Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Very Early Pulsed Dye Laser Intervention for Optimal Cosmetic Outcome in Post-Thyroidectomy Scars
Authors Kim YH, Kim HS , Kim HK, Kim SY, Lee J, Kim YC, Park YJ
Received 15 October 2023
Accepted for publication 31 March 2024
Published 17 April 2024 Volume 2024:17 Pages 877—884
DOI https://doi.org/10.2147/CCID.S444885
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Rungsima Wanitphakdeedecha
Yul Hee Kim,1 Han-Seul Kim,1 Hyeung Kyoo Kim,2 Soo Young Kim,2 Jeonghun Lee,2 You Chan Kim,1 Young Joon Park1
1Department of Dermatology, Ajou University School of Medicine, Suwon, Republic of Korea; 2Department of Surgery, Ajou University School of Medicine, Suwon, Republic of Korea
Correspondence: Young Joon Park, Department of Dermatology, Ajou University Hospital, 164, World Cup-ro, Yeongtong-gu, Suwon-si, Gyeonggi-Do, Suwon, Republic of Korea, Tel +82-31-219-5187, Email [email protected]
Purpose: Early intervention of surgical scars with a pulsed dye laser is known to effectively prevent pathologic scars. Despite multiple reports on the effectiveness of the treatment, very few studies have demonstrated its appropriate initiation timing. In this study, our objective was to determine the optimal timing for initiating laser treatment following thyroidectomy.
Methods: This study retrospectively analyzed 91 patients undergoing pulsed dye laser treatment post-thyroidectomy, grouping them by treatment initiation timing. The patients underwent treatment at intervals of 3– 4 weeks with at least five sessions. Those with a high pliability score were injected with intralesional corticosteroids. The Antera 3D® skin imaging analyzer was used to assess biophysical parameters.
Results: The total Vancouver Scar Scale score significantly reduced after treatment in all groups. The Vancouver Scar Scale score reduction rate was significantly higher after treatment in the group for which the treatment was initiated within 3 weeks of surgery. The pigmentation and erythema score analyzed by Antera 3D® was also lower in this group.
Conclusion: Early intervention using a pulsed dye laser within 3 weeks of thyroidectomy can substantially inhibit pathological scar development, providing physicians with a guide for optimal treatment commencement.
Keywords: hypertrophic scars, laser treatment of scar, scar, pulsed dye laser
Introduction
Surgical scars are a matter of concern for surgeons and patients. A cosmetic concern is not the only issue because pathological scars will likely accompany symptoms such as pruritus and pain. Pathologic scars include both hypertrophic scars and keloids. A hypertrophic scar is firm with elevated scars at the injury site and may cause pain.1 Excessive connective tissue differentiates the scar from a keloid, as the former is restricted to the area within the original wound, whereas the latter extends beyond the wound. Researchers have developed numerous therapies, including lasers, for treating hypertrophic scars.2 Pulsed dye laser (PDL) is one of the most widely used lasers for scar remodeling, reducing scar volume and height, and improving texture and pliability.3 PDL is commonly employed for both preventing and treating various scars including hypertrophic scar and burn scar.4 Early intervention using PDL before scar maturation, within the first-year post-injury, effectively prevents hypertrophic scar formation.5,6 This effect of PDL is based on its role as an angiogenesis regulator, which plays a crucial role in abnormal scar formation.7 Thus, the timing of initiating scar prevention therapy using PDL is critical. Here, we aimed to determine the appropriate time to initiate PDL after thyroidectomy for scar prevention.
Materials and Methods
Patients and Study Design
This single-center retrospective study was conducted at the Department of Dermatology, Ajou University Hospital, from June 2021 to October 2022. The study design was reviewed and approved by the Institutional Review Board of Ajou University Hospital (AJOUIRB-DB-2023-091) and was conducted in accordance with the Declaration of Helsinki. Informed consent was waived because of the retrospective nature of the study and the analysis used anonymous clinical data. The data of adults older than 19 years who underwent PDL treatment after thyroidectomy were reviewed retrospectively. The exclusion criteria were: patients with a history of keloid or hypertrophic scarring, a history of contact dermatitis during the treatment, and those who underwent initial PDL treatment 6 weeks after thyroidectomy (Figure S1 depicts patient selection flowchart). All participants were photographed at every visit. On the final visit, the Antera 3D® camera (Miravex Limited, Dublin, Ireland) was used to analyze the characteristics of the surgical scar.
