Back to Journals » International Journal of General Medicine » Volume 15
Vancomycin Loading Doses and Nephrotoxicity on Medicine Teaching Services
Authors Wagner P , Arnold J, Sheridan K
Received 8 July 2022
Accepted for publication 14 September 2022
Published 6 October 2022 Volume 2022:15 Pages 7685—7692
DOI https://doi.org/10.2147/IJGM.S380017
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Phillip Wagner,1,2 Jonathan Arnold,1 Kathleen Sheridan3
1Department of Internal Medicine, University of Pittsburgh Medical Center, Pittsburgh, PA, USA; 2Department of Internal Medicine, Johns Hopkins Hospital, Baltimore, MD, USA; 3Department of Infectious Diseases, Pittsburgh Infectious Diseases, Pittsburgh, PA, USA
Correspondence: Phillip Wagner, Johns Hopkins Hospital, 600 North Wolfe Street, Meyer 8, Room 134A, Baltimore, MD, 21287, USA, Tel +1-412-841-8522, Email [email protected] Correspondence: Kathleen Sheridan, Pittsburgh Infectious Disease, Pittsburgh Pennsylvania 101 Drake Street, Pittsburgh, PA, 15241, USA, Tel +1 412-347-0057, Email [email protected]
Background: Infectious Disease Society of America (IDSA) guidelines recommend the usage of a loading dose when using vancomycin for seriously ill patients. While the relationship between vancomycin and nephrotoxicity is the focus of many studies, few studies have examined the relationship between vancomycin loading doses and nephrotoxicity.
Methods: We performed a retrospective cohort study examining vancomycin dosing for internal medicine teaching services’ patients over the 2014– 15 academic year at one academic medical center. We generated a list of all hospitalized patients aged 18– 85 who received vancomycin and were admitted to a teaching service. Nephrotoxicity was determined by 7-day acute kidney injury (AKI) rate. Patients in the loading dose cohort were compared with those in the standard-dose cohort. Primary modeling used multivariable logistic regression with AKI as our outcome of interest.
Results: Four hundred and thirty-eight patients were included for analysis. The loading dose (n = 365, 83%) and standard dosing (n = 73, 17%) cohorts were not significantly different regarding demographics, GFR, nephrotoxic drug exposure, total vancomycin received, trough levels, or comorbidities and were only significantly different regarding body mass index (BMI). The 7-day AKI rate was not significantly different between the two arms (6.3% in the standard dosing arm and 8.2% in the loading dose arm, p = 0.6).
Conclusion: Few studies have examined the relationship between nephrotoxicity and vancomycin loading doses. The results of this study provide evidence that the use of loading doses is not significantly associated with increased 7-day AKI rate.
Keywords: vancomycin, loading dose, nephrotoxicity
Introduction
The Infectious Disease Society of America (ISDA) guidelines recommend considering a loading dose when using vancomycin in complicated or serious infections to rapidly achieve therapeutic trough concentrations.1,2 Previous studies have demonstrated that exposure to subtherapeutic serum vancomycin concentrations has produced strains of staphylococcus functionally similar to vancomycin-intermediate staphylococcus (VISA).3,4 VISA strains are associated with treatment failure as well as higher morbidity and mortality, requiring higher doses to eradicate infection.5,6 As loading doses limit the amount of time methicillin-resistant staphylococcus aureus (MRSA) is exposed to these subtherapeutic concentrations, their use not only theoretically leads to faster microbiologic cure and improved outcomes for individual patients but also shows promise as a way of limiting the spread of staphylococcal vancomycin resistance.1
A preponderance of evidence maintains vancomycin as a minor, but likely causal, agent in the development of nephrotoxicity. This includes a dose–response relationship demonstrated with increasing duration of treatment, trough level, Area Under the Curve (AUC) thresholds, and dose. Little evidence exists, however, regarding the relationship between the use of vancomycin loading doses and nephrotoxicity.7,8,14 There are, however, few studies examining the use of vancomycin loading doses. The studies on which the 2009 IDSA recommendation was based had only small sample sizes and were not powered to assess patient outcomes.9,15 As nephrotoxicity is among the clinically significant complications of vancomycin dosing, elucidating any increased acute kidney injury (AKI) rate associated with loading dose use may help guide providers in choosing their dosing regimens.
