tHcy levels. Folate deficiency was associated with elevated tHcy. Elevated tHcy was associated with lower vitamin B12 and folate levels. IL-6 elevation was associated with elevated CRP and TNF-α. CRP elevation was associated with older age, higher BMI, and current drinking.
Conclusion: Lower vitamin B12 levels were correlated with a higher risk of severe anxiety or depressive symptoms. Weak correlations were observed between the severity of some symptoms of anxiety or depression and vitamin B12, folate, tHcy, IL-6, and CRP levels. Vitamin B12, folate, and tHcy levels were related to each other. IL-6 elevation was associated with elevated CRP and TNF-α. CRP elevation was associated with older age, higher BMI, and current drinking.
Keywords: vitamin B12, adolescents, inflammatory mediators, depression, anxiety
Introduction
Depressive and anxiety disorders are serious and common mental disorders in adolescence. In China, the prevalence of depressive symptoms was estimated as 21.9% in junior secondary school and 24.2% in senior secondary school.1 Meanwhile, 32.0% Chinese adolescents have anxiety symptoms.2 Depressive and anxiety disorders in adolescence may extend into adulthood.3 Depressive disorders in adolescence were associated with poor adult psychiatric and functional outcomes such as higher levels of adult anxiety, illicit drug problems, and worse health, criminal, and social function.4 Furthermore, comorbid anxiety and depression is common in children and adolescents, which leads to a more difficult diagnostic assessment and increased symptom severity.5 More severe anxiety and depression symptoms are related to potential consequences such as suicide, self-harm, refusal to eat, poorer treatment outcomes, and treatment resistance.6–8 Therefore, it is indispensable to explore modifiable risk factors to decrease the prevalence or severity of depressive and anxiety symptoms in adolescence.
Depressive and anxiety symptoms are related to numerous factors. Diet plays an important role in the occurrence and progress of depression and anxiety.9 Vitamin B12 is derived from animal products and folate is mainly from plants. They play a critical role in one-carbon metabolism and DNA, protein, and lipid methylation.10 The classic manifestations of vitamin B12 or folate deficiencies were hematological abnormalities such as megaloblastic anemia, which can be easily diagnosed by clinicians.11 However, clinicians frequently overlook that vitamin B12 or folate deficiencies may also lead to metabolic disturbance of monoamine, hormones, and neurotransmitters, which have been implicated in depression or anxiety.12–14 Vitamin B12 and folate are closely correlated with the metabolism of homocysteine. Methylcobalamin, an activated form of vitamin B12, is a cofactor for methionine synthase which catalyzes the conversion of homocysteine to methionine and 5-methyltetrahydrofolate, a folate derivative, is a substrate.15 Vitamin B12 or folate deficiencies may elevate circulating total homocysteine (tHcy) level which is a valuable parameter for reflecting Hcy level and the diagnosis of vitamin B12 or folate deficiency.16,17 Elevated tHcy may cause oxidative stress, mitochondrial dysfunction, and apoptosis of dopaminergic neurons, which may also induce depression or anxiety.18,19 Several observational studies showed that the severity of anxiety and depression symptoms was negatively correlated with vitamin B12 and folate levels, and was positively correlated with tHcy levels in children and adolescents.20,21
In addition, abnormal vitamin B12, folate, or tHcy levels can affect the immune system function.17–19 For example, hyperhomocysteinemia or deficiency of vitamin B12 or folate may regulate innate immunity via increasing dendritic cells activation,22,23 decreasing cytotoxicity of natural killer cells,24 modulating expression of pro-/anti-inflammatory cytokines.25–27 They may also regulate adaptive immunity by changing the percentage of CD4+CD25+ regulatory T cells and CD4+/CD8+ T lymphocytes,28,29 and affecting immunoglobulin production.30 Persistent chronic inflammation can dysregulate the hypothalamic-pituitary-adrenal (HPA) axis function, disturb neurotransmitter metabolism, impair neurons, and alter neural activity in the brain regions involved in emotion regulation, which results in anxiety and depression symptoms.31–33
The key mediators of innate and adaptive immunity, called inflammatory mediators, especially tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP), play a great role in the pathophysiology of depression and anxiety.32 Evidence supported that patients with depression or anxiety had higher TNF-α, IL-6, or CRP levels, which were positively correlated with the severity of anxiety and depression symptoms.34–37 Meanwhile, clinical researches showed that hyperhomocysteinemia or deficiency of vitamin B12 or folate was positively correlated with higher TNF-α, IL-6, or CRP levels in the general population or patients with diabetes, rheumatoid arthritis, and angiographic coronary artery disease.25–27,38–41
However, no studies investigated the association between vitamin B12, folate, tHcy, TNF-α, IL-6, and CRP levels and symptoms of anxiety and depression in adolescent patients. Here, we hypothesized that the severity of anxiety or depression symptoms was negatively associated with vitamin B12 and folate levels, and positively associated with TNF-α, IL-6, CRP, and tHcy levels in adolescents with anxiety or depressive symptoms. Meanwhile, TNF-α, IL-6, and CRP levels were positively associated with tHcy level, and negatively associated with vitamin B12 and folate levels. To test the hypothesis, we first determined the prevalence of abnormal vitamin B12, folate, tHcy, TNF-α, IL-6, and CRP levels in adolescents with anxiety or depression symptoms. Secondly, we assessed the relationship between vitamin B12, folate, tHcy, TNF-α, IL-6, CRP levels and the severity of anxiety or depression symptoms. Finally, we explored the possible factors (eg demographic or clinical features, hemogram parameters) associated with abnormal vitamin B12, folate, tHcy, TNF-α, IL-6, and CRP levels.