Treatment Procedures
The patients received 595-nm PDL (V-beam, Candela Laser Corporation, Wayland, MA, USA) treatment at intervals of 3–4 weeks for 4–6 treatment sessions by a single dermatologist (YJP). Patients were generally recommended a follow-up period of 4 months or a minimum of 5 treatments. Nevertheless, if a patient refused the 5th treatment or requested an additional session, the physician evaluated the scar and made a decision to either discontinue or extend the treatment based on clinical assessment. Fluences ranging from 5.5 to 8.5 J/cm2 were applied using nonoverlapping 7-mm collimated spots and a laser pulse duration of 1.5–6 ms with cryogen cooling. The endpoint was set to erythema, not purpura. The pulse duration and fluence were changed according to the response to the treatment. YJP injected intralesional corticosteroids into patients with a firm plaque or a definite subcutaneous mass, corresponding to a pliability score higher than two on the Vancouver Scar Scale (VSS).
Scar Evaluation
An independent dermatologist (HSK), who captured high-resolution digital photographs at each visit, performed scar evaluation based on VSS. The VSS measures pigmentation (0 = normal, 1 = hypopigmented, and 2 = hyperpigmented), pliability (0 = normal, 1 = supple, 2 = yielding, 3 = firm, 4 = banding, and 5 = contracture), height (0 = flat, 1 <2 mm, 2 = 2–5 mm, and 3 >5 mm), and vascularity (0 = normal, 1 = pink, 2 = red, and 3 = purple), with a maximum total score of 13.8 Two independent dermatologists recorded the mean scores for each category, except for pliability. An Antera 3D® camera was used to assess the biophysical parameters of the scars by measuring the color variation, scar height, pigmentation (melanin), and vascularity (hemoglobin). The average value derived from two repetitive measurements made by Antera 3D® software was used.
Statistical Analyses
Data were statistically analyzed using R software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA, USA). Normally distributed data in the tables were expressed as the mean ± standard deviation, while not normally distributed data were expressed as median with interquartile range. Paired t-test or unpaired t-test was used for statistical analysis when the two groups of dependent variables were normally distributed, otherwise the Wilcoxon test was used. One-way analysis of variance was used for comparing more than three groups. All p-values less than 0.05 were considered statistically significant.
Results
Patient Demographics and Baseline Characteristics
A total of 91 patients who received PDL treatment after thyroidectomy were selected for retrospective analysis. We grouped them based on the time from surgery to PDL initiation in weeks (Figure S1 shows the patient selection flowchart). Table 1 summarizes their demographic and clinical characteristics. During the treatment, none of the patients complained of adverse events, such as a heating sensation, pain, or rash.
 |
Table 1 Demographic and Clinical Characteristics of the Study Population |
PDL Treatment Significantly Decreased Total VSS Scores Irrespective of the Initiation Timing
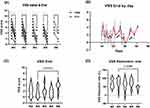
The total VSS score decreased significantly after PDL treatment in all patients who started PDL within 6 weeks, regardless of the initiation time (Figure 1A). We observed an increasing tendency of the total VSS score between weeks 3 (>21 days) and 4 (>28 days) to week 6 (blue arrows, Figure 1B). We observed a significantly lower final VSS score in the week 3 group than in the week 6 group (Figure 1C, p=0.0374). In contrast, there was no significant tendency or difference in the initial total VSS score (Figures S2A and S2B, (A) VSS score at the initiation of treatment by date and (B) VSS at the initiation of treatment grouped by the initiation week of therapy). The week 3 group also demonstrated a significantly greater reduction in VSS scores than the week 6 group (Figure 1D, p=0.0286).
