Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Vessels That Encapsulate Tumor Clusters (VETC) Predict cTACE Response in Hepatocellular Carcinoma
Authors Lin C , He Y, Liu M, Wu A, Zhang J, Li S, Li S, Cao Q, Liu F
Received 10 November 2022
Accepted for publication 19 January 2023
Published 7 March 2023 Volume 2023:10 Pages 383—397
DOI https://doi.org/10.2147/JHC.S395903
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr David Gerber
Chunyu Lin,1,2,* Yuan He,3,* Mengnan Liu,1,4 Aihua Wu,1 Jing Zhang,4 Shurong Li,5 Shuqi Li,6 Qinghua Cao,6 Fang Liu1,2
1State Key Laboratory of Organ Failure Research, Guangdong Provincial Key Laboratory of Viral Hepatitis Research, Department of Infectious Diseases, Department of Liver Tumor Center, Nanfang Hospital, Southern Medical University, Guangzhou, 51051, People’s Republic of China; 2Department of Oncology, Nanfang Hospital, Southern Medical University, Guangzhou, 51051, People’s Republic of China; 3Department of Radiotherapy, The First Affiliated Hospital of University of Science and Technology of China, Hefei, 23000, People’s Republic of China; 4Department of Radiology, Nanfang Hospital, Southern Medical University, Guangzhou, 51051, People’s Republic of China; 5Department of Radiology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, 51008, People’s Republic of China; 6Department of Pathology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, 51008, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qinghua Cao, Department of Pathology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, 51008, People’s Republic of China, Email [email protected] Fang Liu, Department of Oncology, Nanfang Hospital, Southern Medical University, Guangzhou, 51051, People’s Republic of China, Email [email protected]
Background: To investigate the correlation between hepatocellular carcinoma (HCC) pathological types and conventional transarterial chemoembolization (cTACE), and to evaluate the predictive value of the pathological types for efficacy of cTACE.
Methods: We investigated 186 naive HCC patients from 2 hospitals, including 63 patients with recurrence after surgical resection, and 123 unresectable cases, who underwent at least one cTACE procedure as the first treatment. All patients were histologically diagnosed with HCC by surgical resection and/or liver biopsy. Lipiodol deposition rate, ORR (objective response rate), PFS (progression-free survival), OS (overall survival) were compared among different HCC pathological types.
Results: This study evaluated 186 naive HCC patients and 189 tumor nodules. Vessels that encapsulate tumor clusters (VETC), macrotrabecular-massive (MTM), CK19-positive types were identified in 38% (72/189), 40% (76/189), and 28% (53/189) of the whole cohort, respectively. VETC, MTM and CK19-negative HCCs derived significantly better lipiodol deposition rate and ORR. cTACE prolonged the PFS of VETC and CK19-negative HCCs compared with non-VETC and CK19-positive HCCs in the recurrence, liver biopsy and combining whole cohorts, whereas the OSs of different pathological types were not significantly different. Multivariate analysis showed that VETC (OR, 4.671, 95% CI [1.954, 11.166], P< 0.001) and CK19-positive type (OR, 0.127, 95% CI [0.044, 0.362], P< 0.001) were independent predictive factors for the first cTACE response. However, only VETC type was significantly associated with the second cTACE response in multivariate analysis (OR, 3.31, 95% CI [1.24, 8.83], P=0.017), suggesting that VETC might be a more useful predictor of cTACE response.
Conclusion: Our study suggests that VETC is an effective predictor of cTACE response in patients with HCC.
Keywords: hepatocellular carcinoma, vessels that encapsulate tumor clusters, CK19-positive, conventional transarterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC), which accounts for 80% of primary liver cancer, is the sixth most common cancer and the fourth leading cause of cancer-related death world-wide.1 Unfortunately, approximately 70% of HCCs, who are first diagnosed at an intermediate or advanced stage, almost lose access to curative options such as liver transplantation, surgical resection, and local ablative therapy.
