Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 16
VEGF Expression in Umbilical Cord MSC Depends on the Patient’s Health, the Week of Pregnancy in Which the Delivery Took Place, and the Body Weight of the Newborn – Preliminary Report
Authors Bieńko K , Leszcz M , Więckowska M , Białek J , Petniak A, Szymanowski R, Wilińska A, Piszcz B, Krzyżanowski A, Kwaśniewska A, Płachno BJ , Gil-Kulik P , Kocki J
Received 7 December 2022
Accepted for publication 15 February 2023
Published 27 April 2023 Volume 2023:16 Pages 5—18
DOI https://doi.org/10.2147/SCCAA.S399303
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Karolina Bieńko,1 Monika Leszcz,1 Marta Więckowska,1 Justyna Białek,1 Alicja Petniak,2 Rafał Szymanowski,2 Agnieszka Wilińska,2 Bartosz Piszcz,3,4 Arkadiusz Krzyżanowski,3 Anna Kwaśniewska,3 Bartosz J Płachno,5 Paulina Gil-Kulik,2 Janusz Kocki2
1Student Scientific Society of Clinical Genetics, Medical University of Lublin, Lublin, Poland; 2Department of Clinical Genetics, Medical University of Lublin, Lublin, Poland; 3Department of Obstetrics and Pathology of Pregnancy, Medical University of Lublin, Lublin, Poland; 4Doctoral School, Medical University of Lublin, Lublin, Poland; 5Department of Plant Cytology and Embryology, Institute of Botany, Faculty of Biology, Jagiellonian University, Kraków, Poland
Correspondence: Paulina Gil-Kulik, Department of Clinical Genetics, Medical University of Lublin, 11 Radziwillowska Str., Lublin, 20-080, Poland, Email [email protected]
Introduction: Cells collected from Wharton’s jelly are a rich source of mesenchymal stem cells. They can be easily obtained and grown using the adhesive method. They produce many types of proteins, including VEGF. Their role is to participate in angiogenesis, vasodilation, stimulation of cells to migrate, and chemotactic activity. The aim of this study was to evaluate expression of genes from the vascular endothelial growth factor family: VEGFA, VEGFB and VEGFC in MSC and the analysis of dependence of the expression of the studied genes on clinical factors related to the course of pregnancy and childbirth, and health of mother and child.
Material and Methods: The research material was an umbilical cord obtained from 40 patients hospitalized in the Department of Obstetrics and Pathology of Pregnancy of the Independent Public Clinical Hospital No.1 in Lublin. The age of the women was 21– 46, all gave birth by cesarean section. Some of the patients suffered from hypertension and hypothyroidism. Material collected from patients immediately after delivery was subjected to enzymatic digestion with type I collagenase. The isolated cells were then cultured in adherent conditions, and then gene expression was assessed using qPCR and the immunophenotype of the cells was assessed cytometrically.
Results: Conducted studies have shown significant differences in expression of VEGF family genes depending on clinical condition of mother and child. Significant differences in VEGF-family gene expression level in umbilical cord MSC collected from women with hypothyroidism, hypertension, time of labor and birth weight of the baby were shown.
Conclusion: Probably due to hypoxia (caused, for example, by hypothyroidism or hypertension), the MSCs found in the umbilical cord may react with an increased expression of VEGF and a compensatory increase in the amount of secreted factor, the aim of which is, i.a., vasodilation and increase of blood supply to the fetus through the umbilical vessels.
Keywords: VEGF, mesenchymal stem cells, Wharton’s jelly, angiogenesis
Introduction
MSC are non-specialized cells in the human body,1 showing the following characteristics: the possibility of long-term self-renewal and differentiation into any cell lines in the human body,1,2 at least to chondrogenic, osteogenic, and adipogenic lines.3 MSCs are present in the majority adult organism tissues.4,5 Typically, main source of MSC was the bone marrow, however due to inconveniences of its sourcing, its use is limited. At present, MSC can be isolated from a lot of tissues, especially from adipose tissue and perinatal tissues – umbilical cord, umbilical cord blood. Population of MSC is usually isolated using tissue homogenisation or enzymatical digestion and growing cells on plastic dish. Derived cells are then placed in culture dish containing growth medium.1,6
Our research was conducted on MSC derived from umbilical cord Wharton’s jelly, other sources confirm that it is more abundant source of MSC than umbilical cord blood.7–10 Obtaining MSC from Wharton’s jelly is characterized by easy availability, simple isolation method, high proliferation index, and good tolerance in allogeneic transplantation. WJ-MSC present expression of surface markers specific for stem cells, and also produce significant amounts of tissue growth promoting factors, such as vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), insulin-like growth factor-I and IL-8, interleukin (IL) – 6. These factors have a significant influence on apoptosis, cells migrations and capillary rebuilding.4
MSCs isolated from the umbilical cord may have higher differentiation and stem properties than other traditional adult sources. Studies show that the use of MSC in the umbilical cord, among other things, reduces the expression of pro-inflammatory cytokines, inhibits neuronal apoptosis, reduces inflammation and increases regenerative capacity. There are high hopes for the use of MSCs derived from the umbilical cord, among others, in the treatment of ischemic stroke or myocardial damage.11–13
However, there is still a lack of studies evaluating how clinical parameters related to the course of pregnancy and delivery affect the secretory properties of mesenchymal stem cells of the umbilical cord.