PDL Treatment Initiated Within 3 Weeks of Surgery Significantly Reduced VSS Scores More Than That Initiated Between 4 and 6 Weeks
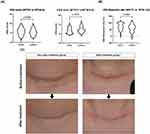
We observed a significant difference in the VSS score reduction between weeks 3 and 6 and a trend of increased VSS scores after weeks 3 and 4. This led us to hypothesize that initiating PDL treatment very early could potentially influence the cosmetic outcomes of hypertrophic scars. Consequently, we established the following two groups: (i) the very early treatment group, in which PDL treatment was initiated within 3 weeks post-surgery, and (ii) the early treatment group, in which the treatment was initiated between weeks 4 and 6 post-surgery. We observed no significant differences in baseline demographics between these groups (Table S1 provides a comparison of the demographic and clinical characteristics between the very early and early treatment groups). There was also no significant difference in total VSS scores at the start and the end of PDL treatment (Figure 2A). However, the reduction rate (calculated as (initial VSS – end VSS)/initial VSS * 100) of the total VSS score was significantly higher in the very early treatment group than in the early treatment group (Figure 2B, 71.30 ± 24.42 vs 59.68 ± 26.81, p=0.0363). A higher reduction rate suggests that the very early PDL treatment might lead to better cosmetic outcomes. Interestingly, no single category within the four VSS categories showed a significant difference when analyzed separately (Table 2). Taken together, our findings suggest that while a high reduction rate indicates that the very early PDL treatment might yield improved cosmetic outcomes, the better outcome is not attributable to a single category. Representative clinical photographs from each group are provided in Figure 2C.
 |
Table 2 Statistical Comparison of the Very Early Treatment Group and Early Treatment Group Using Vancouver Scar Scale Parameters |
ANTERA 3D-Assessed Erythema and Pigmentation Score Was Lower in the Very Early Treatment Group Than in the Early Treatment Group
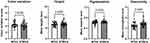
Antera 3D® cameras can quantify various aspects of scars, such as skin texture, scar height, pigmentation, and vascularity. Representative photographs are shown in Figure 3. In total, 53 patients were assessed using the 3D camera at the end of the sessions. The mean hemoglobin (Hb) level, which indicates vascularity and/or erythema of the lesion, was significantly lower in the very early treatment group compared to the early treatment group (Figure 4, 1.25 ± 0.25 vs 1.45 ± 0.28, p=0.0067). Additionally, the very early treatment group had a significantly reduced average melanin level, which is associated with the pigmentation of the lesion, compared to the early treatment. (Figure 4, 0.62 ± 0.06 vs 0.66 ± 0.63, p=0.0435). Other factors such as color variation, scar height, and variations in Hb and melanin levels were comparable between the groups (Figures 4 and S3).
PDL Treatment is Critical for Scar Prevention, Proving Effective Within Three Weeks, Regardless of TAILI Injections
We also observed the differences in VSS score according to triamcinolone intralesional injection (TAILI) to scar (Table S2). The group that received TAILI had significantly higher initial VSS scores (p=0.0046), indicating more severe scars initially. However, they experienced a smaller percentage improvement over time compared to the group who did not receive TAILI group (59.30±22.04 vs 75.11±26.80, p=0.0036). Measurements from the Antera® device revealed that scar color variation and scar height were significantly more pronounced in the TAILI received group (p=0.0094 and p=0.0080, respectively), with a trend towards increased vascularity score. This suggests a higher propensity for developing hypertrophic scars among these patients, potentially justifying the choice of TAILI treatment on them.
The analysis based on the timing of treatment indicated that those treated within the first three weeks (WTH3 group) saw a larger improvement from their baseline compared to those treated between four and six weeks (BTW4-6 group), regardless of TAILI injection (64.26±22.54 vs 50.43±18.69, p=0.059 in the TAILI (+) group; 78.58±22.18 vs 69.73±32.61, p=0.2540 in the TAILI (-) group), although the differences were not statistically significant. This was paralleled by a lower vascularity score in the WTH3 group, regardless of TAILI injection status (1.38±0.27 vs 1.47±0.26, p=0.4110 in the TAILI (+) group; 1.20±0.26 vs 1.42±0.32, p=0.0642 in the TAILI (-) group), again indicating the importance of early intervention.
Discussion and Conclusion
Early laser intervention before scar maturation effectively minimizes hypertrophic scar formation.5 Several studies have investigated the timing of initiating PDL treatment for scar prevention; however, the exact timing of treatment initiation remains highly variable, ranging from the date of stitch removal to within 3 months of surgery.9–12 In our study, treatment initiation within 3 weeks of surgery (the very early treatment group) generates better cosmetic outcomes than that within 4 to 6 weeks (the early treatment group), verified by the higher reduction rate of VSS scores. In addition, a quantitative analysis using the Antera 3D camera demonstrated a similar significant tendency, indicating lower erythema and pigmentation in scars following PDL treatment in the very early treatment group than in the early treatment group.