Methods
We performed a retrospective cohort study of patients admitted to a resident-led general internal medicine service of a single tertiary care academic medical center between July of 2014 and July of 2015. Patients were included in the trial if they were inpatients on medical teaching services for greater than 24 hours, received greater than or equal to three doses of vancomycin with at least two of those doses happening on the medical floor, and were either direct admissions or admitted from the emergency department. Patients were excluded if they fell outside the age range of 18–85, were pregnant, had a vancomycin dosing interval greater than 24 hours, had an initial vancomycin dose less than 10 mg/kg, had an initial trough less than 10mcg/mL, received vancomycin in the last 7 days, or had a glomerular filtration rate (GFR) less than 50 mL/min. Patients were also excluded if they failed to have a trough drawn, or had a trough that was drawn inappropriately, defined as a trough drawn more than 90 minutes before vancomycin was given (Figure 1).
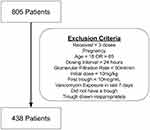 |
Figure 1 Exclusion criteria. |
Data were obtained from the inpatient electronic medical record (EMR) and included demographic information, body mass index (BMI), medical comorbidities, dosage-specific parameters such as dosing interval and amount, concurrent nephrotoxic drug use, vancomycin trough level, as well as daily creatinine and Intensive Care Unit (ICU) transfer.
Loading dose was defined as 20.0–30.0 mg/kg based on institutional guidance during the study period. Actual body weight was used in these calculations. Nephrotoxicity was defined by the IDSA Guidelines as an increase in baseline creatinine by 50% or 0.5 mg/dL over the baseline at least two consecutive days after starting vancomycin.1 Nephrotoxic burden was determined by exposure to known nephrotoxic agents while concurrently exposed to vancomycin and included aminoglycosides, acyclovir, calcineurin inhibitors, cisplatin, ifosfamide, colistin, ketorolac, lithium, protease inhibitors, tenofovir, or trimethoprim-sulfamethoxazole (TMP-SMX). TMP-SMX was included given the evidence it has the potential to cause both renal damage as well as artifactual increase in serum creatinine.13
The primary aim was to determine the odds of developing nephrotoxicity in the loading dose group cohort compared to the standard-dose cohort as measured by 7-day AKI rate. Controlling variables included age, race, BMI, amount of vancomycin administered, comorbidity burden, nephrotoxic burden, and baseline GFR. Comorbidity burden was scored via the Charlson Comorbidity Index and controlled for after grouping into low (0–2), moderate, (3–4) and high (>5) strata. Multivariable linear regression was used to determine the relationship between vancomycin dosing strategy, trough level, and AKI. Primary Modeling used logistic regression with AKI as the outcome of interest. A vancomycin trough level over 25.0 mcg/mL was considered to be a high trough level. Manual chart review was performed to ensure that recorded troughs were drawn at appropriate times. Patients without appropriately drawn trough levels were excluded from the analysis. The relationship between AUC and AKI rate was not assessed as AUC monitoring was not performed at this institution during the study period. T-test and chi squared were performed as appropriate.
Analysis was performed in Stata 14.2 to generate odds ratios (ORs). A 95% confidence interval was used, and a p-value less than 0.05 was considered significant. In order to have a power of 0.8 to detect and an absolute difference in AKI rate of 10%, the cohorts needed 70 and 280 patients in the loading dose and standard-dose arms, respectively. This was based on assuming a 10% AKI rate in the standard-dosing cohort.18
Patient Consent Statement: This study did not contain factors requiring patient consent. The study protocol was approved by the Institutional Review Board of the University of Pittsburgh (PRO15050204). Data was de-identified and anonymized in keeping with data protection and privacy regulations.
Results
During the study period, 805 hospitalized patients received vancomycin. The final list after the application of the exclusion criteria contained 438 patients (Figure 1). Patients in the loading dose cohort were compared to those in the standard-dose cohort. The cohorts were not significantly different with regard to demographic profile, age, race, baseline glomerular filtration rate, nephrotoxic drug exposure, or Charleston Severity Index (Table 1). The cohorts differed significantly only with regard to BMI, with the standard-dose cohort having a mean BMI of 30, while the loading dose cohort had a mean BMI of 24 (p < 0.001).
 |
Table 1 Patient Demographics and Baseline Clinical Variables |
Patients in neither the standard-dose nor the loading dose cohort differed significantly in terms of cumulative dose of vancomycin received (9.4g versus 10.1g, p = 0.37), total days on vancomycin (3.8 versus 3.8, p = 0.50), first vancomycin trough (13.7mcg/mL versus 13.9mcg/mL, p = 0.78), or percentage within the cohort experiencing a supratherapeutic trough (6.8 vs 5.2, p > 0.99) (Table 2).
