Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Vitamin B12 Deficiency and Foot Ulcers in Type 2 Diabetes Mellitus: A Case–Control Study
Authors Badedi M , Darraj H , Hummadi A, Solan Y, Zakri I, Khawaji A, Daghreeri M, Budaydi A
Received 7 October 2019
Accepted for publication 27 November 2019
Published 6 December 2019 Volume 2019:12 Pages 2589—2596
DOI https://doi.org/10.2147/DMSO.S233683
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Jing Sun
Mohammed Badedi,1 Hussain Darraj,1 Abdulrahman Hummadi,1 Yahia Solan,1 Ibrahim Zakri,1 Abdullah Khawaji,1 Mohammed Daghreeri,1 Ahmed Budaydi2
1Jazan Diabetes & Endocrine Center, Ministry of Health, Jazan, Saudi Arabia; 2Abu Arish General Hospital, Ministry of Health, Hanoi, Saudi Arabia
Correspondence: Mohammed Badedi
Tel +966 559154136
Email [email protected]
Objective: To assess the association between vitamin B12 deficiency and the development of diabetic foot ulcers (DFU) in type 2 diabetes mellitus (T2DM).
Methods: This is a case–control study that enrolled 323 Saudi adults with T2DM randomly selected from the Jazan Diabetes & Endocrine Center, Saudi Arabia from January 1, 2019, to July 31, 2019. The sample included 108 newly diagnosed cases with DFU and 215 control participants with T2DM unaffected by and free of foot ulcers (1:2 ratio). Logistic regression analysis was performed to determine the DFU predictors and to examine the association of DFU and vitamin B12 deficiency.
Results: The highest DFU rates were found among the male participants and the participants older than 45 years. Neuropathy, vasculopathy, vitamin B12 deficiency, poor glycemic control, poor feet self-care, Charcot foot, physical inactivity, and spending long time standing at work were significantly associated with DFU, and all except physical inactivity and spending long time standing at work were independent predictors of DFU. After adjustment for the covariates, vitamin B12 deficiency was significantly associated with DFU (odds ratio 3.1), indicating that the patients with T2DM and vitamin B12 deficiency had a three times higher risk of developing DFU than those with normal vitamin B12 levels.
Conclusion: Vitamin B12 deficiency had a significant association with DFU among the Saudi participants with T2DM. Establishing the causality and clarifying the biological role of vitamin B12 deficiency in DFU is important aims for future studies.
Keywords: vitamin B12 deficiency, foot ulcer, type 2 diabetes mellitus, Jazan, Saudi Arabia
Introduction
Diabetic foot ulcers (DFU) are a serious complication of diabetes mellitus characterized by full-thickness wounds accompanied by skin necrosis.1 DFU affect more than 18% of the people with type 2 diabetes mellitus (T2DM) in Jazan, Saudi Arabia.2 DFU constitute a public health concern because they increase health-care costs and can lead to lower limb amputations,3,4 resulting physical limitations and disabilities that can affect work productivity and quality of life. The development of DFU is associated with a variety of potential risk factors, which must be determined to prevent its occurrence. Most previous studies in the literature exploring those factors have been conducted in Western countries, so further research is needed to explore the risk factors associated with DFU in different communities. This need is especially acute in large countries, such as Saudi Arabia whose regions have different behaviors, cultures, and lifestyles.
Vitamin B12 plays an important function in the development of the central and peripheral nervous systems.5 Deficiency of this vitamin is prevalent among patients with T2DM (30%) and can be assessed by measuring serum vitamin B12 levels.6 The main cause of this deficiency is the use of metformin as the first-line pharmacological treatment for T2DM in clinical practice.7 Accumulating evidence8–12 suggests that long-term use of metformin lowers serum vitamin B12 levels. Furthermore, bariatric surgery, which is commonly used with obese patients with T2DM, is also a risk factor for malabsorption of vitamin B12.13 As this vitamin is essential for proper functioning of the peripheral nervous system, its deficiency can be associated with peripheral neuropathy, a prominent risk factor for DFU development in patients with T2DM.14 To the authors’ knowledge, no single study in the literature has explored the possible association between vitamin B12 deficiency and DFU development. This study, therefore, investigated whether vitamin B12 deficiency is associated with DFU in patients with T2DM in Jazan, Saudi Arabia, taking into consideration other associated risk factors of DFU.