Materials and Methods
Subjects
We recruited adolescent (aged 12−18 years) outpatients who presented self-reported anxiety or depressive symptoms assessed with the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) from July 2021 to August 2022 in the Department of Neurology and Psychiatry, the First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Subjects were excluded if they had infectious diseases, CRP ≥10 mg/L, severe hepatic or renal impairment, tumor, severe aphasia, current pregnancy, psychiatric diagnoses of anorexia nervosa or schizophrenia and other psychotic disorders, a history of severe nervous system diseases (eg dementia, mental retardation, epilepsy), acute or chronic medical illness potentially affecting the HPA axis, hypothalamic-pituitary-thyroid (HPT) axis, systemic immune system (eg autoimmune diseases), or recent use of medications affecting the immune system (eg corticosteroids and nonsteroidal inflammatory drugs, antibiotics and antiviral medications). The study was approved by the Ethical Committee of Chongqing Medical University, in accordance with the principles expressed in the Declaration of Helsinki (approval No.2022-K491). All subjects (adolescents and their parents) provided written informed consent before all the procedures.
A priori power analysis was performed to determine the minimal sample size. Based on the prevalence of severe anxiety in the vitamin B12 <300 pg/mL group (55.0%, n = 20) and in the vitamin B12 ≥ 300 pg/mL group (26.7%, n = 30) in the first 50 samples in our preliminary survey, we needed to recruit at least 100 subjects to achieve a power of 0.80 and an alpha of 0.05 in the survey.
Assessment of Anxiety or Depressive Symptoms
An in-person psychological questionnaire was performed. The severity of depressive symptoms was measured with the Chinese version of the patient health questionnaire-9 (PHQ-9), which showed good reliability and validity in Chinese adolescents.42 It includes questions about each of the nine DSM-IV criteria for depression in the previous seven days. Each question was scored between 0 (not at all) and 3 (nearly every day). The total scores ranged from 0 to 27, and higher scores indicated greater depressive symptoms. The scores ≥ 20 represented severe depression (SD) and < 20 represented mild depression (MD).43 The Cronbach’s alpha coefficient is a credibility rating marker. It is considered acceptable when the Cronbach’s alpha coefficient was more than 0.7.44 In our study, the Cronbach’s alpha coefficient of the PHQ-9 is 0.835.
The severity of anxiety was measured with the Chinese version of the generalized anxiety disorder scale-7 (GAD-7), which showed good reliability and validity in Chinese adolescents.45 The scale includes 7 questions to reflect the frequency of anxiety symptoms during the past two weeks. Scores ranged from 0 (not at all) to 3 (nearly every day). The total scores ranged from 0 to 21, and higher scores indicated more severe anxiety. The scores ≥ 15 represented severe anxiety (SA) and < 15 represented mild anxiety (MA).46 In our study, the Cronbach’s alpha coefficient of GAD-7 is 0.868.
Data Collection
All patients underwent clinical history, neurologic and psychological examination, and complete blood work. Demographic or clinical features included age, gender, body mass index (BMI), course of the disease, parental marriage and education level, smoking, drinking, family history of psychiatric illness, the use of antidepressants or antipsychotics, PHQ-9 and GAD-7.
Blood samples were collected from the antecubital vein after an 8-hour fast (between 2 and 4 PM) and laboratory data were obtained using standard laboratory methods at the Central Laboratory and Endocrine Laboratory of our hospital. Serum vitamin B12 and folate levels were measured with an electro-chemiluminescence method (Unicel DxI 800 Immunoassay System, Beckman Coulter, USA). The tHcy was determined with enzymatic methods in an autoanalyzer (HITACHI 7180, Ichige, Japan). Red blood cells (RBC), hemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), hematocrit (HCT), white blood cells (WBC), and platelet count (PLT) were measured with an automatic blood cell counter hematology analyzer (Mindray BC-6600, China). IL-6 and TNF-α were measured with chemiluminescent immunoassay technology (Siemens IMMULITE 1000, Germany). CRP was assessed with immunonephelometry (Beckman Immage 800, USA).
Classification of Vitamin B12, Folate, tHcy, IL-6, TNF-α, CRP Levels
Based on the laboratory reference and previous literature, vitamin B12 levels were classified as deficiency (< 180 pg/mL), margin (180–300 pg/mL), and sufficiency (≥ 300 pg/mL).47 Folate levels were classified as deficiency (< 4.0 ng/mL) and sufficiency (≥ 4.0 ng/mL).48 The tHcy levels were classified as severe (> 100 μmol/L), moderate (30.1–100.0 μmol/L), mild (15.1–30.0 μmol/L) hyperhomocysteinemia (HHcy), and normal levels (5.0–15.0 μmol/L).49 Elevated TNF-α, IL-6, and CRP levels were defined as >8.1 pg/mL, >3.4 pg/mL, and > 3 mg/L, respectively.50
Statistical Analysis
The assumption for the normal distribution of variables was made with the Shapiro–Wilk test. Continuous variables with normal distribution were expressed as mean ± standard deviation (SD). Non-normal distribution of data was expressed as median (interquartile range). Categorical variables were expressed as numbers and percentages. Missing data were handled with full multiple imputation. It generated 20 data sets with 50 iterations to impute missing values of the outcome and covariates based on demographic and clinical features and laboratory data.51 The significance of the differences between groups was determined by independent sample t-test, Chi-square tests (Bonferroni post-hoc correction for pairwise comparison), one-way ANOVA (Welch’s ANOVA in case of unequal variances, SNK-q test for pairwise comparison), or Kruskal–Wallis H-tests (Bonferroni post-hoc correction for pairwise comparison) according to the type of variables and the number of groups.
