Back to Journals » Infection and Drug Resistance » Volume 16
Value of Laboratory Indicators in Predicting Pneumonia in Symptomatic COVID-19 Patients Infected with the SARS-CoV-2 Omicron Variant
Authors Zhu K , Ma S, Chen H, Xie J, Huang D, Fu C, Ma G, Huang Y
Received 11 November 2022
Accepted for publication 21 February 2023
Published 28 February 2023 Volume 2023:16 Pages 1159—1170
DOI https://doi.org/10.2147/IDR.S397231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Kongbo Zhu,1 Shaolei Ma,2 Hui Chen,3 Jianfeng Xie,3 Dan Huang,1 Cuiping Fu,4 Genshan Ma,1 Yingzi Huang3
1Department of Cardiology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, People’s Republic of China; 2Department of Emergency and Critical Care Medicine, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, People’s Republic of China; 3Department of Critical Care Medicine, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, People’s Republic of China; 4Department of Respiratory Medicine, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, People’s Republic of China
Correspondence: Yingzi Huang, Department of Critical Care Medicine, Zhongda Hospital, School of Medicine, Southeast University, No. 87 Dingjiaqiao Road, Nanjing, 210009, People’s Republic of China, Email [email protected]
Background: The pathogenicity of Omicron is different from that of the previous strains. The value of hematological indicators in patients at high risk of Omicron infection remains unclear. We need rapid, inexpensive and widely available biomarkers to guide the early detection of people at risk of pneumonia and to provide early intervention. We aimed to assess the value of hematological indicators as risk factors for pneumonia in symptomatic COVID-19 patients infected with the SARS-CoV-2 Omicron variant.
Patients and Methods: The study enrolled 144 symptomatic COVID-19 patients with Omicron infection. We collected available clinical details, including laboratory tests and CT examinations. Univariate and multivariate logistic analyses and receiver operating characteristic (ROC) curve analyses were used to assess the value of laboratory markers in predicting the development of pneumonia.
Results: Among the 144 patients, 50 (34.7%) had pneumonia. The ROC analysis revealed that the areas under the ROC curve (AUC) for leukocytes, lymphocytes, neutrophils, and fibrinogen were 0.603 (95% confidence interval (CI): 0.501– 0.704, P=0.043), 0.615 (95% CI: 0.517– 0.712, P=0.024), 0.632 (95% CI: 0.534– 0.730, P=0.009) and 0.635 (95% CI: 0.539– 0.730, P=0.008), respectively. The AUC for neutrophil to lymphocyte ratio (NLR), monocyte to lymphocyte ratio (MLR), fibrinogen to lymphocyte ratio (FLR), and fibrinogen to D-dimer ratio (FDR) were 0.670 (95% CI: 0.580– 0.760, P=0.001), 0.632 (95% CI: 0.535– 0.728, P=0.009), 0.669 (95% CI: 0.575– 0.763, P=0.001) and 0.615 (95% CI: 0.510– 0.721, P=0.023), respectively. Univariate analysis showed that elevated levels of NLR (odds ratio (OR): 1.219, 95% CI: 1.046– 1.421, P=0.011), FLR (OR: 1.170, 95% CI: 1.014– 1.349, P=0.031) and FDR (OR: 1.131, 95% CI: 1.039– 1.231, P=0.005) were significantly correlated with the presence of pneumonia. Multivariate analysis indicated elevated NLR (OR: 1.248, 95% CI: 1.068– 1.459, P=0.005) and FDR (OR: 1.160, 95% CI: 1.054– 1.276, P=0.002) levels were associated with the existence of pneumonia. The AUC for the combination of NLR and FDR was 0.701 (95% CI: 0.606– 0.796, P< 0.001, sensitivity 56.0%, specificity 83.0%).
Conclusion: NLR and FDR can predict the presence of pneumonia in symptomatic COVID-19 patients infected with the SARS-CoV-2 Omicron variant.