 |
Table 2 Dosing Covariates and Primary Outcome |
The unadjusted 7-day AKI rate, the primary outcome in this study, was not significantly different (6.3% and 8.2%, p = 0.6) for the standard-dose and loading dose cohorts, respectively (Table 2). The initial dosing strategy was not associated with vancomycin trough level with the loading dose cohort having only a 0.28 mcg/mL higher trough than the standard dosing cohort, 95% CI −1.7 to 2.26, p = 0.78. Vancomycin trough level was associated with AKI. When dichotomized into high (>25 mcg/mL) vs not high (<25 mcg/mL) trough levels, the unadjusted odds ratio for AKI was 5.5 with the higher trough levels, 95% CI 1.95 to 15.4, p = 0.001. In our fully adjusted model, the OR of loading versus standard dosing for AKI was 1.3, 95% CI 0.41 to 3.93, p = 0.69. AKI rate did, however, significantly increase in the setting of concurrent Piperacillin-tazobactam administration, OR 3.1, 95% CI 1.4 to 6.9, p = 0.006. The adjusted OR of an AKI associated with a high vancomycin trough was 8.7, 95% CI 2.6 to 29.2, p < 0.001 (Table 3).
 |
Table 3 Adjusted Odds Ratios of AKI with Loading Dosing, Concurrent Piperacillin-Tazobactam Use, and High Vancomycin Trough |
Discussion
This study suggests that modern formulations of vancomycin given in loading doses did not lead to a significantly higher 7-day AKI rate than standard 10–20 mg/kg dosing. It should be noted, however, that the first vancomycin trough level, the total cumulative dose of vancomycin received, and total days of therapy were not significantly different between cohorts, indicating that total exposure was similar. For comparison, those with a supratherapeutic trough in either cohort had a significantly higher 7-day AKI rate with an OR of 8.7 (95% CI 2.6–29.2, p < 0.001). As trough level and duration of exposure have a larger body of evidence supporting their association with vancomycin-induced nephrotoxicity, this finding is consistent with adjacent existing evidence.7,8,14 There was a significant association between AKI and vancomycin with concurrent piperacillin-tazobactam in our study, a finding corroborated by several other studies specifically designed to look for this outcome.NaN,11 This finding strengthens the evidence that a higher AKI-rate in those who received empiric vancomycin and piperacillin-tazobactam may be due to a synergistic reaction between the two medications and that this should be considered when deciding on an empiric antimicrobial regimen.
A number of studies performed during the same time frame have also failed to show an association between vancomycin loading doses and nephrotoxicity.
While this study did not include those with a GFR less than 50mL/m, a study by Marvin et al, 2019 focused on patients receiving vancomycin loading doses who had a creatinine clearance (CrCl) of less than 30mL/s. The study was also a single-center retrospective design, with the primary outcome being a 5-day AKI rate. Despite concerns of increased risk of nephrotoxicity in those with diminished renal function, the loading dose group had significantly lower adjusted and unadjusted AKI rates (Relative Risk (RR) 0.61; 95% confidence interval 0.39–0.93). This population was decidedly more severely ill than our study population, as over 40% of patients in both cohorts were eventually transferred to the ICU.19
A larger retrospective study by Rosini et al, 2016 examined loading-dose vancomycin and AKI rate in septic patients. Like the aforementioned study, the loading dose cohort had a significantly lower rate of nephrotoxicity (5.8% versus 11.1%) with an adjusted RR of 0.60; 95% confidence interval = 0.44 to 0.82.22 However, adjacent studies examining the same relationship in ICU patients did not show a protective effect of loading doses against AKI rate. Rates between cohorts were still not significantly different, suggesting that if there is a counterintuitive benefit from loading doses and nephrotoxicity, it may not be realized in severely ill patients as seen in an ICU population.23,24
While these studies are limited by their retrospective design and presumed unequal distribution of confounders, Rosini et al, 2015 explored the relationship between the use of vancomycin loading doses in patients in the emergency department and time to appropriate trough concentrations relative to a standard dosing control group via a prospective randomized control study. Nephrotoxicity was assessed as a secondary outcome, and despite faster attainment of appropriate 12-hour trough concentrations (34% vs 3%, P < 0.01), no difference in nephrotoxicity was observed. Although the sample size studied was relatively small (n = 99), concurrent nephrotoxic drug burden was not assessed or controlled for, and the study period was relatively short (36 hours), the RCT design allowed for a more equal distribution of confounders, particularly weight, between study arms, something not achieved in the previously discussed studies.21