Methodology
Study Design and Population
This case–control study was conducted with Saudi adults in Jazan City who were at least 18 years old and had T2DM. In southwestern Saudi Arabia, Jazan is a small city with a relatively homogenous population with similar ethnic and socioeconomic characteristics. Every Saudi patient with T2DM and DFU in Jazan City is registered at the Jazan Diabetes & Endocrine Center, the main center delivering care services for those conditions in the city.
Sampling Technique
All patients who had T2DM and were newly diagnosed at the onset of DFU over January 1, 2019, to July 31, 2019 (n=278) were identified through the registry department of the Jazan Diabetes & Endocrine Center. The control participants were identified as all patients with T2DM who registered at the Jazan Diabetes & Endocrine Center and were unaffected by and free of foot ulcer at the diagnosis date of the affected cases (n=6722). All the cases and control samples were included in two sampling frames as the case and control lists. Simple random selection of the participants was then performed. Patients receiving any form of vitamin B12 supplements were excluded (Figure 1).
Sample Size
The sample size was estimated using Epi Info software15 with a confidence interval (CI) of 95%, power of 80%, and case–control ratio of 1:2. A hypothetical risk assumption of 40% among the controls and an odds ratio of 2 were used in the calculation. This procedure yielded a total sample size of 323, with 108 cases and 215 control participants. Each case with DFU was compared to two controls without DFU.
 |
Figure 1 Participant enrollment and the sampling technique in the current study. Abbreviations: T2DM, type 2 diabetes mellitus; DFU, diabetic foot ulcer. |
Definition of Diabetic Foot Ulcers
A foot ulcer in a patient with T2DM was defined as a new, non-healing or poorly healing, partial- or full-skin thickness wound below the ankle.1
Definition of Vitamin B12 Deficiency
The majority of the published studies in the literature used the value <148 pmol/L as a cut-off point for vitamin B12 deficiency.16–21 In the current study, therefore, vitamin B12 deficiency was defined as serum levels of vitamin B12 <148 pmol/L. Vitamin B12 levels are measured routinely in the Jazan Diabetes & Endocrine Center for patients with T2DM who are on metformin medication and those who have neurological manifestations.
Covariates and Data Collection
Face-to-face interviews were held to collect data from the participants. The interviews used a structured questionnaire designed by an interdisciplinary team at the Jazan Diabetes Center consisting of an endocrinologist, a podiatrist, and family and community medicine consultants. The questionnaire covered serum vitamin B12 levels and other risk factors (the confounding variables) shown in the literature to be associated with DFU development or other possible factors noted by the author in clinical practice. These factors were age, sex, occupation, work activity levels, diabetes duration, foot trauma, neuropathy, vasculopathy, foot self-care (including daily foot care and appropriate footwear), physical activity, body mass index (BMI), glycated hemoglobin (A1C), vitamin B12 levels, dry foot skin, Charcot joints, and foot fissures.