Using flow cytometry allowing to evaluate cultured cells phenotype is crucial in studies on stem cells.10,14,15 Human MSC can be identified based on the expression of a panel of surface markers defined by the International Society for Cell Therapy:3,4 CD105+, CD90+, CD73+, CD14-, CD34-, HLA-DR-3,6 or CD19-,3 CD45-,6 CD11b-,3,16 PE-.16 Cell viability can be determined using annexin V and propidium iodide (PI). PI does not stain living cells and early-apoptotic. Ki-67 protein is used as a cell proliferation marker.17–19
Vascular endothelial growth factor (VEGF) is a family of polypeptides that includes VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PlGF).20 VEGF expression is detected in many body cells and in cancer cells. VEGF shows paracrine and autocrine properties and can act intracellularly, not secreting to the extracellular space, participating in the regulation of the cell-cycle and metabolism of cells. VEGF has strong proangiogenic factors, has significant effects on tissue vascularization. The most well-known molecule of the VEGF family is VEGF-A. Its functions are vasodilation, participation in angiogenesis and immune mechanisms. It affects T lymphocytes and dendritic cells. It can regulate the migration of nerve cells.21,22 VEGF-B differs significantly from VEGF-A because its main feature is antioxidant and only slight angiogenic activity.23 Increased VEGF values are found in many diseases, including cancer or diabetic retinopathy. The association of diseases with abnormal VEGF levels allowed for the creation of monoclonal antibodies that block the action of the vascular endothelial growth factor and thus enable the treatment of patients.21 Placental Growth Factor (PlGF) functions differently than other factors. It is significant in disease states related to ischemia or tumors. Elevated values are helpful in the diagnosis of pre-eclampsia. Contrary to VEGF-A, it is not necessary for the proper development of the organism.24
In our research, cultured cells are MSC, significant differences in VEGF-family gene expression levels in women with, hypertension, hypothyroidism, time of labor and birth weight of the baby were shown. Abnormal VEGF expression may be related to clinical factors of maternal origin, such as hypertension, gestational diabetes, pre-eclampsia and preterm childbirth. Assessment of VEGF expression in MSC may be important in the future for predicting or even preventing preterm childbirth or IUGR. Potential of MSC may be also considered for using in the processes of therapeutic angiogenesis, eg, in the treatment of ischemia of the heart muscle or other organs. So far, we have a very small amount of studies on this subject. In our research, we marked the expression of three genes from the VEGF family, which is a new approach to this issue.
The aim of this study was to evaluate the expression of genes from the vascular endothelial growth factor family: VEGFA, VEGFB and VEGFC in human MSC and to analyze the dependence of gene expression on clinical factors related to the course of pregnancy and childbirth, and health of mother and child.
Materials and Methods
The study was conducted on a group of 40 patients hospitalized in the Department of Obstetrics and Pathology of Pregnancy of the Independent Public Clinical Hospital No. 1 in Lublin. The age of the patients ranged from 21 to 46 years. Immediately after delivery, umbilical cord was collected from the patients, from which mesenchymal stem cells were then isolated.
All examined patients gave birth by Caesarean Section, for 17 patients it was the first delivery, 23 patients gave birth again. The women gave birth to 20 boys and 20 girls.
The average gestation week, in which the child was born, is 38 weeks, 9 babies were born prematurely, before the 37th week of pregnancy. Six patients were diagnosed with arterial hypertension in pregnancy, 13 patients with gestational diabetes, and 14 patients with hypothyroidism. Fourteen patients were classified as “healthy”, did not have any comorbidities and gave birth to healthy babies on time.
The collection of the material and the analysis were performed according to the protocol approved by the Bioethics Committee at the Medical University of Lublin no. KE-0254/128/2014. All methods were carried out in accordance with relevant guidelines and regulations. Each patient gave her written consent to collect the material and conduct the study. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Bioethics Committee at the Medical University of Lublin.
The characteristics of the study group are presented in Table 1.
 |
Table 1 The Characteristics of the Study Group |
MSC Isolation and Cell Culture
An enzymatic method using collagenase type I (Gibco by Life Technologies, USA Grand Island, NY) was used to isolate umbilical cord stem cells. The material collected from the patients immediately after delivery was placed in the culture medium, and then the umbilical cord was cut into smaller pieces. The cut pieces were placed in a type I collagenase solution and digested 2–3 h at 37°C. Then, it was rinsed with PBS solution and filtered. Cells isolated from the umbilical cord were grown up to 14 days under appropriate conditions (37°C, 15% O2, 5% CO2) in a culture medium (DMEM/F-12 Corning, USA, Manassas, VA) supplemented with 10% fetal serum (FBS, ATCC, UK Teddington) and 1% antibiotics (Penicilin-Streptomycin, Sigma-Aldrich, Israel). Isolation and cell culture procedure described in the paper.25 The cultured cells were then used for cytometric analysis and RNA isolation.