A recurrent finding in several of the aforementioned studies as well as ours is the unequal distribution of BMI between dosing cohorts. This difference is particularly striking given the close similarity in all other measured clinical parameters. While possibly a confounder of unclear significance, BMI and its recurrent maldistribution between cohorts in separate studies may be reflective of the actual dosing patterns of vancomycin. Despite the IDSA’s first recommendation for using a loading dose in 2009,1 and reiterating the recommendation again with more evidence in 2020,2 it seems probable given data patterns in numerous studies that loading doses are often administered agnostic of severity of illness and happen rather by virtue of a patient having low enough BMI for the dose they receive to incidentally meet criteria as a loading dose. This possibility is supported by a cross-sectional study by Davis et al, 2013, showing adherence to the loading dose recommendation is minimal and that some of that poor adherence is likely related to ongoing concerns of increased risk of nephrotoxicity.20
Another contributing factor is that the maximum dose of vancomycin given in an initial dose in the manufacturer’s guidelines is recommended to not exceed 3 grams, but the institutional pharmacy guideline at the study hospital while the study was being conducted recommended a 2.5g maximum loading dose.13 Accordingly, no single patient received higher than 2.5 grams in any one dose in this study. This guideline therefore makes it unlikely for any patient weighing between 83 and 125kg, and impossible for any patient weighing above 125kg, to receive a full loading dose, depending on the intended mg/kg dosing target of each patient (83kg corresponds to a 30mg/kg loading dose and 125kg corresponds to a 20mg/kg loading dose). While BMI was controlled for in analysis, a larger prospective randomized study would provide a higher quality of evidence to more completely deal with this potential confounder.
Limitations of this study include that this was a single-center retrospective study that took place between 2014 and 2015, and dosing patterns and proportions noted in this study may not accurately reflect how vancomycin is dosed in other institutions. Further, dosing by trough is becoming less common due to updated recommendations instead of promoting AUC dosing.2 Despite now being considered suboptimal for clinical dosing, as trough still correlates with AUC, it is still a reasonable proxy by which to compare cohorts.16
Another possible limitation may be the duration of treatment studied. AKI rate was only recorded over the first seven days of vancomycin dosing, and differential nephrotoxicity may not develop to a detectable amount unless a longer course of treatment is monitored. Further, current definitions of AKI rely on changes in creatinine and urine output cutoffs, proxies for renal function that do not always capture renal injury.17 However, the previous study duration in similar loading dose studies has been historically between 36 hours and 5 days,19,21–24 so this study has a chance to capture more potential AKIs. As discussed previously, however, there is stronger evidence of an association between duration of vancomycin exposure and AKI. By prolonging the study period, the chances of capturing AKIs due to duration of vancomycin exposure rather than those due to loading doses inevitably increases and further confounds analysis.
Finally, while the cohorts were similar with regard to comorbidity index, the study was not adjusted for severity of illness on presentation. They were all, however, not critically ill as they were deemed stable enough to be admitted to a medical floor rather than an ICU, and both cohorts likewise had similar transfer rates to the ICU.
Conclusion
Further studies on vancomycin loading dosing should preferably be prospective randomized controlled trials rather than observational studies. So far, a significant association between vancomycin loading doses and nephrotoxicity has not been found in numerous inpatient populations of varying etiology or severity of illness as well as renal function. Administering a loading dose of vancomycin was not associated with higher 7-day rates of AKI. These findings provide more evidence for IDSA recommendations regarding the use and safety of loading doses.
Summary
Administering a loading dose of vancomycin was not associated with higher 7-day rates of AKI.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Patient Consent, Trial Registration, and Ethics Approval Statement
This study did not contain factors requiring patient consent. The study protocol was approved by the Institutional Review Board of the University of Pittsburgh (PRO15050204).