Following the World Health Organization,22 BMI was calculated as a person’s weight in kilograms divided by height in meters. BMI was categorized as underweight for values <18.50, normal for 18.50–24.99, overweight for 25–29.99, and obese for ≥30. Physical activity of less than 150 mins per week was categorized as physically inactive, following the American Diabetes Association guidelines.7 A1C ≥7% was categorized as poor glycemic control, and A1C <7% as good glycemic control.7 When the patients exhibited loss of protective sensation, neuropathy was considered through clinical examination using 10 g monofilament assessment, vibration, and temperature sensation.23 Peripheral arterial disease was defined based on patient history of intermittent claudication and absent or diminished feet pulses (posterior tibial artery and dorsalis pedis artery) with assessment of ankle-brachial index measurements using a Doppler device. Values of 0.70–0.90 were considered to be mild obstruction, and values <40 were severe obstruction.24 Charcot joint was considered when the bones, joints, and soft tissues of the foot and ankle were inflamed in the presence of neuropathy and characterized by bone destruction, subluxation, and dislocation.25
Ethical Approval
The Jazan Hospital Institutional Review Board (reference number: H-10-Z-068)26 granted ethical approval (No. 1905) for the study, which also complied with the Helsinki Declaration. Written informed consent was obtained from all the participants before enrolment.
Statistical Analysis
Data entry and analysis were performed by the principal investigator using Statistical Package for the Social Sciences software version 24.27 The data were coded with anonymous identification numbers in order to guarantee the privacy of the participants. The continuous variables were described by means and standard deviation (SD) and the categorical variables by percentages and frequencies. A chi-square test for independence was used to assess the associations among the categorical variables. Binary logistic regression was used to explore the predictors of DFU including the significant variables in univariate regression. The assumptions of the binary logistic regression were checked for the presence of outliers and multicollinearity (high inter-correlations among independent variables). P values <0.05 were considered to be statistically significant.
Results
The study included 323 Saudi participants with T2DM, including 108 cases with DFU and 215 controls without DFU.
Diabetic Foot Ulcers Risk Factors
DFU was most common in the participants aged 45–64 years (Table 1). The mean age of the cases with DFU was 56.9 years (SD ±12.2), with a range of 31–81 years. The mean age of the controls without DFU was 54 years (SD ±9.8), with a range of 34–76 years. More men (61.5%) than women (38.5%) had DFU (Table 1). The type and nature of their occupations had significant associations with DFU (Table 1). It was found that the patients who had T2DM and worked in positions that required standing for long periods of time (e.g., police, security, and teachers) were more likely to have DFU than those who spent more time sitting or walking at work (Table 1).
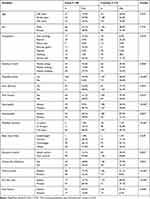 |
Table 1 Risk Factors of Diabetic Foot Ulcers Using Chi-Square Test |
DFU was found to have a significant association with the cases who practiced poor feet self-care, such as inspecting feet, cleaning, and drying between the toes, using moistening creams, and wearing appropriate shoes (Table 1). In addition, physical inactivity and history of foot trauma had significant associations with DFU (Table 1). Table 1 shows that diabetes complications, peripheral neuropathy, and peripheral vascular disease also had significant associations with DFU development. Long duration of T2DM was also associated with DFU (Table 1). The T2DM mean duration was 19.1 years (SD ±7.5) and 11.1 years (SD ±4.9) for the cases and the controls, respectively.
The mean BMI of the cases was 28.9 (SD ±5.3), with a range of 16.9–44.4. The mean BMI of the controls was 28.8 (SD ±4.3), with a range of 18–40. More cases (90.8%) had poor glycemic control than controls (69.8%). The mean HbA1c of the cases with DFU was 10.5 (SD ±2), with a range of 6.3–14. The controls who did not have DFU had a mean HbA1c of 8.5 (SD ±2.1), with a range of 5.5–12.6. Poor glycemic control was observed to be a significant risk factor associated with DFU development (Table 1), as were Charcot joint (Table 1), dry skin, and foot fissures (Table 1). Most cases with DFU were on metformin medication (87%) and others were insulin. Similarly, about 88% of control group were on metformin and 12% were on insulin and other anti-diabetic medications.