Cytometric Analysis
To assess the MSC phenotype, cytometric analysis was performed using Dura clone SC Mesenchymal Tube antibodies (Beckman Coulter, India, Bangalore, Karnataka). DURA Clone MSC tubes contain lyophilisate of a panel of 9 monoclonal antibodies (CD90-FITC, CD73-PE, CD34-ECD, CD146-PC5.5, CD105-PC7, CD45-APC-AF750, CD31-Pacific Blue, CD14-Krome Orange, CD19-Krome Orange) dedicated to the determination of characteristic MSC antigens.10,26,27 Cell proliferation was assessed using the anti Ki67-PE eBioscience antibody (Invitrogen by Life Technologies, USA, Carlsbad, CA) and viability of the cultured cells using propidium iodide and annexin V. Staining with annexin-V, which can bind to phosphatidylserine on the surface of apoptotic cells, was used as a marker of apoptosis. Annexin V was detected by flow cytometry in FITC and PI channels. For this purpose, a commercially available annexin-V staining detection kit was used to measure apoptosis and cell viability (Invitrogen, USA, Eugene, Oregon). MSC immunophenotyping was performed on a Navios flow cytometry (BeckmanCoulter). The cytometric analysis procedure of Ki67 and annexin V is described in the work Fathi et al.28
Gene Expression Analysis
RNA was isolated from cells by the modified method of Chomczyński and Sacchi29 using the TRIzol from Invitrogen (by Thermo Fisher Scientific, USA). 0.5 mL of TRI was added to a defined portion of cells after culture, then homogenized and incubated for 5 minutes at room temperature. Subsequently, 0.1mL of chloroform was added and shaken for 10 seconds, then incubated for 15 minutes at room temperature, then centrifuged for 15 minutes at 4 degrees Celsius. After centrifugation, the aqueous phase was collected and 0.25 mL of isopropanol was added thereto, then mixed and incubated at room temperature for 20 minutes. It was then centrifuged for 20 minutes at 4 degrees Celsius. After centrifugation, the precipitate was purified in 75% ethyl alcohol and then dissolved in ultrapure RNAase-free water. Properly prepared RNA samples were subjected to qualitative and quantitative analysis. The extracted RNA was measured by NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, USA). For all samples analyzed, A260/A280 ratio was between 1.8 and 2.0.
The next step was to perform a reverse transcription reaction using a commercial High Capacity cDNA Re-verse Transcription Kits (Applied Biosystems by Thermo Fisher Scientific, Lithuania). Approximately 1 µg total RNA was used for cDNA synthesis. The reverse transcription reaction was carried out in a volume of 20 μL, consisting of: 2 μL (10xRT Buffer), 0.8 μL (10xdNTPs (100 mM)), 2 μL (10xRT Random Primer), 1 μL (RNasin 40 U/μL), 1 μL (Reverse transcriptase 50 U/μL), 3.2 μL (RNAz- and DNAz-free ultrapure water), and 10 μL (1 μg RNA dissolved in 10 μL ultrapure water). The reaction was carried out in a thermal cycler (Verit Thermal Cycler, Life Technologies); initially, the reaction mixture was incubated for 10 min at 25°C, then for 2 h at 37°C, followed by 5 min at 95°C. The resulting cDNA was used for real-time PCR.
Expression of VEGFA, VEGFB and VEGFC genes was investigated using the qPCR technique using commercially available TaqMan probes (VEGFA: Assay ID: Hs00900055_m1, Refseq; NM_001025366; VEGFB: Assay ID: Hs00173634_m1, Refseq; NM_001243733.1; VEGFC: Assay ID: Hs00153458_m1, Refseq; NM_005429.4; GAPDH: Assay ID: Hs99999905_m1, Refseq; NM_001289746.1, endogenous control), as a protocol the manufacturer (Applied Biosystems, USA).
The reactions were performed in MicroAmp Fast Optical 96-Well Reaction Plate 0.1 mL (Applied Biosystems, USA) in a volume of 10 μL/well, consisting of 4.5 μL cDNA synthesized in the reverse transcription reaction with RNAz- and DNAz-free ultrapure water, 0.5 μL gene-specific probe, and 5 μL TaqMan Gene Expression Master Mix (Applied Biosystems, USA). The real-time PCR reaction, after the initial 10-minute denaturation at 95°C, was carried out according to the following scheme—40 cycles: 15 seconds at 95°C and 60 seconds at 60°C. Expression of mRNA was detected using StepOnePlus System (Applied Biosystems). The level of relative expression was assessed according to the Livak formula RQ = 2−ddCt.30 To normalize the expression of test genes, the CT value against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reference gene was determined for each sample. Final gene expression was determined against the sample used to calibrate the entire experiment. The procedure for RNA isolation, reverse transcription reaction and gene expression assessment is described in the works of Gil-Kulik et al.27,31–34
Statistical Analysis
Non-parametric tests were used to test the differences between the studied samples, due to the lack of a normal distribution, the differences between the study groups were assessed using the Mann Whitney U-Test, the correlation analysis was performed using the Spearman’s test, the significance level was p < 0.05.