HCC is the hypervascular tumor and receives its blood supply predominantly from the hepatic artery. Conventional transarterial chemoembolization (cTACE), which could reduce or eliminate blood flow to the tumor by injecting lipiodol-based small embolic particles with cytotoxic agent into hepatic artery and result in tumor cell necrosis, is the first-line treatment for patients with intermediate stage HCC. Moreover, cTACE, as a locoregional palliative therapy together with other treatment strategies (targeted therapy, immunotherapy, etc.), is usually applied on advanced HCCs, which might ease symptoms, improve the quality of life and overall survival. However, many patients respond poorly to cTACE.2 Therefore, it is necessary to find useful factors that could predict effectiveness before cTACE treatment.
Recently, HCC is further subclassified into distinct histologically pathological subtypes, such as macrotrabecular-massive (MTM), vessels that encapsulate tumor clusters (VETC) and CK19-positiveness.3 Pathological types of HCC could better guide patients’ personalized treatment. For example, VETC type is a predictor of sorafenib benefit in HCC patients.4 FGF19-positive HCCs are more sensitive to FGFR4 inhibitor treatment.5 CK19-positive HCCs are related to high recurrence after radiofrequency ablation.6 MTM and VETC types are associated with angiogenesis,4,7,8 which plays important role in the effectiveness of cTACE.9 Increased plasma levels of VEGF have been associated with the development of metastasis after cTACE.10 However, the correlation between pathological types and cTACE response has not been elucidated fully. This study aims to evaluate the value of pathological types in predicting cTACE efficacy.
Patients and Methods
Patients and Specimens
We retrospectively included 186 naive HCC patients who underwent a first session of cTACE at the First Affiliated Hospital of Sun Yat-sen University between January 2015 and December 2020 (n=110, 60 surgical resection, 50 liver biopsy, 6 both of them, 2 male patients with two lesions both confirmed HCC by biopsy), and Nanfang Hospital, Southern Medical University between June 2019 and December 202 (n=76, 3 surgical resection, 73 biopsy, 3 both of them, 1 female patient with two lesions confirmed HCC by biopsy). In surgical resection cohort, the largest nodules were chosen as the target lesion if recurrent cases were multifocal tumor nodules. In liver biopsy cohort, the biopsy lesions were investigated. Therefore, 186 naive HCC with 189 nodules were investigated in our study. Patients in two cohorts underwent routine follow-up until patients missed visits or died.
Inclusion criteria for all patients were as follows: (1) cTACE was the first treatment for the patients with HCC recurrence and/or unresectable HCC evaluated by HCC multidisciplinary team (MDT). Patients who received any anti-tumor drug or local treatment before cTACE were excluded from the present study; (2) the patients were histologically confirmed HCC by hepatectomy or liver needle biopsy; (3) tumor tissue sections were sufficient for CD34, CK19, Ki67, AFP, Glypican-3 immunohistochemical (IHC) staining (Figure 1).
 |
Figure 1 Patient selection. |
Immunohistochemistry Analysis
Immunohistochemical staining was carried out according to the manufacturer’s instructions. All sections were dewaxed, soaked in ethanol, and treated with 3% hydrogen peroxide to block endogenous peroxidase activity. Nonspecific immunoreactivity was blocked by incubating the sections in normal rabbit serum at room temperature. The tumor tissue sections were then incubated with primary monoclonal antibodies CD34, CK19, Ki67, AFP, Glypican-3 (working solution, Gene Tech Company limited). Then, secondary biotinylated antimouse immunoglobulin (Dako, Denmark) was applied and then reacted with streptavidin biotinylated horseradish peroxidase complex (Dako, Denmark). The sections were stained with diaminobenzidine solution and then counterstained with Mayer’s hematoxylin. The negative control was obtained by substituting the primary antibodies with mouse immunoglobulin G. The nuclear fraction of Ki67 positivity was quantitatively measured as previously described.11 All patients were divided into positive and negative Ki67 groups based on the median value. Expression of CD34, AFP, GPC3 with moderated or strong staining in tumor cells was defined as positive expression.11
Pathology Types
Macrotrabecular-Massive (MTM) Type
For each surgical resection specimen, all available histological slides were reviewed, and tumors with a predominant (>50%) macrotrabecular (trabeculae of more than six cells thick) architectural pattern were classified as MTM-HCC. For all biopsy samples, tumors were classified as MTM-HCC if foci of macrotrabecular architectural pattern were observed.7
Vessels That Encapsulate Tumor Clusters (VETC) Type
CD34 immunoreactivity of a continuous lining around tumor clusters (at least 55% of the tumor area) was defined as VETC-HCC.8
CK19-Positive Type
Cytokeratin 19 (CK19) is well acknowledged as a biliary/progenitor cell marker. CK19-positive HCC, regarded as a more aggressive tumor, is defined by the immunohistochemical expression of CK19 more than 5% of tumor cells.12 All pathological classifications and IHC evaluations were performed by two pathologists who specialize in liver diseases.