Keywords: COVID-19, Omicron, pneumonia, neutrophil to lymphocyte ratio, fibrinogen, D-dimer
Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global public health concern since December 2019.1,2 In late February 2022, a wave of SARS-CoV-2 Omicron variant infections rapidly emerged in Shanghai, China. Phylogenetic characterization of SARS-CoV-2 viral genomes from 129 patients in Shanghai indicated that all new viral genomes clustered in the SARS-CoV-2 BA.2.2 sublineage. The Omicron variant of SARS-CoV-2 emerged in South Africa in 2021, spreading more readily than other variants.3 Therefore, Omicron rapidly replaced other variants as the dominant variant worldwide.4 However, Omicron is less virulent and thus less pathogenic, resulting in a lower risk of hospitalization and a lower prevalence of pneumonia.5–7
Pneumonia is a common cause of hospitalization and death in COVID-19 patients, and computed tomography (CT) images play a vital role in determining the severity and extent of the disease.8–12 However, the incidence of pneumonia in Omicron itself is low, not all patients can receive immediate CT screening during a pandemic, and it is not necessary to perform CT scans on all Omicron-infected patients. In addition, the CT suites are high-risk areas for hospital-related transmission of COVID-19 and require infection control.13 Male gender, increasing age, smoking and chronic comorbidities are associated with a higher risk of pneumonia following Omicron infection.14–18 COVID-19 vaccine is less effective against symptomatic Omicron infection but provides strong protection against pneumonia, hospitalization and death associated with COVID-19.16,19–21
Dysregulation of immune response is present in COVID-19 patients22 and hematological indicators such as neutrophil to lymphocyte ratio (NLR) are independent predictors of disease progression and mortality in patients with COVID-19.23–28 Hypercoagulable states and elevated levels of D-dimer have been proven in COVID-19 patients. D-dimer levels are valuable predictors of severity and mortality and may be associated with inflammation in SARS-COV-2 pneumonia prior to coagulopathy/thrombosis.28–31
However, the pathogenicity of Omicron is different from that of the previous strains.3,5–7 Omicron is less virulent. The infectivity of the SARS-CoV-2 Omicron variant is more than Alpha, but Omicron patients are less likely to be admitted to ICU. Are the previous hematological indicators still valuable in the Omicron era? The value and threshold of these indicators could be more precise. In the Omicron era, we have also focused on symptomatic, elderly patients with comorbidities. The value of these indicators in patients at high risk of Omicron infection remains unclear. A previous study found that only aging was associated with pneumonia.15 We need rapid, inexpensive and widely available biomarkers to guide the early detection of people at risk of pneumonia and to provide early intervention. Therefore, further studies of hematological indicators associated with pneumonia in COVID-19 patients infected by Omicron are needed and may improve patients’ clinical management in the Omicron era. In the present study, we aimed to assess the value of hematological indicators as risk factors for pneumonia in symptomatic COVID-19 patients infected with the SARS-CoV-2 Omicron variant.
Materials and Methods
Study Design and Participants
This retrospective cohort study enrolled patients with a confirmed diagnosis of COVID-19 admitted to Shanghai Lingang Shelter Hospital in China between April 21, 2022 and May 20, 2022. Symptomatic COVID-19 patients aged 18 years or older with complete clinical data, blood and CT examinations were included. Patients were excluded if they met one of the following criteria: 1) past or existing history of chronic medical conditions affecting inflammatory markers, such as autoimmune disease, chronic inflammatory disease, malignancy under treatment, gastrointestinal bleeding, chronic hematological disease, recent acute myocardial damage, recent surgery, HIV infection and cirrhosis of the liver; 2) recent disease affecting the coagulation system, such as acute pulmonary embolism, lower limb deep vein thrombosis, hematological disorders, prior to COVID-19 infection; 3) during pregnancy and lactating women; 4) ongoing treatment with drugs that affect indicators of inflammation and coagulation system, such as corticosteroids and anticoagulants. This study was authorized by the Ethics Commission for Clinical Research of Zhongda Hospital, affiliated to Southeast University. Informed consent was waived due to the nature of the study as a retrospective study. The study was conducted in accordance with the principles described in the Declaration of Helsinki and the confidentiality of patients was guaranteed.