Results
Cell Culture and Cytometric Analysis
Mesenchymal stem cells (MSC) were isolated from the umbilical cord by digestion in a type I collagenase solution, and the cells were then cultured under adherent conditions for a period of 10–14 days. During the cell culture, the ability of the tested cells to adherence to the plastic walls was confirmed, a fibroblast-like shape of the analyzed cells was also observed (Figure 1).
 |
Figure 1 Mesenchymal stem cells from a 4-day culture bright field microscopy (BF), 200× magnification, using Xcellence RT system with an IX81 inverted microscope (Olympus). |
After the cultivation period, cells were cytometrically analyzed for the presence of surface antigens characteristic of MSC, including: CD73, CD90, CD105, CD146 (Figure 2); the cell viability analysis was performed using propidium iodide and annexin V (Figure 3), the proliferation potential of cells was determined using Ki67 (Figure 4), and the cell cycle phase analysis was also performed (Figure 5).
 |
Figure 2 Cytometric evaluation of the presence of CD146, CD195, CD90, CD73 surface antigens on the analyzed MSCs. Navios Cytometer (Beckman Coulter). |
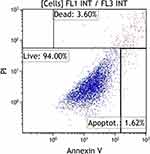 |
Figure 3 Cytometric evaluation of cell viability using propidium iodide and annexin V. Navios cytometer (Beckman Coulter). |
 |
Figure 4 Cytometric evaluation of cell proliferation using Ki67 in analyzed MSCs. Navios Cytometer (Beckman Coulter). |
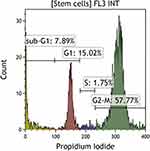 |
Figure 5 Cytometric evaluation of the cell cycle phases of the analyzed MSCs. Navios Cytometer (Beckman Coulter). |
Based on the analyzes carried out so far, it has been shown that over 91% of mesenchymal stem cells are present in the population after cell culture. Mean cell viability during cytometric analysis was 92 ± 2.1%. Mean 72 ± 5.8% of cells are positive for Ki67. The analysis of the phases of the cell cycle showed that, on average, approx. 8% was in the sub-G1 phase, approx. 15% in the G1 phase, approx. 4% in the S phase, approx. 57% in the G2-M phase.
Molecular Analysis
Our study showed that umbilical cord mesenchymal stem cells, at the mRNA level, express genes encoding the vascular endothelial growth factor family: VEGFA, VEGFB and VEGFC.
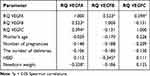
The study analyzes the dependence of the expression of the studied genes on factors re-lated to pregnancy and childbirth, such as: the number of pregnancies and deliveries, the woman’s age, the week of pregnancy in which the delivery took place, the body weight of the newborn, as well as comorbidities in the woman giving birth. Positive relationships were observed between the expression level of the studied genes: VEGFA with VEGFB (r = 0.523 p < 0.05), VEGFA with VEGFC (r = 0.394 p < 0.05) (Table 2).
There was no significant relationship between the expression of VEGFA, VEGFB and VEGFC genes with the maternal age, number of pregnancies and deliveries, and the sex of the newborn.
It was observed that VEGFA gene expression significantly negatively correlated with the body weight of the newborn (r = −0.328 p < 0.05) (Figure 6), the higher the body weight of the newborn, the lower the levels of VEGFA in the umbilical cord MSC were observed (Table 2).
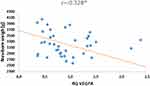 |
Figure 6 Graph of distribution newborn weight and VEGFA gene expression. *p < 0.05 Spearman correlations. |
The analysis of the dependence of the expression of genes under study on comorbidities showed significant differences.
Analyzing the dependence of the expression of genes from the time of delivery, it was shown that the expression of the VEGFB gene is statistically significantly higher in MSCs taken from preterm deliveries compared to full term deliveries (p = 0.001) (Figure 7). Correlation analysis showed a significant and moderate negative correlation between the level of VEGFB expression and the week of pregnancy (HBD) (r = −0.345 p < 0.05), the later the delivery, the lower the expression of the VEGFB gene in MSC was observed (Table 2. Figure 8). Expression of VEGFA and VEGFC genes did not show any significant correlation with the date of delivery.
 |
Figure 7 Mean (RQ±SE) expression VEGFB gene in MSC, depending on the time of childbirth, *p < 0.05 U Mann Whitney Test. |
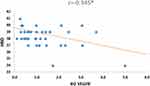 |
Figure 8 Graph of distribution HBD and VEGFB gene expression. *p < 0.05 Spearman correlations. |
The analysis of the relationship between the expression of studied genes in the MSC of the umbilical cord and the presence of hypertension in women showed a statistically significantly higher expression of VEGFA (p = 0.004) and VEGFC (p = 0.001) in mesenchymal stem cells taken from the umbilical cord obtained from women diagnosed with hypertension compared to healthy women (Figure 9). There was no significant relationship between the expression level of the VEGFB gene in MSC and the presence of hypertension.
 |
Figure 9 Mean (RQ±SE) expression of VEGFA and VEGFC genes in MSC, depending on the prevalence of hypertension in pregnant women, *p < 0.05 U Mann Whitney Test. |
The analysis of the relationship between the expression of genes in the examined patients and the occurrence of hypothyroidism in the studied patients showed a statistically significantly higher expression of the VEGFA gene in the MSC of the umbilical cord collected in patients with hypothyroidism compared to healthy patients (p = 0.018) (Figure 10).
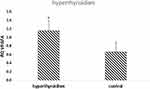 |
Figure 10 Mean (RQ±SE) expression VEGFA gene in MSC, depending on prevalence of hyperthyroidism, *p < 0.05 U Mann Whitney Test. |
There were no significant differences in the expression of tested genes depending on the prevalence of gestational diabetes in patients.