cTACE Procedure
cTACE was performed by experienced doctor after pathological diagnosis of HCC. Using the Seldinger technique, an arterial catheter (5-Fr) was inserted into the femoral artery after local anesthesia. The catheter was then advanced in the hepatic artery. The tumor-feeding vessels were superselected using the catheter or microcatheter (2.8-Fr) to infuse a suspension containing 2–20 mL of iodized oil (Laboratoire Guerbet, Roissy-Charles de Gaulle, France) and cytotoxic agents such as raltitrexed, oxaliplatin, epirubicin, and doxorubicin hydrochloride. Gelfoam sponge embolization was performed following iodized oil embolization. The dosages of drugs and iodized oil were determined by body surface area, liver function and tumor characteristics.
Tumor Response and Lipiodol Deposition Assessment on the First Follow-Up CT and/or Gd-EOB-DTPA MRI
The tumor response to cTACE was evaluated by modified Response Evaluation Criteria in Solid Tumors (mRECIST), which measure alive tumor on enhanced CT. However, some studies have found that the high focus of high concentration lipiodol on CT might mask the potential high enhancement part of tumors.13,14 Therefore, if there was controversy about the alive tumor after cTACE, the patients were administered Gd-EOB-DTPA (gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid) MRI, which could help eliminate the influence of lipiodol deposition.
In our study, tumor response was evaluated by contrast-enhanced CT and/or Gd-EOB-DTPA MRI according to mRECIST,15 which included: (1) complete response (CR): disappearance of any intratumoral arterial enhancement in the target lesion; (2) partial response (PR): at least a 30% decrease in the diameter of target lesion; (3) stable disease (SD): any cases that did not qualify as either PR or progressive disease (PD); and (4) PD: an increase of at least 20% in the diameter of the target lesions or new lesions (enhancement in the arterial phase). In addition, objective response rate (ORR) was defined as the sum of CR and PR. The prognostic value of the lipiodol deposition rate and pattern after cTACE has been shown in some studies. However, there is no unified standard about lipiodol deposition, especially for its shape characteristics. Therefore, in our study, we classified quantitative types: ≥50% and <50% lipiodol deposition by CT 4–6 weeks after cTACE treatment.16
Statistical Analysis
Statistical analyses were performed using SPSS software (version 19.0). Comparisons between qualitative and quantitative data were performed using chi-square tests. PFS and OS curve plotting used the Kaplan–Meier method, and the association between variables and tumor response was investigated using univariate and multivariate Cox proportional hazards regression models used the R4.0.3 package. P<0.05 was considered statistically significant.
Results
Patients Histologically Diagnosed with HCC by Surgical Resection
The main clinical features of the 63 patients with HCC recurrence after surgical resection are summarized in Table 1. There were 53 (83.8%) males, and mean age was 50 years±12; HBV infection (92%) and cirrhotic livers (65%) were the main etiology of HCC; HBV DNA copies (>1000IU/mL) and elevated serum AFP levels (>400 ng/mL) were detected in 48% and 28% of recurrent cases, respectively. Disease stage was BCLC A and BCLC B/C in 11% and 89% of the cases. ECOG score 0 and 1 were 56% (35/63), 44% (28/63). Recurrent tumor nodules were multifocal (58%) and solitary (42%), and 50% of nodules were more than 5 cm. Cases with satellite nodules, microvascular invasion, portal vein tumor thrombus (PVT), lymph node metastasis were 9.5% (6/63), 40% (25/63), 7.9% (5/63), 6.3% (4/63), respectively (Table 1).
 |
Table 1 Clinical and Pathological Features of the Patients According to MTM, VETC, CK19-Positive Type in Recurrence after Surgical Resection Cohort |
In recurrent cohort, 6 cases with recurrent lesions were administered liver biopsy and pathological characteristics of recurrent lesions were similar to corresponding primary surgical tissues. Therefore, 63 largest recurrent tumor nodules were observed as target lesions. VETC, MTM, CK19-positive types were 38% (24/63), 44% (28/63), 27% (17/63), respectively. MTM and VETC types were significantly associated with several features, such as: HBV DNA (>1000IU/mL) (P= 0.000, P =0.0341, respectively), high AFP level (>400 ng/mL) (P=0.001, P=0.0173, respectively), and microvascular invasion (P=0.011, P=0.014, respectively). CK19-positive types were not significantly correlated with above factors, but related to lymph node metastasis (P=0.047). (Table 1).