The relationship between vitamin B12, folate, tHcy, inflammatory mediators, and the severity of anxiety or depressive symptoms was evaluated with Spearman correlation tests. The degree of correlation was considered as strong (Rho ≥ 0.7), moderate (0.3 < Rho < 0.7), or weak (Rho ≤ 0.3) after taking significant correlation (p < 0.05) values into consideration. Moreover, logistic regression analyses were performed to estimate the odds ratio (OR) of the severity of anxiety or depressive symptoms according to vitamin B12, folate, tHcy, and inflammatory mediators’ levels. The above independent variables were analyzed as continuous variables (per standard deviation increase) and categorical variables (deficiency or elevation). Model 1 was the unadjusted model. Model 2 was adjusted for age and gender. The results were expressed as OR with a 95% confidence interval (CI).
Multivariate logistic regression was used to identify factors associated with abnormal vitamin B12, folate, tHcy, and inflammatory mediators levels. Univariate logistic regression was used to screen out variables, and we selected those with overall p-values < 0.1 as candidate variables for the multivariable analysis. The variance inflation factor (VIF) is a marker for assessing multicollinearity. If VIF > 10, the multicollinearity is present and variable(s) were removed, and the multivariate logistic regression was conducted again. Statistical analysis was performed with SPSS 25.0 statistical package. P < 0.05 by two-tailed tests was considered significant.
Results
Demographic and Clinical Features and Laboratory Data of the Subjects
We recruited 139 adolescent outpatients with anxiety or depressive symptoms from July 2021 to August 2022 in our Neurology and Psychiatry departments. 11 patients were eventually excluded for CRP ≥ 10 mg/L (2 cases), epilepsy (3 cases), abnormal thyroid function (4 cases), and autoimmune diseases (2 cases). Finally, 128 patients (64.1% girls; mean age 15.4 ± 1.6 years) were eligible. Table S1 showed the percentage of subjects with missing data for the continuous and categorical variables, which ranged from 0% (almost all variables) to 3.12% (father’s education level and mother’s education level).
Table 1 displayed demographic and clinical features and laboratory data of subjects according to the severity of anxiety or depression for 128 subjects which included 71 with mild depression (MD) and mild anxiety (MA), 11 with severe depression (SD) and MA, 14 with MD and severe anxiety (SA), and 32 with SD and SA. Vitamin B12, folate, tHcy, IL-6, TNF-α, and CRP levels were not significantly different in the 4 groups.
 |
Table 1 Demographic and Clinical Features and Laboratory Data |
Vitamin B12 deficiency and marginal vitamin B12 status patients were 11 (8.6%) and 42 (32.8%), respectively. Folate deficiency was 12 (10.2%) subjects. Mild and moderate HHcy was 29 (22.7%) and 4 (3.1%) subjects, respectively. Elevated TNF-α, IL-6, and CRP were 19 (14.8%), 28 (21.9%), and 13 (10.2%) subjects, respectively. Figure 1 showed the details of vitamin B12, folate, tHcy, TNF-α, IL-6, and CRP levels based on age and gender.
 |
Figure 1 Vitamin B12, folate, tHcy, TNF-α, IL-6 and CRP levels based on age and gender. |
The Relationship Between Vitamin B12, Folate, tHcy, Inflammatory Mediators, and the Severity of Anxiety or Depressive Symptoms
Table 2 displayed the relationship between vitamin B12, folate, tHcy, TNF-α, IL-6, CRP levels and the risk of severe anxiety or depressive symptoms. After adjustment for age and gender, vitamin B12 < 300 pg/mL was associated with an increased risk of severe anxiety symptoms (OR = 2.39, 95% CI = 1.06, 5.35). Moreover, each per standard deviation increase (165 pg/mL) in vitamin B12 levels was associated with a lower risk of severe depressive symptoms (OR = 0.61, 95% CI = 0.38, 0.98). However, the risk of severe anxiety or depressive symptoms was not associated with folate, tHcy, TNF-α, IL-6, and CRP levels.
 |
Table 2 Binary Logistic Regression Analysis for Severe Depressive and Anxiety Symptoms |
Correlation Between Vitamin B12, Folate, tHcy Levels and the Severity of Each Symptom of Anxiety or Depression
Vitamin B12 levels were weakly negatively correlated with scores of GAD-7 (Rho = −0.184, p = 0.04), not being able to stop or control worrying (Rho = −0.187, p = 0.04), worrying too much about different things (Rho = −0.178, p = 0.04), trouble relaxing (Rho = −0.190, p = 0.03). A weak positive correlation was found between folate levels and scores of not being able to stop or control worrying (Rho = 0.190, p = 0.03). A weak negative correlation was observed between tHcy levels and scores of feeling tired or having little energy (Rho = −0.209, p = 0.02) (Table 3).
 |
Table 3 Spearman Correlation Analysis Between Vitamin B12, Folate, tHcy, IL-6, TNF-α, CRP Levels and Anxiety or Depressive Symptoms |
In addition, the tHcy levels were moderately negatively correlated with vitamin B12 levels (Rho = −0.346, p < 0.01) and folate levels (Rho = −0.611, p < 0.01). Vitamin B12 levels were weakly positively correlated with folate levels (Rho = 0.179, p = 0.04) (Figure 2).
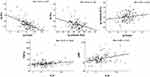 |
Figure 2 Graphics of significant correlations. |
Correlation Between Inflammatory Mediators and the Severity of Each Symptom of Anxiety or Depression
IL-6 showed a weak positive correlation with fidgeting (Rho = 0.190, p = 0.03) and annoyed or irritable scores (Rho = 0.190, p = 0.03). A weak negative correlation was observed between CRP levels and scores of slowly moving or speaking or restless (Rho = −0.189, p = 0.03). TNF-α levels were not associated with the severity of any symptoms (Table 3). In addition, IL-6 levels were weakly positively correlated with TNF-α levels (Rho = 0.175, p = 0.049) and CRP levels (Rho = 0.299, p < 0.01) (Figure 2).