Discussion
The conducted studies have shown that human umbilical cord MSC exhibit expression of genes encoding the family of vascular endothelial growth factors: VEGFA, VEGFB and VEGFC. Moreover, the conducted analyzes have shown that this expression depends on the patient’s health condition, as well as on the week of pregnancy in which the child was born and the body weight of the newborn. In our study, we observed that VEGFA expression in MSC of the umbilical cord collected from patients with hypothyroidism, is significantly higher compared to MSC collected from healthy patients.
The other scientists studies indicate that VEGF-A is an important factor in the proliferation of vascular cells as it is involved in physiological processes as well as in pathological neovascularization. VEGF expression is affected by the local oxygen concentration; therefore, vascular remodeling occurs under hypoxic conditions.35
Elevated VEGF levels are detected in patients who have not been treated for Hashimoto's and Graves-Basedov disease. The relationship was positively correlated with thyroid vascularization.36 Normal thyroid cells show minimal VEGF expression, which may be increased upon stimulation by increased TSH levels. Studies have shown increased VEGF expression in thyroiditis and thyroid cancer. Moreover, elevated VEGF values have been associated with metabolic disorders, type 2 diabetes and polycystic ovary syndrome.37 Studies on trabecular bone derived mesenchymal stem cells (hTBC) showed increased expression of VEGF-A and VEGFR-1 mRNA during osteogenesis and hypoxia.38 Hypothyroidism in pregnant women may be associated with a lower oxygen supply for the fetus and, consequently, an increase in VEGF-A expression. These results are consistent with the cited studies, thanks to the compensatory mechanisms by increasing the amount of VEGF-A in cells, there is an increase in oxygen supply to cells, the mechanism of vasodilation or increased angiogenesis. Thyroid hormones are a bioactive element that supports the survival of human pluripotent stem cells. Clinically, hormone activity may influence VEGF expression to ensure proper angiogenesis for developing tissues. Other studies prove that the influence of thyroid hormones on VEGF expression is related not so much to the function of the hormones as to the amount of endocrine-active tissue.39,40
In our study, we observed that the expression of VEGFA and VEGFC genes in the MSC of the umbilical cord collected from hypertensive women is statistically significantly higher compared to healthy women. A team of researchers from India assessed the expression of VEGF in umbilical cord tissues depending on the severity of hypertension. In healthy and mild gestational hypertension patients, the staining intensity was lower than in severe pre-eclampsia. Our studies confirm that there is an increase in the expression of VEGFA and VEGFC at the mRNA level in MSC in patients with hypertension. Increasing the amount of VEGF in tissues may be a compensatory mechanism related to hypoxia. Untreated HT in pregnancy may lead to underdevelopment of the placenta, early detachment of placenta, and defects of the umbilical vessels and blood flow. As a result of increasing VEGF, it is possible, i.a., dilatation of blood vessels, which grants better blood supply to the fetus.41
Our research showed that along with increasing weight of the newborn the expression of VEGFA was decreasing. One of the possible cause of IUGR is insufficient blood perfusion in the placenta. The child is not provided with the right amount of nutrients for proper growth. Our research showed that the lower the birth weight of the baby, the higher VEGFA expression. Perhaps, it is due to an increase in the development and remodeling of blood vessels in the placenta. This is to increase blood flow to the developing fetus. Studies of local VEGF expression indicate that it has a positive effect on fetal growth in the case of diagnosed IUGR.42 Almasry et al evaluated umbilical cords from patients with PE and healthy ones in terms of morphology and immunohistochemistry. In patients diagnosed with pre-eclampsia, VEGF immunoexpression was higher than in the control group. Increased levels of expression were also observed in arteries than in umbilical veins. In our study, umbilical cord MSC expression was also higher in patients with preeclampsia. Elevated VEGF levels can be explained by 2 theories. The first says that as a result of hypoxia, cells contained in the umbilical cord secrete VEGF and other substances that cause vascular proliferation and compensatory changes due to increased pressure in the umbilical vessels. The second concept is that elevated VEGF levels may contribute to the development of hypertension. Elevated levels of VEGF-A have been found in the placentas of IUGR patients. However, VEGF-A levels did not correlate with the degree of intrauterine growth retardation. Our study showed a negative correlation between birth weight and VEGF-A expression level. The lower the child’s weight, the higher the VEGF levels.43,44
Preterm babies had lower birth weight, which was associated with an increase in VEGF-A expression. Research and clinical case reports show that premature babies are more likely to develop retinopathy of premature infants (ROP). However, there are no studies that would indicate a correlation between the vascular endothelial growth factor derived from Wharton’s jelly MSC and the development of this disease. So far, only the relationship between disease development and an increase in VEGF derived from retinal cells secreted in response to neuronal hypoxia has been demonstrated.45