According to mRECIST, the total CR, PR, SD and PD were 22% (14/63), 16% (10/63), 25% (16/63), 37% (23/63), respectively; ORR was 38%. Lipiodol deposition: 64% (31/63) in ≥50% group and 36% (32/63) in < 50% group. Better ORR and lipiodol deposition rate were related to VETC type (P=0.000, P=0.001, respectively) and negatively associated with CK19-positive type (P=0.004, P=0.031, respectively) (Tables S1 and S2). VETC and CK19-negative HCCs showed significantly longer PFS (VETC versus non-VETC, median survival 250 days versus 109 days, P=0.026; CK19-negative versus positive, median survival 256 days versus 53 days, P<0.001), suggesting that non-VETC and CK19-positive HCCs are resistant to cTACE therapy. It was interesting to find that, although the OS of VETC and non-VETC, MTM and non-MTM HCCs were not significantly different (VETC versus non-VETC, median survival 515 days versus 526 days, P=0.555; MTM versus non-MTM, median survival 568 days versus 394 days, P=0.283), CK19-negative HCCs derived a significant survival benefit compared with CK19-positive HCCs (median survival 569 days versus 165 days, P=0.005) (Figure S1). The reason may be that 64% (11/17) of CK19-negative HCCs did not received sequential treatments for reasons such as liver failure, infection and high medical expenses.
Patients’ Histologically Confirmed HCC by Liver Needle Biopsy
Among the 123 patients with 126 tumor nodules histologically diagnosed with HCC, including 3 cases with two lesions, similar results were observed: predominance of males, HBV infection and cirrhotic livers (84%, 89%, 60%). BCLC B/C, tumor size (>5 cm), AFP (>400 ng/mL), PVT and extrohepatic metastasis were observed in 88%, 64%, 30%, 6%, 4% of the patients (Table 2).
 |
Table 2 Clinical and Pathological Features of the Patients According to MTM, VETC, CK19-Positive Type in Liver Biopsy Cohort |
Among 126 needle biopsy tissues examined, MTM, VETC, CK19-positive types were 37% (46/126), 38% (48/126), 28% (35/126), respectively. As observed in recurrent cohort, MTM and VETC types were also closely associated with HBV DNA (>1000 IU/mL) (P=0.0221, P=0.042, respectively), high AFP level (>400 ng/mL) (P=0.001, P=0.025, respectively), and PVT (P=0.002, P=0.033, respectively). Moreover, there was very significant correlation for CK19-positive type and lymph node, extrohepatic metastasis (P=0.012, P=0.026, respectively) (Table 2). Similarly, CR, PR, SD and PD were 14% (18/126), 28% (35/126), 36% (45/126), 22% (28/126), respectively; ORR was 33%; lipiodol deposition: ≥50% group 45% (57/126) and <50% group 55% (69/126) (Tables S3 and S4).
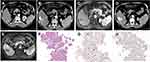
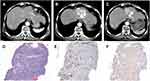
Moreover, 3 cases with two lesions both diagnosed with HCC by needle biopsy were studied carefully, pathological patterns of 2 cases were almost identical. However, the pathological types were not completely uniform in two lesions of a female HCC patient, whose lipiodol deposition and tumor response were different (VETC lesion, ≥50% lipiodol deposition, PR; non-VETC lesion, <50% lipiodol deposition, SD) (Figure 2).