Diabetic Foot Ulcers and Vitamin B12 Deficiencies
Table 1 shows a significant correlation between DFU and vitamin B12 deficiency in the patients with T2DM: lower vitamin B12 levels led to higher risk for DFU development. Table 2 presents the results of the multivariate binary logistic regression analysis examining DFU predictors in patients with T2DM. A significant association with a 95% CI existed between vitamin B12 deficiency and DFU after adjusting for the other covariates. The patients with T2DM and vitamin B12 deficiency had 3.1 higher odds of reporting DFU than the patients with T2DM but without vitamin B12 deficiency.
 |
Table 2 Significant Predictors for Diabetic Foot Ulcers in Logistic Regression^ |
Discussion
The potential association between vitamin B12 deficiency and DFU is an attractive topic whose potential clinical implications have aroused researchers’ interest. However, the literature lacks studies exploring this association. This case–control study, therefore, was aimed at exploring the association between vitamin B12 deficiency and DFU among the patients with T2DM in Jazan City, Saudi Arabia, taking into consideration other DFU risk factors. The study compared 108 cases with DFU to 215 controls without DFU.
Diabetic Foot Ulcers and Vitamin B12 Deficiency
Vitamin B12, also known as cobalamin, is a vitamin essential to the proper functioning and development of the central and peripheral nervous systems,5 ensuring effective nerve-impulse transmission.5 Vitamin B12 deficiency can cause demyelination and nerve damage with clinical presentation of neurological manifestations and peripheral neuropathy with the associated symptoms of pain, tingling, numbness, paresthesia, and loss of sensation.28 Improvement of neurological symptoms after vitamin B12 supplements is a clear indication of this relationship.9 Peripheral neuropathy is one of microvascular complications of T2DM.7,29 In the current study, the vast majority of the cases with DFU (90.7%) had peripheral neuropathy, manifested clinically with a loss of sensation, especially in the feet. Peripheral neuropathy increased the risk of DFU development as the patients with feet hypoesthesia were vulnerable to foot trauma.
Metformin (biguanide derivative) is the recommended first-line anti-diabetic agent commonly used for treatment of T2DM.7 Accumulating evidence, however, has confirmed that its long-term use contributes to vitamin B12 deficiency in patients with T2DM.8–12 Metformin has been reported to lead to malabsorption of vitamin B12 and reduce its uptake in the terminal ileum, thereby decreasing the concentration of serum vitamin B12 10–30%.30 Manifestation of vitamin B12 deficiency starts three to 6 months after starting use of metformin medication.12,31 Furthermore, most obese patients with T2DM undergo bariatric surgery, which is also a risk factor for nutrients and vitamins deficiency including vitamin B12 due to malabsorption.13 Therefore, there is an association between vitamin B12 deficiency and DFU in patients with T2DM, especially among those who use metformin medication and undergo bariatric surgery.
Diabetic Foot Ulcers and Other Risk Factors
The patients who had T2DM and were older than 45 years old had the highest DFU rate, possibly because diabetes complications, including foot ulcer, increase with age and diabetes duration. The male cases also had a high DFU rate, possibly due to their vulnerability to foot trauma, especially those who wear unsuitable shoes such as the open styles of footwear (known as sandals) popular in Jazan’s culture.2 In contrast, female residents of Jazan are more likely to spend more time indoors as housewives, and they may be more capable of foot self-care than males, making them less likely to experience foot trauma. Furthermore, it was noticed that some jobs that required standing for long periods of time were associated with DFU (e.g. police, security, and teachers). Obesity is an associated comorbidity with T2DM, so high weight may increase foot pressure when standing for long periods, which may increase the risk of the progression of DFU pathogenesis.
In addition, it is believed that proper feet self-care, such as cleaning feet regularly, using moistening creams, and wearing appropriate shoes, may prevent the occurrence of foot ulcers in people with diabetes.2 This study found that a lack of feet self-care in patients with T2DM increased risk of DFU development. The physically inactive participants also tended to have DFU. Physical activity contributes to good glycemic control, reducing the risk of diabetes complications such as foot ulcers.32,33 Poor glycemic control also increases risk of microvascular complications such as neuropathy and DFU development. The UK Prospective Diabetes Study, therefore, has recommended controlling blood glucose levels to reduce risk of microvascular complications in patients with diabetes.29 The presence of dry skin, Charcot foot, and plantar calluses contributes to progression of DFU in patients with T2DM, as the current findings indicate. Plantar calluses are another important contributor to the pathogenesis of DFU. The likely explanation is that in the presence of neuropathy complications, sweating of lower limbs may decrease, leading to dry skin. Consequently, foot calluses and fissures can form under weight-bearing areas, which may cause foot ulcers in areas exposed to increased foot pressure.