Several scientific studies have shown that VEGF levels are higher in premature babies than in full-term babies. T. Kaukola showed high VEGF values in studies on the development of the nervous system of premature babies with cardiovascular disorders and placental insufficiency. Tissue hypoxia, probably caused by a decreased cardiac output and an increase in venous pressure, triggered compensatory processes in the form of an increase in VEGF expression. On the other hand, Krukier in his research observed a decrease in VEGF expression both in the umbilical cord and placenta during full term pregnancy. Our reports confirm an increase in VEGFB in pre-term deliveries.46–48
The effect of hypoxia on mouse mesenchymal stem cells cultured in a medium supplemented with 5% and 20% mouse serum was assessed. The researchers found an increase in VEGF expression in the medium with 5% mouse serum under hypoxic conditions, which proves that VEGF expression may vary depending on environmental factors such as oxygen concentration or the amount of serum in the medium.49 The presence of VEGF affects the survival of cells. VEGF-deficient haematopoietic stem cells showed decrease in survival, repopulation, and colony formation.22 Also, Chinnici in their research show that activation of VEGF-A derived from MSC can stimulate the process of endogenous angiogenesis.50
Fathi et al studied the effect of MSC on the telomerase activity of KG1 AML cells. In their research, they used a control group, which was a bone marrow cell culture, and a research group, which was a culture of MSCs obtained from adipose tissue with bone marrow cells (it was a co-culture). Thanks to this culture, they obtained a decrease in the expression of ERK proteins, a decrease in telomerase activity and telomere length as well as hTERT gene expression in leukemic cells, and thus a decrease in AML cell proliferation. For subsequent studies, they used a different type of cells, these were CML cells of the K562 line, which were cultured alone as a control group, and BMSC (bone marrow mesenchymal stem cells) were used for the research group. The co-culture also inhibited the proliferation of the CML cell line by reducing the expression of ERK proteins. It can be assumed that MSCs inhibit the proliferation of cancer cells, it is worth assessing whether MSCs from other sources, such as Wharton’s jelly, also have such properties. This phenomenon is probably due to the production of cytokines and growth factors.51,52
Conclusions
Hypothyroidism in pregnant women may be associated with hypoxia and fetal hypoxia. On this basis, we can assume that, due to hypoxia, the MSCs found in the umbilical cord may react with an increased expression of VEGF and a compensatory increase in the amount of secreted factor, the aim of which is, i.a., vasodilation and increase of blood supply to the fetus through the umbilical vessels. Hypertension in pregnant women may lead to abnormalities in blood flow through the placenta. The body tries to correct these deficiencies at the cellular level, probably by increasing VEGFA and VEGFC expression.
Factors such as prematurity and low birth weight are, in most cases, related to each other, and here we also observe an increase of the expression of proteins from the VEGF family. In these children, it is possible that compensatory mechanisms must work, due to insufficient oxygen being delivered to the organs. Lower baby weight may be associated with earlier delivery due to fetal hypoxia, placental pathology or maternal disease.
Reports give a new look at MSCs, in the future using the properties of these cells for therapeutic angiogenesis in wound healing or ischemic organ diseases in connection with VEGF expression is worth considering. However, the ability to neovascularize cells depending on VEGF expression was not assessed in this preliminary study and must be confirmed in our subsequent analyzes. In the future, it is worth expanding the research with the method of co-culture of MSCs from Wharton's jelly with cancer cell lines in order to compare the properties that inhibit the development of cancer cells.
Abbreviations
DMEM, Dulbecco’s Modified Eagle Medium; FBS, Fetal Bovine Serum; IUGR, Intrauterine Growth Restriction; MSCs, mesenchymal stem cells; PDGF, platelet-derived growth factor; PE, preeclampsia; PlGF, placental growth factor; RQ, relative quantification; TGF-β, transforming growth factor beta; WJ-MSC, Mesenchymal stem cells derived from Wharton jelly; VEGF, vascular endothelial growth factor.
Funding
This study was supported in part of project “Analysis of the phenotype of Wharton jelly mesenchymal stem cells in correlation with clinical data on the course of pregnancy and labor” realize in 2021–2022, financed by Medical University of Lublin, project leader: Karolina Bieńko.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68. doi:10.1186/s13287-019-1165-5
2. Mens MMJ, Ghanbari M. Cell cycle regulation of stem cells by microRNAs. Stem Cell Rev Rep. 2018;14(3):309–322. doi:10.1007/s12015-018-9808-y
3. Uder C, Brückner S, Winkler S, Tautenhahn HM, Christ B. Mammalian MSC from selected species: features and applications. Cytometry A. 2018;93(1):32–49. doi:10.1002/cyto.a.23239
4. Bandekar M, Maurya DK, Sharma D, Sandur SK. Preclinical studies and clinical prospects of Wharton’s Jelly-derived MSC for treatment of acute radiation syndrome. Curr Stem Cell Rep. 2021;7(2):85–94. doi:10.1007/s40778-021-00188-4
5. Sobhani A, Khanlarkhani N, Baazm M, et al. Multipotent stem cell and current application. Acta Med Iran. 2017;55(1):6–23.
6. Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93(1):19–31. doi:10.1002/cyto.a.23242
7. Rein ID, Notø HØ, Bostad M, Huse K, Stokke T. Cell cycle analysis and relevance for single-cell gating in mass cytometry. Cytometry A. 2020;97(8):832–844. doi:10.1002/cyto.a.23960
8. Nery AA, Nascimento IC, Glaser T, Bassaneze V, Krieger JE, Ulrich H. Human mesenchymal stem cells: from immunophenotyping by flow cytometry to clinical applications. Cytometry A. 2013;83(1):48–61. doi:10.1002/cyto.a.22205
9. Ranjbaran H, Abediankenari S, Mohammadi M, et al. Wharton’s Jelly derived-mesenchymal stem cells: isolation and characterization. Acta Med Iran. 2018;56(1):28–33.