Consistent with the findings from recurrence cohort, compared with non-VETC and CK19-positive HCCs, VETC and CK19-negative HCCs in the biopsy cohort had a significantly better lipiodol deposition rate (P=0.002, P=0.001, respectively), ORR (P=0.003, P=0.000, respectively) (Tables S3 and S4), and longer PFS (VETC versus non-VETC, median survival 289 days versus 118 days, P=0.014; CK19-negative versus CK19-positive, median survival 179 days versus 108 days, P=0.026), whereas the OS of different pathological types were not significantly different (VETC versus non-VETC, median survival 342 days versus 303 days, P=0.65; CK19-negative versus CK19-positive, median survival 330 days versus 295 days, P=0.848; MTM versus non-MTM, median survival 308 days versus 329 days, P=0.878) (Figure S2).
ORR After the First cTACE, PFS and OS Among Different Pathological Types in the Whole Cohort
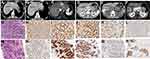
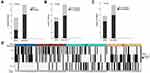
In the whole cohort, the total CR, PR, SD and PD were 17% (32/189), 24% (45/189), 32% (61/189), 27% (51/189), respectively; ORR was 40.7%; VETC, MTT and CK19-positive types were identified in 38% (24 recurrent and 48 unresectable cases), 40% (28 recurrent and 48 unresectable cases), 27% (17 recurrent and 35 unresectable cases) of 189 cases. Of VETC cases, 58% (42/72) were observed in the group of MTT type; VETC and MTT types were significantly associated with each other (P=0.001); VETC, not MTM HCCs, expressed fewer CK19 tumor cells (P<0.05) (Figure 3A–C). Moreover, 20 out of all 32 CR cases were VETC type, and 31 were CK19-negative type (Figures 3D and 4).
The different lipiodol deposition rate, ORR and PFS benefits of cTACE treatment for VETC and CK19-negative HCCs were further verified in the patients combining recurrence and biopsy cohorts. In agreement with the above results, VETC and CK19-negative HCCs tended to derive greater lipiodol deposition rate (P=0.001, P=0.002, respectively), better ORR (P=0.001, P=0.001, respectively) (Figure 5), and PFS benefits from cTACE (VETC versus non-VETC, median survival 255 days versus 117 days, P=0.001; CK19-negative versus CK19-positive median survival 213 days versus 98 days, P<0.001) (Figure 6A and C), which does not switch to OS benefits (VETC versus non-VETC, median survival 361 days versus 353 days, P= 0.636; CK19-negative versus CK19-positive, median survival days 394 versus 281 days, P=0.151) (Figure 6D and F). The PFS and OS were not significantly different in MTM and non-MTM groups (PFS, median survival 361 days versus 353 days, P=0.636; OS, median survival days 409 versus 339 days, P=0.3) (Figure 6B and E).
Considering MTM and VETC types show elevated serum AFP in our study, and AFP, GPC3, Ki67 are believed to play a crucial regulatory role in cellular proliferation and tumor progression,17,18 we also analyzed AFP, GPC3 and Ki67 expression among different pathological types. There were no significant relations between the expression of Ki67, GPC3 and different pathological types. The expression in situ of AFP were significantly correlated with MTM and VETC types (P=0.002, P=0.032, respectively) (Table S5).
Variables associated with ORR for the first cTACE in univariate analysis were C-P stage (OR 2.519, 95% CI [1.113, 5.697], P=0.027), ≥50% lipiodol deposition (OR 6.667, 95% CI [3.488, 12.742], P<0.001), VETC type (OR 4, 95% CI [2.148, 7.449], P<0.001), and CK19-positive type (OR 0.149, 95% CI [0.063, 0.353], P<0.001). Multivariate analysis showed that VETC (OR, 4.671, 95% CI [1.954, 11.166], P<0.001) and CK19-positive type (OR, 0.127, 95% CI [0.044, 0.362], P<0.001) were independent predictors of better ORR of the first cTACE (Table 3).