Acknowledgments
The abstract of this paper was presented at the 2019 ID Week Conference as a poster presentation with interim findings. The poster’s abstract was published in Open Forum Infectious Diseases, Volume 6, Issue Supplement_2, October 2019, Page S575, https://doi.org/10.1093/ofid/ofz360.1439. Clinical And Translational Science Institute, University of Pittsburgh provided statistical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was not funded.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rybak M, Lomaestro B, Rotschafer J., et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. American journal of health-system pharmacy. AJHP. 2009;66:82–98. doi:10.2146/ajhp080434
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Sys Pharmacy. 2020;77(11):835–864. doi:10.1093/ajhp/zxaa036
3. Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2004;38:448–451. doi:10.1086/381093
4. Tsuji BT, Rybak MJ, Lau KL, et al. Evaluation of accessory gene regulator (agr) group and function in the proclivity towards vancomycin intermediate resistance in Staphylococcus aureus. Antimicrobial Agents Chemotherapy. 2007;51:108991. doi:10.1128/AAC.00671-06
5. Hiramatsu K. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect Dis. 2001;1:147–155. doi:10.1016/S1473-3099(01)00091-3
6. Sakoulas G, Moise-Broder PA, Schentag J, et al. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398–2402. doi:10.1128/JCM.42.6.2398-2402.2004
7. Van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrobial Agents Chemotherapy. 2013;57:734–744. doi:10.1128/AAC.01568-12
8. Chow AW, Azar RM. Glycopeptides and nephrotoxicity. Intensive Care Med. 1994;20(Suppl 4):S23–S29. doi:10.1007/BF01713979
9. Wang JT, Fang CT, Chen YC, et al. Necessity of a loading dose when using vancomycin in critically ill patients. J Antimicrobial Chemotherapy. 2001;47:246. doi:10.1093/jac/47.2.246
10. Balcı C, Ö U, Arıcı M, Hayran SA, Yüce D, Ünal S. Nephrotoxicity of piperacillin/tazobactam combined with vancomycin: should it be a concern? Int J Antimicrobial Agents. 2018;52(2):180–184. doi:10.1016/j.ijantimicag.2018.03.024
11. Rutter WC, Burgess DR, Talbert JC, et al. Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: a retrospective cohort analysis. J Hosp Med. 2017;12(2):77. doi:10.12788/jhm.2684
12. Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrology Renovascular Dis. 2014;7:457–468. doi:10.2147/IJNRD.S39747
13. Vancomycin.2019. Prescriber’s Digital Reference. Available from: https://www.pdr.net/drug-summary/Vancocin-vancomycin-hydrochloride-802.
14. Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019;69(11):1881–1887. doi:10.1093/cid/ciz051
15. Mohammedi I, Descloux E, Argaud L, et al. Loading dose of vancomycin in critically ill patients: 15 mg/kg is a better choice than 500 mg. Int J Antimicrobial Agents. 2006;27:259–262. doi:10.1016/j.ijantimicag.2005.11.009
16. Ayuthaya S, Katip W, Oberdorfer P, Lucksiri A. Correlation of the vancomycin 24-h area under the concentration-time curve (AUC24) and trough serum concentration in children with severe infection: a clinical pharmacokinetic study. Int J Infect Dis. 2020;92:151–159. doi:10.1016/j.ijid.2019.12.036
17. Ostermann M, Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care. 2016;20:299. doi:10.1186/s13054-016-1478-z
18. Elyasi S, Khalili H, Dashti-Khavidaki S, et al. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. Eur J Clin Pharmacol. 2012;68:1243–1255. doi:10.1007/s00228-012-1259-9
19. Marvin JL, Levine BJ, Papas M, Rosini JM. An evaluation of the incidence of nephrotoxicity after a loading dose of vancomycin in patients with severe renal impairment. J Emerg Med. 2019;56(6):701–708. doi:10.1016/j.jemermed.2019.03.020
20. Davis SL, Scheetz MH, Bosso JA, Goff DA, Rybak MJ. Adherence to the 2009 consensus guidelines for vancomycin dosing and monitoring practices: a cross-sectional survey of U.S. Hospitals Pharmacotherapy. 2013;33(12):1256–1263. doi:10.1002/phar.1327
21. Rosini JM, Laughner J, Levine BJ, Papas MA, Reinhardt JF, Jasani NB. A randomized trial of loading vancomycin in the emergency department. Ann Pharmacother. 2015;49(1):6–13. doi:10.1177/1060028014556813
22. Rosini JM, Davis JJ, Muenzer J, et al. High single-dose vancomycin loading is not associated with increased nephrotoxicity in emergency department sepsis patients. Acad Emerg Med. 2016;23(6):744–746. doi:10.1111/acem.12934
23. Flannery AH, Wallace KL, Rhudy CN, et al. Efficacy and safety of vancomycin loading doses in critically ill patients with methicillin-resistant Staphylococcus aureus infection. Therapeutic Adv Infect Dis. 2021;8:20499361211005965. doi:10.1177/20499361211005965
24. Hodiamont CJ, Juffermans NP, Berends SE, et al. Impact of a vancomycin loading dose on the achievement of target vancomycin exposure in the first 24 h and on the accompanying risk of nephrotoxicity in critically ill patients. J Antimicrobial Chemother. 2021;76(11):2941–2949. doi:10.1093/jac/dkab278
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