Study Limitation
Multiple factors interact and contribute to DFU development, such as environmental hazards, lower limb pathology, and genetic factors. In this study, it was not possible to account for genetic factors and explore their associations with DFU. Furthermore, despite the exciting findings on a potential, new risk factor of DFU, this study design cannot prove causation. Establishing the causal association between vitamin B12 deficiency and DFU and clarifying the biological role of vitamin B12 deficiency in DFU are important aims for future studies such as cohort studies.
Conclusion
The study results indicate that vitamin B12 deficiency was associated with DFU development in the Saudi patients with T2DM in Jazan, Saudi Arabia. The patients with T2DM and vitamin B12 deficiency had 3.1 higher odds of reporting DFU than the patients with T2DM but without vitamin B12 deficiency.
Data Sharing Statement
The data that support the findings of this study are available on request from the author, Mohammed Badedi.
Acknowledgments
We would like to express our gratitude to the team who helped us in completing this research. To begin with, we would like to thank Jazan Diabetes Center staff; endocrinologists, podiatrists, family and community medicine consultants, nurses, and technicians in the registry department for their help. Finally, we would like to thank all participants in this study for their response and cooperation.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflict of interest in this work.
References
1. Bakker K, Schaper N; on behalf of the International Working Group on the Diabetic Foot Ulcer Editorial Board. The development of global consensus guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28(1):116–118. doi:10.1002/dmrr.v28.S1
2. Solan Y, Kheir H, Mahfouz M, et al. Diabetic foot care: knowledge and practice. J Endocrinol Metab. 2016;6:172–177. doi:10.14740/jem388e
3. Sargen M, Hoffstad O, Margolis D. Geographic variation in medicare spending and mortality for diabetic patients with foot ulcers and amputations. J Diabetes Complications. 2013;27(2):128–133. doi:10.1016/j.jdiacomp.2012.09.003
4. Boulton A, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724. doi:10.1016/S0140-6736(05)67698-2
5. Scalabrino G. The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: lessons learned from its deficiency. Prog Neurobiol. 2009;88(3):203–220. doi:10.1016/j.pneurobio.2009.04.004
6. Khan A, Shafiq I, Hassan Shah M. Prevalence of vitamin B12 deficiency in patients with type II diabetes mellitus on metformin: a study from Khyber Pakhtunkhwa. Cureus. 2017;9(8):e1577.
7. American Diabetes Association (ADA). Introduction: standards of medical care in diabetes 2019. Diabetes Care. 2019;42(1):61–66. doi:10.2337/dc19-S006
8. Alharbi T, Tourkmani A, Abdelhay O, et al. The association of metformin use with vitamin B12 deficiency and peripheral neuropathy in Saudi individuals with type 2 diabetes mellitus. PLoS One. 2018;13(10):e0204420. doi:10.1371/journal.pone.0204420
9. Reinstatler L, Qi Y, Williamson R, Garn J, Oakley G. Association of biochemical B12 deficiency with metformin therapy and vitamin B12 supplements: the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care. 2012;35(2):327–333. doi:10.2337/dc11-1582
10. de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo-controlled trial. BMJ. 2010;340:c2181. doi:10.1136/bmj.c2181
11. Aroda V, Edelstein S, Goldberg R, et al. Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J Clin Endocrinol Metab. 2016;101(4):1754–1761. doi:10.1210/jc.2015-3754
12. Wulffelé M, Kooy A, Lehert P, et al. Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med. 2003;254:455–463. doi:10.1046/j.1365-2796.2003.01213.x
13. Bloomberg R, Fleishman A, Nalle J, Herron D, Kini S. Nutritional deficiencies following bariatric surgery. Obes Surg. 2005;15(2):145–154. doi:10.1371/journal.pone.0204420