 |
Table 3 Univariate and Multivariate Analyses of Clinical, Biological, and Pathological Types for ORR of the First cTACE Treatment by Logistic Regression Analysis in the Whole Cohort |
ORR and Lipiodol Deposition After the Second cTACE Session in the Whole Cohort
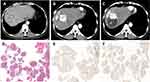
After the first cTACE, 100 patients received the second cTACE, and 16 patients who received ablation or molecular drugs before the second cTACE were excluded. Finally, 84 cases were further investigated, including 31 MTM, 33 VETC, 9 CK19-positive HCCs. Compared with non-VETC lesions, more lipiodol continued to depose in the alive part of the lesions in VETC lesion (P=0.000) (Figures 7A, 8 and 9), whereas lipiodol depositions were not increased by repeated cTACE in CK19-positive group (P=0.005) (Figure 7C). The whole ORR to the second cTACE was 32% (27/84). However, better ORR was observed in VETC group but not MTM and CK19-negative group (P=0.01, P=0.11, P=0.129, respectively) (Figure 7D–F). Moreover, VETC type, not MTM and CK19-positive, was significantly associated with ORR for the second cTACE in univariate analysis and multivariate analysis (OR 3.86, 95% CI [1.49, 10.04], P=0.006; OR, 3.31, 95% CI [1.24, 8.83], P=0.017, respectively) (Table S6). Our results showed that naive VETC pathological type, not CK19-positiveness and MTM, remained predicting the second cTACE efficacy. It can be explained that hypoxia after cTACE triggers the expression of CK19 of tumor cells,17,18 and might change the naive pathological types.
Discussion
Due to the fact that HCC is a unique solid tumor that can be diagnosed only by imaging and medical history, there are few reports about the correlation between HCC pathological types and cTACE. In our study, VETC, MTM, CK19-positive types, which can be easily confirmed by IHC and pathological evaluation, were identified in 38%, 40%, 28% of the whole cohort respectively, and the prevalence of MTM and VETC types was higher than foreign reports (less than 20%),7,8 which is consistent with Chinese researches.4,19 This may be explained by the difference in main etiology (HBV infection in our study versus alcohol and HCV in those foreign studies). Moreover, both of MTM and VETC types were correlated with high HBV DNA copies (>1000 IU/mL) in our study. The underlying oncogenic pathways associated with etiology and particular HCC subtype may help us better understand the occurrence and development of liver tumors.
The efficacy of cTACE is affected by many factors, including liver cirrhosis, liver function, serum AFP level, tumor size, whether the tumor capsule is complete, portal vein tumor thrombus, ECOG score and individual heterogeneity.20 Although the volume, rate and shape of lipiodol deposition play very important roles in the effectiveness of cTACE and local progression,13,21 the exact mechanisms are not clear. It is well known that lipiodol deposition and washout slowly from HCC embolized lesions because of sufficient angiogenesis, absence of Kupffer cells, and lack of lymphatic cells and vessels.22,23 We found HCC patients with VETC, MTM and CK19-negative types have greater lipiodol deposition. Kurebayashi et al reported that immune-high subtype of HCC, characterized by increased B-/plasma-cell and T cell infiltration, was associated with CK19-positiveness,24 but MTM and VETC types demonstrated high angiogenesis and low lymphocytic infiltration.25 Therefore, we speculated that the microenvironment characterized by VETC and MTM is fit for more lipiodol deposition and less washout.
2019 WHO Classification of Tumors suggests that Ck19-positive HCCs have higher rates of resistance to cTACE, but the number of naive HCC patients in the paper are few.26 In our study, we investigated the association between CK19-positiveness and cTACE response by 186 naive patients, and found a similar result of worse cTACE response in CK19-positive group; moreover, CK19 expression of tumor cells was negative with VETC type. Some scholars found that hypoxia may trigger the expression of CK19.26,27 Conversely, VETC types with a terminal vessel encapsulating a tumor cluster, correlated with angiogenesis, are inclined to supply tumor cells with a relatively oxygen-enriched microenvironment, which may decrease the expression of CK19. Moreover, the tumor microenvironment (TME) of VETC is low-immune and CK19-positive type is high-immune,24,25 and TME can have a profound impact on tumor cell types,28 implying that tumor cells of VETC would express less expression of CK19 due to low-immune TME. The detailed molecular mechanisms between VETC and CK19 need to be further investigated.