10. Walecka I, Gil-Kulik P, Krzyżanowski A, et al. Phenotypic characterization of adherent cells population CD34+ CD90+ CD105+ derived from Wharton’s Jelly. Med Sci Monit. 2017;23:1886–1895. doi:10.12659/msm.902186
11. Adugna DG, Aragie H, Kibret AA, Belay DG. Therapeutic application of stem cells in the repair of traumatic brain injury. Stem Cells Cloning. 2022;15:53–61. doi:10.2147/SCCAA.S369577
12. Worku MG. Pluripotent and multipotent stem cells and current therapeutic applications: review. Stem Cells Cloning. 2021;14:3–7. doi:10.2147/SCCAA.S304887
13. Abou-ElNaga A, El-Chennawi F, Ibrahim Kamel S, Mutawa G. The potentiality of human umbilical cord isolated mesenchymal stem/stromal cells for cardiomyocyte generation. Stem Cells Cloning. 2020;13:91–101. doi:10.2147/SCCAA.S253108
14. Donnenberg VS, Ulrich H, Tárnok A. Cytometry in stem cell research and therapy. Cytometry A. 2013;83(1):1–4. doi:10.1002/cyto.a.22243
15. Riordon DR, Boheler KR. Immunophenotyping of live human pluripotent stem cells by flow cytometry. Methods Mol Biol. 2018;1722:127–149. doi:10.1007/978-1-4939-7553-2_9
16. Kaminska A, Wedzinska A, Kot M, Sarnowska A. Effect of long-term 3D spheroid culture on WJ-MSC. Cells. 2021;10(4):719. doi:10.3390/cells10040719
17. Rieger AM, Nelson KL, Konowalchuk JD, Barreda DR. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp. 2011;(50):2597. doi:10.3791/2597
18. Sun X, Kaufman PD. Ki-67: more than a proliferation marker. Chromosoma. 2018;127(2):175–186. doi:10.1007/s00412-018-0659-8
19. Podgorny O, Peunova N, Park JH, Enikolopov G. Triple S-phase labeling of dividing stem cells. Stem Cell Rep. 2018;10(2):615–626. doi:10.1016/j.stemcr.2017.12.020
20. Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol. 2012;24(2):188–193. doi:10.1016/j.ceb.2012.02.002
21. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi:10.1016/j.cell.2019.01.021
22. Wiszniak S, Schwarz Q. Exploring the intracrine functions of VEGF-A. Biomolecules. 2021;11(1):128. doi:10.3390/biom11010128
23. Chen R, Lee C, Lin X, Zhao C, Li X. Novel function of VEGF-B as an antioxidant and therapeutic implica-tions. Pharmacol Res. 2019;143:33–39. doi:10.1016/j.phrs.2019.03.002
24. Dewerchin M, Carmeliet P. PlGF: a multitasking cytokine with disease-restricted activity. Cold Spring Harb Perspect Med. 2012;2(8):a011056. doi:10.1101/cshperspect.a011056
25. Świstowska M, Gil-Kulik P, Krzyżanowski A, et al. Potential effect of SOX2 on the cell cycle of wharton’s jelly stem cells (WJSCs). Oxid Med Cell Longev. 2019;2019:5084689. doi:10.1155/2019/5084689
26. Gil-Kulik P, Świstowska M, Krzyżanowski A, et al. Evaluation of the impact of pregnancy-associated factors on the quality of Wharton’s Jelly-derived stem cells using SOX2 gene expression as a marker. Int J Mol Sci. 2022;23:7630. doi:10.3390/ijms23147630
27. Gil-Kulik P, Leśniewski M, Bieńko K, et al. Influence of perinatal factors on gene expression of IAPs family and main factors of pluripotency: OCT4 and SOX2 in human breast milk stem cells—a preliminary report. Int J Mol Sci. 2023;24:2476. doi:10.3390/ijms24032476
28. Fathi E, Vandghanooni S, Montazersaheb S, Farahzadi R. Mesenchymal stem cells promote caspase-3 expression of SH-SY5Y neuroblastoma cells via reducing telomerase activity and telomere length. Iran J Basic Med Sci. 2021;24(11):1583–1589. doi:10.22038/IJBMS.2021.59400.13187
29. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi:10.1016/0003-2697(87)90021-2
30. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2011;25:402–408. doi:10.1006/meth.2001.1262
31. Gil-Kulik P, Chomik P, Krzyżanowski A, et al. Influence of the type of delivery, use of oxytocin, and maternal age on POU5F1 gene expression in stem cells derived from Wharton’s Jelly within the umbilical cord. Oxidative Med Cell Longev. 2019;2019:1027106. doi:10.1155/2019/1027106
32. Gil-Kulik P, Krzyżanowski A, Dudzińska E, et al. Potential involvement of BIRC5 in maintaining pluripotency and cell differentiation of human stem cells. Oxid Med Cell Longev. 2019;2019:8727925. doi:10.1155/2019/8727925
33. Gil-Kulik P, Świstowska M, Kondracka A, et al. Increased expression of BIRC2, BIRC3, and BIRC5 from the IAP family in mesenchymal stem cells of the umbilical Cord Wharton’s Jelly (WJSC) in younger women giving birth naturally. Oxidative Med Cell Longev. 2020;2020:9084730. doi:10.1155/2020/9084730
34. Gil-Kulik P, Dudzińska E, Radzikowska-Büchner E, et al. Different regulation of PARP1, PARP2, PARP3 and TRPM2 genes expression in acute myeloid leukemia cells. BMC Cancer. 2020;20:435. doi:10.1186/s12885-020-06903-4
35. Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol. 2007;177(3):489–500. doi:10.1083/jcb.200608093
36. Kajdaniuk D, Marek B, Fołtyn W, Kos-Kudła B. Vascular endothelial growth factor (VEGF) in endo-crinology and oncology. Endokrynologia Polska. 2011;62(32):423.