Data Collection
Health information stored in the electronic medical record system was analyzed. We collected available clinical details, including demographic characteristics, vaccination status, comorbidities, nucleic acid test results, laboratory tests and CT examinations. Comorbidities included hypertension, diabetes mellitus, cardiovascular disease, chronic respiratory disease (asthma, chronic obstructive pulmonary disease or interstitial lung disease) and chronic kidney disease. Laboratory indicators were collected, including leukocytes, neutrophils, lymphocytes, monocytes, eosinophils, platelets, hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, direct bilirubin, blood urea nitrogen, serum creatinine, fibrinogen and D-dimer. This study also calculated and collected derived hematological indicators, including NLR, monocyte to lymphocyte ratio (MLR), eosinophil to lymphocyte ratio (ELR), platelet to lymphocyte ratio (PLR), fibrinogen to lymphocyte ratio (FLR), fibrinogen to D-dimer ratio (FDR). Nasopharyngeal swab specimens were collected daily from each patient during hospitalization. The criterion for negative conversion was two consecutive negative nucleic acid tests within a 24-hour minimum sampling interval. The date of the first two consecutive negative nucleic acid tests after admission to the hospital was defined as the date of nucleic acid negativity. The nucleic acid negative conversion time (NCT) was calculated as the number of days between the date of positive nucleic acid on the pre-admission community screen and the date of negative nucleic acid after admission.32,33
Statistical Analysis
Categorical variables are presented as the number and percentage of the total. Continuous variables are shown as the median (Quartile 25%, 75%). The distribution of continuous variables was assessed using the Shapiro–Wilk test. The chi-squared test or Fisher’s exact test was used to compare the significance of the differences between categorical variables. We compared continuous variables between different groups using the Student’s t-test or Mann–Whitney U-test as appropriate. Univariate and forward stepwise multivariate logistic analyses were conducted to examine risk factors for the presence of pneumonia. Receiver operating characteristic (ROC) curve analysis was used to assess the value of laboratory markers in predicting the development of pneumonia. Optimal cut-off levels were determined and their sensitivities and specificities were calculated. All analyses were performed using SPSS 25.0 software (SPSS Inc, Chicago, IL). Two-tailed P values of <0.05 were considered statistically significant.
Results
Clinical Characteristics
151 symptomatic COVID-19 patients aged 18 years or older with complete clinical data, blood tests and CT examinations met the inclusion criteria. Of these, 3 patients were excluded from the study due to autoimmune diseases. In addition, 2 patients were ruled out due to ongoing treatment for malignancy. Furthermore, 2 patients were excluded because they were being treated with anticoagulants for a recent thrombotic disease. The study ultimately enrolled 144 symptomatic COVID-19 patients with Omicron infection (Figure 1). Asymptomatic infected individuals were not included in this study.
 |
Figure 1 The flowchart of patients according to inclusion and exclusion criteria. |
Table 1 shows the clinical characteristics of symptomatic COVID-19 patients infected with Omicron by pneumonia status classification. The median age of the patients was 66 years, and the majority of patients were elderly. 50 patients (34.7%) had pneumonia, and 79 (54.9%) were male. Figure 2 shows common CT findings in pneumonia COVID-19 patients infected by the SARS-CoV-2 Omicron variant. Comorbidities were common in these patients, including 51 patients (35.4%) with hypertension, 24 (16.7%) with diabetes, 6 (4.2%) with cardiovascular disease, 4 (2.8%) with chronic respiratory diseases and 9 (6.3%) with chronic kidney disease. 80 patients (64.2%) received at least one dose of the inactivated vaccine (vero cell), of which 42 patients (29.2%) received a third (booster) dose. Only 4 patients (2.8%) received one dose of the inactivated vaccine, and 34 patients (23.6%) got two doses of the inactivated vaccine. There were still 64 patients in this study who did not receive any vaccination. None of the patients had a history of COVID-19 infection. The median time to NCT of nucleic acids was 8 (5,10) days. The clinical data were compared according to the presence or absence of pneumonia. Age, gender, comorbidities and doses of the vaccine showed no significant differences between the two groups of pneumonia patients and non-pneumonia patients (all P>0.05). The median time to NCT of nucleic acid was longer in patients with pneumonia than those without pneumonia, with 9 and 7 days in the two groups, respectively (P=0.040). 2 patients in the pneumonia group were admitted to ICU, and these two patients were not vaccinated. In this study, no patients had secondary complications or died.