Correlation Between Vitamin B12, Folate, tHcy and Inflammatory Mediators
Table 4 showed vitamin B12, folate, tHcy and inflammatory mediators levels according to demographic and clinical features and laboratory data. Girls, subjects with younger ages, lower tHcy levels had higher vitamin B12 levels. Vitamin B12 levels were higher in adolescents aged 12–14 years compared with adolescents aged 17–18 years after Bonferroni post-hoc correction (p = 0.03). Girls with lower Hb, MCV, HCT, tHcy levels and divorced parents had higher folate levels. Boys with higher RBC, Hb, MCHC, HCT levels and lower vitamin B12 and folate levels had higher tHcy levels. Subjects with higher RBC levels had higher TNF-α levels. Subjects with older ages, higher BMI, and higher CRP levels had higher IL-6 levels. IL-6 levels were higher in adolescents aged 17–18 years compared with adolescents aged 12–14 years after Bonferroni post-hoc correction (p = 0.03). Subjects with higher BMI and IL-6 levels had higher CRP levels. In addition, CRP levels were higher in adolescents aged 17–18 years compared with adolescents aged 12–14 years (p = 0.02), and adolescents aged 15–16 years (p < 0.01) after Bonferroni post-hoc correction.
 |
Table 4 Vitamin B12, Folate, tHcy, TNF-α, IL-6, and CRP Levels According to Demographic and Clinical Features and Laboratory Data |
Spearman correlation tests showed that there was no significant association between vitamin B12, folate, tHcy levels and IL-6, TNF-α, CRP levels in all subjects. Considering the different levels of these parameters in subjects of different ages and genders, we analyzed the relationship between them at different ages and gender.
Subgroup analysis showed that folate levels were moderately negatively correlated with CRP levels in subjects aged 13 years or less (Rho = −0.645, p < 0.01), TNF-α levels were weakly negatively correlated with folate (Rho = −0.178, p = 0.049) and weakly positively correlated with tHcy levels (Rho = 0.178, p = 0.049) in subjects aged 13 years or older, tHcy levels were weakly positively correlated with TNF-α levels in subjects aged 14 years or older (Rho = 0.197, p = 0.04), and vitamin B12, folate, tHcy levels were not significantly associated with inflammatory mediators when boys and girls were respectively analyzed.
Odds Ratio of Abnormal Vitamin B12, Folate, tHcy, TNF-α, IL-6 and CRP Levels
As shown in Table 5, multivariate binary logistic regression analyses demonstrated that vitamin B12 deficiency and vitamin B12 < 300 pg/mL were associated with older age (OR = 1.88, 95% CI = 1.04, 3.40), (OR = 1.35, 95% CI = 1.04, 1.75) and higher tHcy levels (OR = 1.08, 95% CI = 1.03, 1.15), (OR =1.13, 95% CI = 1.04, 1.23), respectively. Folate deficiency was associated with higher tHcy levels (OR = 1.11, 95% CI = 1.04, 1.19). HHcy was associated with lower vitamin B12 (OR = 1.009, 95% CI = 1.004, 1.014) and folate levels (OR = 2.68, 95% CI = 1.70, 4.23).
 |
Table 5 Multivariate Binary Logistic Regression for Odds of Abnormal Vitamin B12, Folate, Hcy, IL-6, TNF-α and CRP Levels |
IL-6 elevation was associated with higher CRP (OR = 1.65, 95% CI = 1.17, 2.32) and TNF-α levels (OR = 1.19, 95% CI = 1.00, 1.43). CRP elevation was associated with older age (OR = 3.06, 95% CI = 1.41, 6.65), higher BMI (OR = 1.42, 95% CI = 1.16, 1.76) and current drinking (OR = 11.71, 95% CI = 1.61, 85.12).
Discussion
The present study showed that the prevalence of vitamin B12 and folate deficiency, tHcy, TNF-α, IL-6, and CRP elevation was 8.6%, 10.2%, 25.8%, 14.8%, 21.9%, and 10.2%, respectively, in adolescents with anxiety or depressive symptoms. Lower vitamin B12 levels were correlated with a higher risk for severe anxiety and depressive symptoms. The severity of some symptoms of anxiety or depression was weakly correlated with vitamin B12, folate, tHcy, IL-6, and CRP levels, so these results were unlikely to be clinically meaningful. Although vitamin B12, folate, and tHcy levels were not significantly associated with inflammatory mediators, significant correlations were found between folate and CRP, between folate and TNF-α, between tHcy and TNF-α in age-subgroup analyses. Vitamin B12 deficiency was associated with older age and higher tHcy levels. Folate deficiency was associated with higher tHcy levels. HHcy was associated with lower vitamin B12 and folate levels. IL-6 elevation was associated with higher CRP and TNF-α levels. CRP elevation was associated with older age, higher BMI, and current drinking.
In 2006, Gao reported the estimated prevalence of vitamin B12 deficiency (serum vitamin B12 < 200 pg/mL) was 4.5% in Chinese children aged 2 to 7 years.47 In 2014, a meta-analysis showed that the prevalence of HHcy was 27.5% in the Chinese population aged 3–97 years.52 Here, we first reported that the prevalence of vitamin B12 and folate deficiency, tHcy, TNF-α, IL-6, and CRP elevation was 8.6%, 10.2%, 25.8%, 14.8%, 21.9%, and 10.2%, respectively, in the Chinese adolescents with anxiety or depressive symptoms, which was an extension of previous research.
In individuals with depression or anxiety, lower levels of vitamin B12, folate, and higher levels of tHcy were often reported compared with healthy controls.21,53 An individually matched case-control study showed that unhealthy eating habits were associated with a higher risk of depression via lower serum levels of folate and vitamin B12.54 Our results further supported the negative association between vitamin B12 and the severity of anxiety or depression symptoms. However, no significant association was observed between folate, tHcy and overall severity of anxiety or depression symptoms.