14. Tesfaye S, Boulton A, Dyck P, et al.; Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi:10.2337/dc10-1303
15. Dean A, Arner T, Sunki G, et al. Epi Info Program for Public Health Professionals. Atlanta: CDC; 2011.
16. Lindenbaum J, Rosenberg I, Wilson P, Stabler S, Allen R. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr. 1994;60(1):2–11. doi:10.1093/ajcn/60.1.2
17. Pfeiffer C, Caudill S, Gunter E, Osterloh J, Sampson E. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82(2):442–450. doi:10.1093/ajcn/82.2.442
18. Morris M, Jacques P, Rosenberg I, Selhub J. Elevated serum methylmalonic acid concentrations are common among elderly Americans. J Nutr. 2002;132(9):2799–2803. doi:10.1093/jn/132.9.2799
19. von Castel-dunwoody KM, Kauwell GP, Shelnutt KP, et al. Transcobalamin 776C->G polymorphism negatively affects vitamin B12 metabolism. Am J Clin Nutr. 2005;81(6):1436–1441. doi:10.1093/ajcn/81.6.1436
20. Miller J, Garrod M, Rockwood A, et al. Measurement of total vitamin B12 and holotranscobalamin, singly and in combination, in screening for metabolic vitamin B12 deficiency. Clin Chem. 2006;52(2):278–285. doi:10.1373/clinchem.2005.061382
21. Morris M, Jacques P, Rosenberg I, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85(1):193–200. doi:10.1093/ajcn/85.1.193
22. World Health Organization (WHO). Physical status: the use and interpretation of anthropometry: report of a WHO Expert committee. Technical report series 854. Geneva: WHO; 1995.
23. Smieja M, Hunt D, Edelman D, Etchells E, Cornuz J, Simel D; and for the International Cooperative Group for Clinical Examination Research. Clinical examination for the detection of protective sensation in the feet of diabetic patients. J Gen Intern Med. 1999;14(7):418–424. doi:10.1046/j.1525-1497.1999.05208.x
24. Bus S, van Netten J, Lavery L, et al. The International Working Group on the Diabetic Foot. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. Diabetes Metab Res Rev. 2016;32(1):16–24. doi:10.1002/dmrr.2696
25. Rogers L, Frykberg R, Armstrong D, et al. The charcot foot in diabetes. Diabetes Care. 2011;34(9):2123–2129. doi:10.2337/dc11-0844
26. Jazan Hospital IRB. National committee of bio ethics. King Abdulaziz City for Science & Technology (KACST): H-10-Z-068
27. BM Corp. IBM SPSS Statistics for Windows. New York, NY: IBM Corp; 2012.
28. Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349:5226. doi:10.1136/bmj.g5226
29. Stratton I, Adler A, Neil H, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi:10.1136/bmj.321.7258.405
30. Bauman W, Shaw S, Jayatilleke E, Spungen A, Herbert V. Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care. 2000;23(9):1227–1231. doi:10.2337/diacare.23.9.1227
31. Chapman L, Darling A, Brown J. Association between metformin and vitamin B12 deficiency in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2016;42(5):316–327. doi:10.1016/j.diabet.2016.03.008
32. Badedi M, Solan Y, Darraj H, et al. Factors associated with long-term control of type 2 diabetes mellitus. J Diabetes Res. 2016;Article ID 2109542:8.
33. Darraj H, Badedi M, Poore K, et al. Vitamin D deficiency and glycemic control among patients with type 2 diabetes mellitus in Jazan City, Saudi Arabia. Diabetes Metab Syndr Obes. 2019;12:853–862. doi:10.2147/DMSO.S203700
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