In our study, 189 naive intrahepatic HCC lesions were selected as target lesions of cTACE response. The ORR (measured by mRECIST) for the first cTACE was 41% in the whole cohort (38%, recurrence after surgical resection; 42%, liver biopsy cohort), which basically coincides with previous reports.29 Although cTACE prolongs the PFS of VETC and CK19-negative HCCs, which achieved better ORR compared with non-VETC and CK19-positive HCCs, the OS was not significantly different among pathological types in the whole cohort. One of the reasons may be that 70% of patients received sequential treatments besides cTACE, such as ablation, molecular targeted drugs and immunotherapy that could have influence on the OS. It is not uncommon that the significant differences in ORR and PFS do not correlate with differences in OS in Phase III studies. For example, a higher ORR did not correlate with an improved OS in a REFLECT trial.30 Moreover, 4 Phase III trials (EACH, REACH, REFLECT, SILIUS) reporting a positive PFS with a hazard ratio between 0.6–0.8 were associated with no significant survival benefits.31 Considering long-time follow up and different treatments after cTACE, the 2018 EASL guidelines for the diagnosis and treatment of liver cancer recommend the ORR as an alternative index for the observation of the overall survival time.32
After initial cTACE success, the decision on when cTACE therapy should be repeated or interrupted is complex. Sieghart et al reported that the ART score could identify patients who would not profit from retreatment with cTACE pretty well.32 However, their applicability is controversial because the ART score is not effective to select patients for cTACE retreatment.33 In our study, only VETC type was significantly associated with the first and second cTACE response, suggesting that VETC might be more useful to predict cTACE response in hepatocellular carcinoma.
In recent decades, tumor biopsy and pathological diagnosis of HCC aroused no or very little interest for physicians due to the excellent diagnostic performance of imaging procedures and worrying about potential risks of bleeding and needle track seeding. However, it is now widely accepted that the potential risks are infrequent, manageable and do not affect the course of the disease or overall survival.32 Pathological features are closely correlated with transcriptomic classification and genetic alterations, which could help us to get full characteristics of HCC to better guide patients’ precise treatment.12 In the present study, we provide evidence that VETC is a predictor of good response of cTACE and CK19-positiveness is a predictor of cTACE resistance. Therefore, tumor biopsy of HCC helps physicians select patients accurately for cTACE and reduce error costs of unsuitable patients.
Our study had limitations. First, it was retrospectively performed with inherent selection bias. For example, many patients received live biopsy because they could not be diagnosed with HCC, only by medical history and image data in liver biopsy cohort. Second, the results, especially from biopsy cohort, did not represent the full spectrum of HCCs. Finally, the sample size was limited and therefore lacked enough power to detect significance.
Conclusion
Our study suggests that VETC and CK19-positive types were independent predictive factors for the first cTACE. However, only VETC type was significantly associated with the second cTACE response in multivariate analysis, suggesting that VETC might be a more useful predictor of cTACE response.
Abbreviations
HCC, hepatocellular carcinoma; cTACE, conventional transarterial chemoembolization; VETC, vessels that encapsulate tumor clusters; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; MTM, macrotrabecular-massive; PVT, portal vein tumor thrombus; mRECIST, modified response evaluation criteria in solid tumors; Gd-EOB-DTPA, gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Funding
This study was supported by National Natural Science Foundation of China (Grant Nos. 81802431, 81972516), Natural Science Foundation of Guangdong Province (Grant No. 2021A1515012448); President Foundation of Nanfang Hospital, Southern Medical University (Grant No. 2018B012).
Disclosure
Qinghua Cao and Fang Liu contributed equally and were co-corresponding authors. In addition, Chunyun Lin and Yuan He equally contributed and were the co-first authors. The authors report no potential conflicts of interest in relation to this work.
References
1. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462.
2. Calderaro J, Ziol M, Paradis V, et al. Molecular and histological correlations in liver cancer. J Hepatol. 2019;71(3):616–630.
3. Fang JH, Xu L, Shang LR, et al. Vessels that encapsulate tumor clusters (VETC) pattern is a predictor of sorafenib benefit in patients with hepatocellular carcinoma. Hepatology. 2019;70(3):824–839.
4. Kim RD, Sarker D, Meyer T, et al. First-in-human Phase I study of fisogatinib (BLU-554) validates aberrant FGF19 signaling as a driver event in hepatocellular carcinoma. Cancer Discov. 2019;9(12):1696–1707.
5. Tsuchiya K, Komuta M, Yasui Y, et al. Expression of keratin 19 is related to high recurrence of hepatocellular carcinoma after radiofrequency ablation. Oncology. 2011;80(3–4):278–288.
6. Ziol M, Poté N, Amaddeo G, et al. Macrotrabecular-massive hepatocellular carcinoma: a distinctive histological subtype with clinical relevance. Hepatology. 2018;68(1):103–112.