Some studies attempted to explore the possible molecular mechanism on how vitamin B12, folate, and Hcy metabolism are coupled to depressive or anxiety symptoms. A recent study showed that vitamin B12 supplementation reduced the DNA methylation of the Ntrk-2 3′UTR, which increased the expression of Ntrk-2 in vitro. In addition, acute vitamin B12 supplementation reversed the reduction of Ntrk-2 caused by accumulating stressors in the mouse prefrontal cortex.55 Ntrk-2 is the gene for tropomyosin/tyrosine receptor kinase B, the receptor for brain-derived neurotropic factor, which plays an important role in the pathophysiology of depression and the antidepressant response.56 Budni showed that folate exerted antidepressant effects with the involvement of N-methyl-D-aspartate receptors and the L-arginine-NO pathway.57 Duan suggested that HHcy damages dopaminergic neurons directly via exacerbating methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopamine depletion or indirectly via exacerbating oxidative stress, resulting in the increased risk of depression.18 We found that vitamin B12, folate, and tHcy were weakly associated with some symptoms of anxiety or depression symptoms, not all the symptoms. This reminds us that the impact of abnormal vitamin B12, folate, and tHcy levels localized to specific brain regions. Future studies could focus on investigating why vitamin B12, folate, and tHcy were associated with specific anxiety or depression symptoms.
Some studies showed correlations between vitamin B12, folate, tHcy levels and IL-6, TNF-α, CRP levels in general population or patients.25,58,59 We also presented correlations between folate and CRP, between folate and TNF-α, between tHcy and TNF-α in age-subgroup analyses. The animal experiment showed that TNF-α levels were higher in the spinal cord of rats 2 months after total gastrectomy (vitamin B12 deficiency) and the TNF-α levels decreased to control levels after treatment with vitamin B12 in totally gastrectomized rats.60 IL-6 levels decreased in the cerebrospinal fluid of totally gastrectomized rats and increased after treatment with vitamin B12.61 The clinical trials suggested that vitamin B12 was negatively correlated with TNF-α among adolescent girls.58 CRP elevation was found to be related to higher levels of tHcy in multiple diseases such as type 1 diabetes, rheumatoid arthritis, and angiographic coronary artery disease.39–41 However, the exact molecular mechanism between vitamin B12, folate, Hcy and IL-6, TNF-α, CRP remains to be explored.
TNF-α, produced predominantly by macrophages and natural killer cells, may induce anxiety and depressive symptoms by stimulating the HPA axis, endothelial activation, neutrophil activation, and inducing apoptotic cell death.32,62–64 IL-6, produced predominantly by macrophages and T cells, may induce anxiety and depressive symptoms by causing HPA axis dysfunction, altering synaptic neurotransmission, and reducing neurotrophic factors.65–67 CRP, a nonspecific acute-phase protein, whose production was stimulated by IL-6, was the most widely used to measure low-grade inflammation in psychiatric conditions, especially depression.68,69 It was accepted that inflammatory mediators levels were higher in patients with anxiety or depression compared with controls in cross-sectional studies,70,71 and subjects with higher inflammatory mediator levels had a higher risk of anxiety or depression in longitudinal studies.66,72,73 What’s more, alterations in inflammatory mediator levels are associated with antidepressant treatment outcomes and prognosis in interventional studies.74,75 In summary, inflammatory mediators may be potential markers for the diagnosis, treatment, and prognosis of anxiety and depression.
The relationship is complex between vitamin B12, folate, tHcy, IL-6, TNF-α, CRP, and anxiety or depressive symptoms, and our research failed to make a causal inference. Firstly, poor appetite or overeating, one of the common depression symptoms, may result in abnormal IL-6, TNF-α, CRP, vitamin B12, folate, and tHcy levels.76–78 Secondly, abnormal vitamin B12, folate, and tHcy levels may affect IL-6, TNF-α, and CRP levels and result in anxiety or depression symptoms. Lastly, abnormal IL-6, TNF-α, and CRP levels may lead to the occurrence of anxiety or depression symptoms. Therefore, more high-quality prospective cohort studies and network analyses are needed to explore their relationship in the future.
Our study had some limitations. Firstly, the results cannot be extrapolated to the general population of adolescents with anxiety or depressive symptoms for a small sample size. Secondly, vitamin B12, folate, tHcy, IL-6, TNF-α, and CRP levels were not assessed in a healthy control group. Thirdly, causal inference cannot be performed because we only conducted a cross-sectional study without the conditions of temporal precedence and covariation. Lastly, we cannot access the effect and adverse events of vitamin B12 or folate supplements in adolescents with anxiety or depressive symptoms because we did not collect follow-up data.
Conclusion
Above all, the conclusions of the present study are as follows. Firstly, the prevalence of vitamin B12 and folate deficiency, tHcy, TNF-α, IL-6, and CRP elevation was 8.6%, 10.2%, 25.8%, 14.8%, 21.9%, and 10.2%, respectively, in adolescents with anxiety or depressive symptoms. Secondly, lower vitamin B12 levels were correlated with a higher risk of severe anxiety and depressive symptoms. The severity of some symptoms of anxiety and depression were weakly correlated with vitamin B12, folate, tHcy, IL-6, and CRP levels. Vitamin B12, folate, and tHcy levels were not associated with inflammatory mediators. Lastly, Vitamin B12, folate, and tHcy levels were related to each other. IL-6 elevation was associated with higher elevated CRP and TNF-α. CRP elevation was associated with older age, higher BMI, and current drinking.
Data Sharing Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant no. 82171456 and 81971229) and the Natural Science Foundation of Chongqing Science and Technology Commission (Grant no. cstc 2021jcyj-msxmX0263).
Disclosure
The authors declared that they have no competing interests.