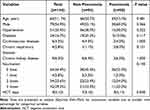 |
Table 1 Clinical Characteristics of Symptomatic COVID-19 Patients Infected by SARS-CoV-2 Omicron Variant |
Laboratory Results
Table 2 shows the laboratory results of symptomatic COVID-19 patients infected by the SARS-CoV-2 Omicron variant classified by pneumonia status. Patients with pneumonic COVID-19 had higher levels of leukocytes (P=0.043), neutrophils (P=0.009), fibrinogen (P=0.008), and lower levels of lymphocytes (P=0.048) compared to those with non-pneumonic COVID-19 infection. In contrast, values for platelets, hemoglobin, ALT, AST, total bilirubin, direct bilirubin, total protein, albumin, blood urea nitrogen, serum creatinine and D-dimer were similar between the two groups (all P>0.05). Derived hematological parameters of symptomatic COVID-19 patients with the SARS-CoV-2 Omicron variant are shown in Table 3. NLR (P=0.001), MLR (P=0.009), FLR (P=0.001) and FDR (P=0.023) were all significantly higher in the pneumonia group than in the non-pneumonia group, while ELR and PLR were statistically indistinguishable (P>0.05).
 |
Table 2 Laboratory Findings of Symptomatic COVID-19 Patients Infected by SARS-CoV-2 Omicron Variant |
 |
Table 3 Derived Hematological Indicators of Symptomatic COVID-19 Patients Infected by SARS-CoV-2 Omicron Variant |
Risk Factors for Pneumonia
ROC curve analyses based on the presence of pneumonia in patients with symptomatic COVID-19 infected by the SARS-CoV-2 Omicron variant were performed to compare the predictive performance of blood parameters (Figure 3). As presented in Table 4, cut-off values and the area under the ROC curve (AUC) also were calculated. The AUC for leukocytes, lymphocytes, neutrophils and fibrinogen were 0.603 (95%confidence interval(CI): 0.501–0.704, P=0.043), 0.615 (95% CI:0.517–0.712, P=0.024), 0.632 (95% CI:0.534–0.730, P=0.009) and 0.635 (95% CI:0.539–0.730, P=0.008), respectively. In contrast, the AUC for monocytes, eosinophils, platelets, hemoglobin and D-dimer were insignificant (all P>0.05). ROC curve analyses also revealed the cut-off levels of leukocytes (cut-off:6.345×109/L, sensitivity 46.0%, specificity 74.5%), lymphocytes (cut-off:1.340×109/L, sensitivity 60.0%, specificity 63.8%), neutrophils (cut-off:2.945×109/L, sensitivity 72.0%, specificity 50.0%) and fibrinogen (cut-off:3.99g/L, sensitivity 40.0%, specificity 83.0%).
 |
Table 4 The Area Under the ROC Curve and Optimal Cut-Off Values for the Blood Parameters Based on the Presence of Pneumonia in Symptomatic COVID-19 Patients Infected by SARS-CoV-2 Omicron Variant |
Then, ROC curve analyses were performed to compare the predictive performances among derived hematological indicators according to pneumonia in symptomatic Omicron patients (Figure 4A). As shown in Table 5, the AUC for NLR, MLR, FLR and FDR were 0.670 (95% CI:0.580–0.760, P=0.001), 0.632 (95% CI:0.535–0.728, P=0.009), 0.669 (95% CI:0.575–0.763, P=0.001) and 0.615 (95% CI:0.510–0.721, P=0.023), respectively. However, ELR and PLR had no statistical differences (P>0.05), and the AUC was relatively small (0.526 and 0.575). The cut-off levels of NLR (cut-off:2.23, sensitivity 62.0%, specificity 62.8%), MLR (cut-off:0.34, sensitivity 54.0%, specificity 74.5%), FLR (cut-off:2.21, sensitivity 70.0%, specificity 57.4%) and FDR (cut-off:10.43, sensitivity 40.0%, specificity 87.2%) were also provided by the analyses.