During wound healing, the inflammation phase (days 1–3) and proliferation phase (days 4–21) are central in preventing the formation of pathological scars.13 Pathological scar development is strongly associated with endothelial dysfunction in both phases of wound healing. Thus, inhibiting abnormal angiogenesis and vascular hyperpermeability in the early stages is a potential therapeutic approach for preventing scar formation.14 PDL targets the blood vessels critical for scar formation; therefore, very early intervention within 3 weeks may result in better cosmetic results, as indicated by the total VSS score and mean Hb level.4
The discrepancy between the VSS score and the Hb and pigmentation level determined by the Antera 3D® camera warrants some discussion. Although the scores of VSS vascularity and pigmentation category did not show a significant difference between the two groups, a notable difference was observed. Such difference could potentially arise from the discrepancy between human perception and the camera’s ability to detect erythema and pigmentation. Specifically, considering the lower vascularity in the very early treatment group compared to the early treatment group, it is suggested that the early initiation of PDL treatment before complete angiogenesis could lead to better cosmetic result in reducing erythema. On the other hand, a reduction in pliability score, which indicates increased softness, was higher in the very early group compared to the early group, despite not being statistically significant (Table 2, p = 0.055). Vascularity, pigmentation, and pliability are crucial factors for detecting early scar formation. These findings suggest that while the human eye might not detect early hypertrophic scar formation, tactile sensations may be more sensitive to it. However, given that not all cases were assessed using the 3D camera, we are cautious in drawing firm conclusions about the correlation between the VSS vascularity score and the camera’s vascularity score.
Subpurpuric fluence significantly improves the outcome of surgical scar treatment without causing any adverse effects beginning on the day of suture removal.9 Nonpurpuric settings of 595-nm PDL significantly improve the appearance of fresh surgical scars along with reduced pain compared to purpuric settings.15 Consistent with previous studies, our treatment protocol with nonpurpuric settings demonstrated a significant reduction in VSS score regardless of its initiation timing. None of the patients complained of persistent erythema, purpura, or pain. Nonpurpuric parameters of PDL may be considered for preventing hypertrophic scarring in early treatment initiated within 3 weeks of surgery.
Our study did have several limitations, primarily due to its retrospective design. First, the laser parameters, such as pulse duration and fluence, were not standardized. Second, despite the effectiveness of intralesional steroid injections in the prevention and treatment of hypertrophic scars, we did not assess this factor while comparing the groups. However, to mitigate these confounding effects, we only included patients who received PDL from a single dermatologist. As a result, we believe all patients were treated by similar standards. Additionally, patients who received TAILI exhibited high initial VSS and vascularity scores as measured by Antera 3D camera, suggesting a greater risk of hypertrophic scar development, potentially justifying the choice of TAILI treatment. Third, we did not consider variable surgical factors, including lesion size, the operating surgeon, and the stage of thyroid cancer. Lastly, the duration of the follow-up was relatively short, and we did not assess the scars over an extended period. Moreover, the study’s design precluded us from incorporating other important measures like patient satisfaction. To overcome these limitations and deepen our understanding, we are planning a prospective study that will provide a thorough assessment of treatment effects, including quality of life aspects. This future study aims to deliver a richer, more detailed exploration of the impact of scar treatments, focusing on both physical outcomes and their influence on patients’ overall well-being.
In conclusion, early intervention using PDL within 3 weeks of thyroidectomy may significantly prevent the formation of pathological scars, resulting in a better cosmetic outcome than a slightly delayed PDL treatment. Notably, the application of PDL with a nonpurpuric setting during early intervention is both safe and well received by patients, suggesting that physicians could consider immediate PDL treatment for enhanced cosmetic results. While these findings are promising, further prospective studies are necessary to validate these results. Particularly, in patients with high VSS scores within the initial 3 weeks, immediate initiation of PDL treatment may yield superior cosmetic results, emphasizing the potential benefits of timely intervention.
Acknowledgments
The authors appreciate all the individuals who participated in these studies and all the researchers and clinicians who enabled this work to be carried out.