References
1. Rao WW, Xu DD, Cao XL, et al. Prevalence of depressive symptoms in children and adolescents in China: a meta-analysis of observational studies. Psychiatry Res. 2019;272:790–796. doi:10.1016/j.psychres.2018.12.133
2. Luo X, Zhou Y, Zheng R, et al. Association of health-risk behaviors and depressive symptoms and anxiety symptoms: a school-based sample of Chinese adolescents. J Public Health. 2020;42(3):e189–e198. doi:10.1093/pubmed/fdz115
3. Ranøyen I, Lydersen S, Larose TL, et al. Developmental course of anxiety and depression from adolescence to young adulthood in a prospective Norwegian clinical cohort. Eur Child Adolesc Psychiatry. 2018;27(11):1413–1423. doi:10.1007/s00787-018-1139-7
4. Copeland WE, Alaie I, Jonsson U, Shanahan L. Associations of childhood and adolescent depression with adult psychiatric and functional outcomes. J Am Acad Child Adolesc Psychiatry. 2021;60(5):604–611. doi:10.1016/j.jaac.2020.07.895
5. Melton TH, Croarkin PE, Strawn JR, McClintock SM. Comorbid anxiety and depressive symptoms in children and adolescents: a systematic review and analysis. J Psychiatr Pract. 2016;22(2):84–98. doi:10.1097/PRA.0000000000000132
6. Kessing LV. Severity of depressive episodes according to ICD-10: prediction of risk of relapse and suicide. Br J Psychiatry. 2004;184:153–156. doi:10.1192/bjp.184.2.153
7. Sonawalla SB, Fava M. Severe depression: is there a best approach? CNS Drugs. 2001;15(10):765–776. doi:10.2165/00023210-200115100-00003
8. Compton SN, Peris TS, Almirall D, et al. Predictors and moderators of treatment response in childhood anxiety disorders: results from the CAMS trial. J Consult Clin Psychol. 2014;82(2):212–224. doi:10.1037/a0035458
9. Kris-Etherton PM, Petersen KS, Hibbeln JR, et al. Nutrition and behavioral health disorders: depression and anxiety. Nutr Rev. 2021;79(3):247–260. doi:10.1093/nutrit/nuaa025
10. Frankenburg FR. The role of one-carbon metabolism in schizophrenia and depression. Harv Rev Psychiatry. 2007;15(4):146–160. doi:10.1080/10673220701551136
11. Yadav MK, Manoli NM, Vimalraj S, Madhunapantula SV. Unmethylated promoter DNA correlates with p53 expression and apoptotic levels only in Vitamin B9 and B12 deficient megaloblastic anemia but not in non-megaloblastic anemia controls. Int J Biol Macromol. 2018;109:76–84. doi:10.1016/j.ijbiomac.2017.12.070
12. Baek JH, Bernstein EE, Nierenberg AA. One-carbon metabolism and bipolar disorder. Aust N Z J Psychiatry. 2013;47(11):1013–1018. doi:10.1177/0004867413502091
13. Borçoi AR, Mendes SO, Gasparini Dos Santos J, et al. Risk factors for depression in adults: NR3C1 DNA methylation and lifestyle association. J Psychiatr Res. 2020;121:24–30. doi:10.1016/j.jpsychires.2019.10.011
14. Bottiglieri T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev. 1996;54(12):382–390. doi:10.1111/j.1753-4887.1996.tb03851.x
15. Molloy AM. Folate and homocysteine interrelationships including genetics of the relevant enzymes. Curr Opin Lipidol. 2004;15(1):49–57. doi:10.1097/00041433-200402000-00010
16. Ueland PM, Refsum H, Stabler SP, Malinow MR, Andersson A, Allen RH. Total homocysteine in plasma or serum: methods and clinical applications. Clin Chem. 1993;39(9):1764–1779. doi:10.1093/clinchem/39.9.1764
17. Amores-Sánchez MI, Medina MA. Methods for the determination of plasma total homocysteine: a review. Clin Chem Lab Med. 2000;38(3):199–204. doi:10.1515/CCLM.2000.028
18. Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J Neurochem. 2002;80(1):101–110. doi:10.1046/j.0022-3042.2001.00676.x
19. Zarrindast MR, Khakpai F. The modulatory role of dopamine in anxiety-like behavior. Arch Iran Med. 2015;18(9):591–603.
20. Chung KH, Chiou HY, Chen YH. Associations between serum homocysteine levels and anxiety and depression among children and adolescents in Taiwan. Sci Rep. 2017;7(1):8330. doi:10.1038/s41598-017-08568-9
21. Esnafoglu E, Ozturan DD. The relationship of severity of depression with homocysteine, folate, vitamin B12, and vitamin D levels in children and adolescents. Child Adolesc Ment Health. 2020;25(4):249–255. doi:10.1111/camh.12387
22. Rutault K, Alderman C, Chain BM, Katz DR. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic Biol Med. 1999;26(1–2):232–238. doi:10.1016/S0891-5849(98)00194-4
23. Kaplan P, Tatarkova Z, Sivonova MK, Racay P, Lehotsky J. Homocysteine and mitochondria in cardiovascular and cerebrovascular systems. Int J Mol Sci. 2020;21:20. doi:10.3390/ijms21207698
24. Partearroyo T, Úbeda N, Montero A, Achón M, Varela-Moreiras G. Vitamin B(12) and folic acid imbalance modifies NK cytotoxicity, lymphocytes B and lymphoprolipheration in aged rats. Nutrients. 2013;5(12):4836–4848. doi:10.3390/nu5124836
25. Mikkelsen K, Stojanovska L, Prakash M, Apostolopoulos V. The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas. 2017;96:58–71. doi:10.1016/j.maturitas.2016.11.012
26. Al-Daghri NM, Rahman S, Sabico S, et al. Association of vitamin B12 with pro-inflammatory cytokines and biochemical markers related to cardiometabolic risk in Saudi subjects. Nutrients. 2016;8(9):460. doi:10.3390/nu8090460
27. Lee YJ, Wang MY, Lin MC, Lin PT. Associations between vitamin B-12 status and oxidative stress and inflammation in diabetic vegetarians and omnivores. Nutrients. 2016;8(3):118. doi:10.3390/nu8030118
28. Boran P, Yildirim S, Karakoc-Aydiner E, et al. Vitamin B12 deficiency among asymptomatic healthy infants: its impact on the immune system. Minerva Pediatr. 2021;73(1):59–66. doi:10.23736/S2724-5276.16.04274-X
29. Funada U, Wada M, Kawata T, et al. Changes in CD4+CD8-/CD4-CD8+ ratio and humoral immune functions in vitamin B12-deficient rats. Int J Vitam Nutr Res. 2000;70(4):167–171. doi:10.1024/0300-9831.70.4.167
30. Funada U, Wada M, Kawata T, et al. Vitamin B-12-deficiency affects immunoglobulin production and cytokine levels in mice. Int J Vitam Nutr Res. 2001;71(1):60–65. doi:10.1024/0300-9831.71.1.60
31. Zainal NH, Newman MG. Prospective network analysis of proinflammatory proteins, lipid markers, and depression components in midlife community women. Psychol Med. 2022:1–12. doi:10.1017/S003329172200232X
32. Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140(3):774–815. doi:10.1037/a0035302
33. Hu P, Lu Y, Pan BX, Zhang WH. New insights into the pivotal role of the amygdala in inflammation-related depression and anxiety disorder. Int J Mol Sci. 2022;23(19):11076.