37. Farhangi MA, Dehghan P, Tajmiri S, et al. The effects of Nigella sativa on thyroid function, serum Vas-cular Endothelial Growth Factor (VEGF) – 1, Nesfatin-1 and anthropometric features in patients with Hashimoto’s thyroiditis: a randomized controlled trial. BMC Complement Altern Med. 2016;2016:471.
38. Mayer H, Bertram H, Lindenmaier W, Korff T, Weber H, Weich H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem. 2005;95(4):827–839. doi:10.1002/jcb.20462
39. Deng C, Zhang Z, Xu F, et al. Thyroid hormone enhances stem cell maintenance and promotes lineage-specific differentiation in human embryonic stem cells. Stem Cell Res Ther. 2022;13(1):120. doi:10.1186/s13287-022-02799-y
40. Dedecjus M, Kołomecki K, Brzeziński J, Adamczewski Z, Tazbir J, Lewiński A. Influence of L-thyroxine administration on poor-platelet plasma VEGF concentrations in patients with induced short-term hypothyroidism, monitored for thyroid carcinoma. Endocr J. 2007;54(1):63–69. doi:10.1507/endocrj.k05-112
41. Bhavina K, Radhika J, Sundara Pandian S. VEGF and eNOS expression in umbilical cord from pregnancy complicated by hypertensive disorder with different severity. Biomed Res Int. 2014;2014:6. doi:10.1155/2014/982159
42. David AL. Maternal uterine artery VEGF gene therapy for treatment of intrauterine growth restriction. Placenta. 2017;59(Suppl 1):S44–S50. doi:10.1016/j.placenta.2017.09.011
43. Almasry SM, Elfayomy AK, Hashem HE. Ultrastructure and histomorphometric analysis of human umbilical cord vessels in preeclampsia: a potential role of VEGF, VEGFR-1 and VEGFR-2. Rom J Morphol Embryol. 2016;57(2Suppl):681–689.
44. Szentpéteri I, Rab A, Kornya L, Kovács P, Joó JG. Gene expression patterns of vascular endothelial growth factor (VEGF-A) in human placenta from pregnancies with intrauterine growth restriction. J Matern Fetal Neonatal Med. 2013;26(10):984–989. doi:10.3109/14767058.2013.766702
45. Daruich A, Bremond-Gignac D, Behar-Cohen F, Kermorvant E. Rétinopathie du prématuré: de la préven-tion au traitement [Retinopathy of prematurity: from prevention to treatment]. Med Sci (Paris). 2020;36(10):900–907. French. doi:10.1051/medsci/2020163
46. Chehroudi C, Kim H, Wright TE, Collier AC. Dysregulation of inflammatory cytokines and inhibition of VEGFA in the human umbilical cord are associated with negative pregnancy outcomes. Placenta. 2019;87:16–22. doi:10.1016/j.placenta.2019.09.002
47. Kaukola T, Räsänen J, Herva R, Patel DD, Hallman M. Suboptimal neurodevelopment in very preterm infants is related to fetal cardiovascular compromise in placental insufficiency. Am J Obstet Gynecol. 2005;193(2):414–420. doi:10.1016/j.ajog.2004.12.005
48. Krukier II, Pogorelova TN. Production of vascular endothelial growth factor and endothelin in the placenta and umbilical cord during normal and complicated pregnancy. Bull Exp Biol Med. 2006;141(2):216–218. doi:10.1007/s10517-006-0131-2
49. Page P, DeJong J, Bandstra A, Boomsma RA. Effect of serum and oxygen concentration on gene expression and secretion of paracrine factors by mesenchymal stem cells. Int J Cell Biol. 7;2014.
50. Chinnici CM, Iannolo G, Cittadini E, et al. Extracellular vesicle-derived microRNAs of human Wharton’s Jelly mesenchymal stromal cells may activate endogenous VEGF-A to promote angiogenesis. Int J Mol Sci. 2021;22(4):2045. doi:10.3390/ijms22042045
51. Fathi E, Valipour B, Farahzadi R. Targeting the proliferation inhibition of chronic myeloid leukemia cells by bone marrow derived – mesenchymal stem cells via ERK pathway as a therapeutic strategy. Acta Med Iran. 2020;2020:199–206.
52. Fathi E, Farahzadi R. Mesenchyaml stem cells as a cell based therapeutic strategy targeting the telomerase activity of KG1 acute myeloid leukemia cells. Acta Med Iran. 2022;60(2):71.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.