7. Renne SL, Woo HY, Allegra S, et al. Vessels Encapsulating Tumor Clusters (VETC) is a powerful predictor of aggressive hepatocellular carcinoma. Hepatology. 2020;71(1):183–195.
8. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103(4):914–921.
9. Li Z, Xue TQ, Chen XY. Predictive values of serum VEGF and CRP levels combined with contrast enhanced MRI in hepatocellular carcinoma patients after TACE. Am J Cancer Res. 2016;6(10):2375–2385.
10. Xu LX, He MH, Dai ZH, et al. Genomic and transcriptional heterogeneity of multifocal hepatocellular carcinoma. Ann Oncol. 2019;30(6):990–997.
11. Zhuo J, Lu D, Wang J, et al. Molecular phenotypes reveal heterogeneous engraftments of patient-derived hepatocellular carcinoma xenografts. Chin J Cancer Res. 2021;33(4):470–479.
12. Kloeckner R, Otto G, Biesterfeld S, et al. MDCT versus MRI assessment of tumor response after transarterial chemoembolization for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33(3):532–540.
13. Dioguardi BM, Sartoris R, Libotean C, et al. Lipiodol retention pattern after TACE for HCC is a predictor for local progression in lesions with complete response. Cancer Imaging. 2019;19(1):75.
14. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
15. Dong X, Chen G, Huang X, et al. Copy number profiling of circulating free DNA predicts transarterial chemoembolization response in advanced hepatocellular carcinoma. Mol Oncol. 2022;16(10):1986–1999.
16. Nishida T, Kataoka H. Glypican 3-targeted therapy in hepatocellular carcinoma. Cancers. 2019;11(9):1339.
17. Ridder DA, Weinmann A, Schindeldecker M, et al. Comprehensive clinicopathologic study of alpha fetoprotein-expression in a large cohort of patients with hepatocellular carcinoma. Int J Cancer. 2022;150(6):1053–1066.
18. Lai JP, Conley A, Knudsen BS, Guindi M. Hypoxia after transarterial chemoembolization may trigger a progenitor cell phenotype in hepatocellular carcinoma. Histopathology. 2015;67(4):442–450.
19. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–442.
20. Kinugasa H, Nouso K, Takeuchi Y, et al. Risk factors for recurrence after transarterial chemoembolization for early-stage hepatocellular carcinoma. J Gastroenterol. 2012;47(4):421–426.
21. Kan Z, McCUSKEY PA, Wright KC, Wallace S. Role of Kupffer cells in iodized oil embolization. Invest Radiol. 1994;29(11):990–993.
22. Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009;10(5):425–434.
23. Kurebayashi Y, Ojima H, Tsujikawa H, et al. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology. 2018;68(3):1025–1041.
24. Kurebayashi Y, Matsuda K, Ueno A, et al. Immunovascular classification of HCC reflects reciprocal interaction between immune and angiogenic tumor microenvironments. Hepatology. 2022;75(5):1139–1153.
25. Rhee H, Nahm JH, Kim H, et al. Poor outcome of hepatocellular carcinoma with stemness marker under hypoxia: resistance to transarterial chemoembolization. Mod Pathol. 2016;29(9):1038–1049.
26. Nishihara Y, Aishima S, Kuroda Y, et al. Biliary phenotype of hepatocellular carcinoma after preoperative transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2008;23(12):1860–1868.
27. Kaymak I, Williams KS, Cantor JR, Jones RG. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell. 2021;39(1):28–37.
28. Chen S, Peng Z, Zhang Y, et al. Lack of response to transarterial chemoembolization for intermediate-stage hepatocellular carcinoma: abandon or repeat? Radiology. 2021;298(3):680–692.
29. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173.
30. Llovet JM, Montal R, Villanueva A. Randomized trials and endpoints in advanced HCC: role of PFS as a surrogate of survival. J Hepatol. 2019;70(6):1262–1277.
31. EASL Clinical Practice. Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
32. Sieghart W, Hucke F, Pinter M, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2261–2273.
33. Kudo M, Arizumi T, Ueshima K. Assessment for retreatment (ART) score for repeated transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2014;59(6):2424–2425.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.