34. Rengasamy M, Marsland A, McClain L, et al. Longitudinal relationships of cytokines, depression and anhedonia in depressed adolescents. Brain Behav Immun. 2021;91:74–80. doi:10.1016/j.bbi.2020.09.004
35. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi:10.1097/PSY.0b013e3181907c1b
36. Liu M, Li Y, Liu X. Serum tumor necrosis factor-α, interleukin-1β, interleukin-6, and interleukin-17 relate to anxiety and depression risks to some extent in non-small cell lung cancer survivor. Clin Respir J. 2022;16(2):105–115. doi:10.1111/crj.13457
37. Lu H, Yang Q, Zhang Y. The relation of common inflammatory cytokines with anxiety and depression and their values in estimating cardiovascular outcomes in coronary heart disease patients. J Clin Lab Anal. 2022;36(6):e24404. doi:10.1002/jcla.24404
38. Abbenhardt C, Miller JW, Song X, et al. Biomarkers of one-carbon metabolism are associated with biomarkers of inflammation in women. J Nutr. 2014;144(5):714–721. doi:10.3945/jn.113.183970
39. Dinleyici EC, Kirel B, Alatas O, Muslumanoglu H, Kilic Z, Dogruel N. Plasma total homocysteine levels in children with type 1 diabetes: relationship with vitamin status, methylene tetrahydrofolate reductase genotype, disease parameters and coronary risk factors. J Trop Pediatr. 2006;52(4):260–266. doi:10.1093/tropej/fmk001
40. Yang X, Gao F, Liu Y. Association of homocysteine with immunological-inflammatory and metabolic laboratory markers and factors in relation to hyperhomocysteinaemia in rheumatoid arthritis. Clin Exp Rheumatol. 2015;33(6):900–903.
41. Schroecksnadel K, Grammer TB, Boehm BO, März W, Fuchs D. Total homocysteine in patients with angiographic coronary artery disease correlates with inflammation markers. Thromb Haemost. 2010;103(5):926–935. doi:10.1160/TH09-07-0422
42. Xing-Chen HU, Zhang YL, Liang W, Zhang HM, Yang SC. Reliability and validity of the patient health questionnaire-9 in Chinese adolescents. Sichuan Ment Health. 2014;27(4):357–360.
43. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
44. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi:10.1016/j.jclinepi.2006.03.012
45. Liu Y, Zhang WB, Cai J. Relation of anxiety and depression to lifestyle in junior high school students. Chin Ment Health J. 2017;12:235–240.
46. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi:10.1001/archinte.166.10.1092
47. Gao MZ, Li HQ. 高美哲, 黎海芪. 重庆地区学龄前儿童血清维生素B12营养状况调查 . 中华儿科杂志. [Vitamin B12 nutritional status in preschool children in Chongqing]. Zhonghua er ke za zhi. 2006;44(1):7–10. Chinese.