Funding
This study was supported by the 2022 Amorepacific grant.
Disclosure
The authors have no conflicts of interest to disclose.
References
1. Rabello FB, Souza CD, Farina Júnior JA. Update on hypertrophic scar treatment. Clinics. 2014;69(8):565–573. doi:10.6061/clinics/2014(08)11
2. Elsaie ML. Update on management of keloid and hypertrophic scars: a systemic review. J Cosmet Dermatol. 2021;20(9):2729–2738. doi:10.1111/jocd.14310
3. Chowdhury B, Kassir M, Salas-Alanis J, et al. Laser in surgical scar clearance: an update review. J Cosmet Dermatol. 2021;20(12):3808–3811. doi:10.1111/jocd.14325
4. Parrett BM, Donelan MB. Pulsed dye laser in burn scars: current concepts and future directions. Burns. 2010;36(4):443–449. doi:10.1016/j.burns.2009.08.015
5. Karmisholt KE, Haerskjold A, Karlsmark T, Waibel J, Paasch U, Haedersdal M. Early laser intervention to reduce scar formation - a systematic review. J Eur Acad Dermatol Venereol. 2018;32(7):1099–1110. doi:10.1111/jdv.14856
6. Deng H, Tan T, Luo G, Tan J, Li-Tsang CWP. Vascularity and Thickness Changes in Immature Hypertrophic Scars Treated With a Pulsed Dye Laser. Lasers Surg Med. 2020. doi:10.1002/lsm.23366
7. Nischwitz SP, Lumenta DB, Spendel S, Kamolz LP. Minimally Invasive Technologies for Treatment of HTS and Keloids: pulsed-Dye Laser. In: Téot L, Mustoe TA, Middelkoop E, Gauglitz GG, editors. Textbook on Scar Management: State of the Art Management and Emerging Technologies. Cham (CH): Springer; 2020:263–269.
8. Baryza MJ, Baryza GA. The Vancouver Scar Scale: an administration tool and its interrater reliability. J Burn Care Res. 1995;16(5):535–538. doi:10.1097/00004630-199509000-00013
9. Nouri K, Jimenez GP, Harrison‐Balestra C, Elgart GW. 585‐nm pulsed dye laser in the treatment of surgical scars starting on the suture removal day. Dermatologic Surg. 2003;29(1):65–73. doi:10.1046/j.1524-4725.2003.29014.x
10. Lee Y, Kim W. Combination laser treatment for immediate post-surgical scars: a retrospective analysis of 33 immature scars. Lasers Med Sci. 2017;32(5):1111–1119. doi:10.1007/s10103-017-2215-9
11. McCraw JB, McCraw JA, McMellin A, Bettencourt N. Prevention of unfavorable scars using early pulse dye laser treatments: a preliminary report. Ann Plastic Surgery. 1999;42(1):7–14. doi:10.1097/00000637-199901000-00002
12. Brewin M, Docherty S, Heaslip V, et al. Early Laser for Burn Scars (ELABS): protocol for a multi-centre randomised, controlled trial of both the effectiveness and cost-effectiveness of the treatment of hypertrophic burn scars with Pulsed Dye Laser and standard care compared to standard care alone. NIHR Open Res. 2022;2:1.
13. Profyris C, Tziotzios C, Do Vale I. Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J Am Acad Dermatol. 2012;66(1):1–10; quiz 1–2. doi:10.1016/j.jaad.2011.05.055
14. Ogawa R, Akaishi S. Endothelial dysfunction may play a key role in keloid and hypertrophic scar pathogenesis–keloids and hypertrophic scars may be vascular disorders. Med Hypotheses. 2016;96:51–60. doi:10.1016/j.mehy.2016.09.024
15. Gladsjo JA, Jiang SIB. Treatment of surgical scars using a 595‐nm pulsed dye laser using purpuric and nonpurpuric parameters: a comparative study. Dermatologic Surg. 2014;40(2):118–126. doi:10.1111/dsu.12406
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Clinical Observation of Sequential Laser Therapy Combined with Tension Reducer for Postoperative Tension Incision Scar Growth
Wang TY, Ye ZQ, Xie W
Clinical, Cosmetic and Investigational Dermatology 2023, 16:59-65
Published Date: 7 January 2023