48. de Benoist B. Conclusions of a WHO technical consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. 2008;29(2 Suppl):S238–S244. doi:10.1177/15648265080292S129
49. Guieu R, Ruf J, Mottola G. Hyperhomocysteinemia and cardiovascular diseases. Ann Biol Clin. 2022;80(1):7–14. doi:10.1684/abc.2021.1694
50. Felger JC, Haroon E, Patel TA, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 2020;25(6):1301–1311. doi:10.1038/s41380-018-0096-3
51. Ji L, Chow SM, Schermerhorn AC, Jacobson NC, Cummings EM. Handling missing data in the modeling of intensive longitudinal data. Struct Equ Modeling. 2018;25(5):715–736. doi:10.1080/10705511.2017.1417046
52. Yang B, Fan S, Zhi X, et al. Prevalence of hyperhomocysteinemia in China: a systematic review and meta-analysis. Nutrients. 2014;7(1):74–90. doi:10.3390/nu7010074
53. Saraswathy KN, Ansari SN, Kaur G, Joshi PC, Chandel S. Association of vitamin B12 mediated hyperhomocysteinemia with depression and anxiety disorder: a cross-sectional study among Bhil indigenous population of India. Clin Nutr ESPEN. 2019;30:199–203. doi:10.1016/j.clnesp.2019.01.009
54. Khosravi M, Sotoudeh G, Amini M, Raisi F, Mansoori A, Hosseinzadeh M. The relationship between dietary patterns and depression mediated by serum levels of Folate and vitamin B12. BMC Psychiatry. 2020;20(1):63. doi:10.1186/s12888-020-2455-2
55. Trautmann C, Bock A, Urbach A, Hübner CA, Engmann O. Acute vitamin B12 supplementation evokes antidepressant response and alters Ntrk-2. Neuropharmacology. 2020;171:108112. doi:10.1016/j.neuropharm.2020.108112
56. Zhang JC, Yao W, Hashimoto K. Brain-derived Neurotrophic Factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol. 2016;14(7):721–731. doi:10.2174/1570159X14666160119094646
57. Budni J, Moretti M, Freitas AE, et al. Behavioral and neurochemical effects of folic acid in a mouse model of depression induced by TNF-α. Behav Brain Res. 2021;414:113512. doi:10.1016/j.bbr.2021.113512
58. Ghatpande NS, Apte PP, Naik SS, Joshi BN, Gokhale MK, Kulkarni PP. Association of B12 deficiency and anemia synergistically increases the risk of high TNF-α levels among adolescent girls. Metallomics. 2016;8(8):734–738. doi:10.1039/C6MT00129G
59. Steluti J, Miranda AM, De Carli E, Palchetti CZ, Fisberg RM, Marchioni DML. Unmetabolized folic acid is associated with TNF-α, IL-1β and IL-12 concentrations in a population exposed to mandatory food fortification with folic acid: a cross-sectional population-based study in Sao Paulo, Brazil. Eur J Nutr. 2021;60(2):1071–1079. doi:10.1007/s00394-020-02307-z
60. Buccellato FR, Miloso M, Braga M, et al. Myelinolytic lesions in spinal cord of cobalamin-deficient rats are TNF-alpha-mediated. FASEB J. 1999;13(2):297–304. doi:10.1096/fasebj.13.2.297
61. Scalabrino G, Corsi MM, Veber D, et al. Cobalamin (vitamin B(12)) positively regulates interleukin-6 levels in rat cerebrospinal fluid. J Neuroimmunol. 2002;127(1–2):37–43. doi:10.1016/S0165-5728(02)00095-4
62. Irie M, Asami S, Ikeda M, Kasai H. Depressive state relates to female oxidative DNA damage via neutrophil activation. Biochem Biophys Res Commun. 2003;311(4):1014–1018. doi:10.1016/j.bbrc.2003.10.105
63. Yin W, Swanson SP, Biltz RG, et al. Unique brain endothelial profiles activated by social stress promote cell adhesion, prostaglandin E2 signaling, hypothalamic-pituitary-adrenal axis modulation, and anxiety. Neuropsychopharmacology. 2022;47(13):2271–2282. doi:10.1038/s41386-022-01434-x
64. Bouzinova EV, Norregaard R, Boedtkjer DM, et al. Association between endothelial dysfunction and depression-like symptoms in chronic mild stress model of depression. Psychosom Med. 2014;76(4):268–276. doi:10.1097/PSY.0000000000000062
65. Ting EY, Yang AC, Tsai SJ. Role of Interleukin-6 in depressive disorder. Int J Mol Sci. 2020;21(6):2194. doi:10.3390/ijms21062194
66. de Baumont A, Bortoluzzi A, Wollenhaupt de Aguiar B, et al. Anxiety disorders in childhood are associated with youth IL-6 levels: a mediation study including metabolic stress and childhood traumatic events. J Psychiatr Res. 2019;115:43–50. doi:10.1016/j.jpsychires.2019.05.011
67. Bannerman DM, Sprengel R, Sanderson DJ, et al. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 2014;15(3):181–192. doi:10.1038/nrn3677
68. Ngwa DN, Pathak A, Agrawal A. IL-6 regulates induction of C-reactive protein gene expression by activating STAT3 isoforms. Mol Immunol. 2022;146:50–56. doi:10.1016/j.molimm.2022.04.003
69. Fernandes BS, Steiner J, Bernstein HG, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry. 2016;21(4):554–564. doi:10.1038/mp.2015.87
70. Ng A, Tam WW, Zhang MW, et al. IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep. 2018;8(1):12050. doi:10.1038/s41598-018-30487-6
71. Renna ME, O’Toole MS, Spaeth PE, Lekander M, Mennin DS. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: a systematic review and meta-analysis. Depress Anxiety. 2018;35(11):1081–1094. doi:10.1002/da.22790
72. Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150(3):736–744. doi:10.1016/j.jad.2013.06.004
73. Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB. The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry. 2021;26(7):3302–3314. doi:10.1038/s41380-020-00867-4
74. Liu JJ, Wei YB, Strawbridge R, et al. Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Mol Psychiatry. 2020;25(2):339–350. doi:10.1038/s41380-019-0474-5
75. Kofod J, Elfving B, Nielsen EH, Mors O, Köhler-Forsberg O. Depression and inflammation: correlation between changes in inflammatory markers with antidepressant response and long-term prognosis. Eur Neuropsychopharmacol. 2022;54:116–125. doi:10.1016/j.euroneuro.2021.09.006
76. Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48(4):677–685. doi:10.1016/j.jacc.2006.03.052
77. Halicioglu O, Sutcuoglu S, Koc F, et al. Vitamin B12 and folate statuses are associated with diet in pregnant women, but not with anthropometric measurements in term newborns. J Matern Fetal Neonatal Med. 2012;25(9):1618–1621. doi:10.3109/14767058.2011.648244
78. Dutheil S, Ota KT, Wohleb ES, Rasmussen K, Duman RS. High-fat diet induced anxiety and anhedonia: impact on brain homeostasis and inflammation. Neuropsychopharmacology. 2016;41(7):1874–1887. doi:10.1038/npp.2015.357
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