By applying logistic regression analysis, we aimed to determine the effect of derived hematological indicators on pneumonia in patients with symptomatic COVID-19 infected by the SARS-CoV-2 Omicron variant. Table 6 summarizes the results of the univariate analysis. As for the derived hematological indicators, elevated levels of NLR (OR:1.219, 95% CI:1.046–1.421, P=0.011), FLR (OR:1.170, 95% CI:1.014–1.349, P=0.031) and FDR (OR:1.131, 95% CI:1.039–1.231, P=0.005) were significantly correlated with an increased risk of pneumonia. In order to find the ideal model, forward stepwise multivariate logistic regression was performed. Ultimately elevated levels of NLR (OR:1.248, 95% CI:1.068–1.459, P=0.005) and FDR (OR:1.160, 95% CI:1.054–1.276, P=0.002) were associated with the development of pneumonia (Table 6).
 |
Table 6 Univariate and Multivariate Logistic Analyses for Determining Risk Factors for Pneumonia in Symptomatic COVID-19 Patients Infected by SARS-CoV-2 Omicron Variant |
Finally, we performed ROC curve analysis to evaluate the value of the combined NLR and FDR indicator for pneumonia. The combined NLR and FDR indicators could also predict pneumonia in symptomatic COVID-19 patients infected by the SARS-CoV-2 Omicron variant, with an AUC of 0.701 (95% CI:0.606–0.796, P<0.001, sensitivity 56.0%, specificity 83.0%) (Figure 4B, Table 5).
Discussion
The study ultimately included 144 patients with symptomatic Omicron infection, 50 of whom had pneumonia. Patients with COVID-19 who had pneumonia had higher levels of leukocytes, neutrophils, fibrinogen, and lower levels of lymphocytes compared to patients without pneumonic Omicron infection. The derived hematological indices NLR, MLR, FLR and FDR were significantly higher in the pneumonia group than in the non-pneumonia group. When ROC analysis was performed, these derived indicators had better results than the original hematological indicators. Univariate analysis of these derived hematological indicators also confirmed that NLR, FLR, and FDR were significantly associated with an increased risk of pneumonia. Furthermore, multivariate analysis showed statistically significant results for NLR and FDR. Ultimately, the ROC analysis suggested that the combination of NLR and FDR was more helpful than other indicators in identifying pneumonia in symptomatic COVID-19 patients infected by Omicron.
Previous studies have identified age and comorbidities as independent risk factors for pneumonia due to Omicron infection.14–16 However, in this study, there was no distinction in age and comorbidity between pneumonia and non-pneumonia groups. It may be due to the advanced age and the high incidence of comorbidities in the enrolled patients. More than half of the patients were elderly with comorbidities. Breakthrough infection with pneumonia has been reported.34 The Omicron variant may evade immunization from previous vaccines or infections,35–37 but vaccination can protect against hospitalization and death.19–21 In this study, the vaccination rate of the pneumonia group is lower than that of the non-pneumonia group, which is not statistically significant. However, this may be limited to the sample size, so it cannot reflect the protective effect of the vaccines on pneumonia. Although the data is limited, there is no evidence that the treatment of breakthrough infection should differ from the treatment used at the time of initial infection. This study also found that the nucleic acid NCT was longer in the pneumonia group, suggesting that patients with lower respiratory tract involvement might carry the virus for a longer time and have a longer duration of illness. This result also prompts that it is more important to investigate the risk factors for pneumonia and identify relevant indicators.
Inflammatory indicators have been studied extensively as reliable predictors of the progression and severity of COVID-19.25–27,38,39 In our study, we found elevated levels of leukocytes and neutrophils with decreased levels of lymphocytes in patients with Omicron-induced pneumonia compared to those with non-pneumonic Omicron infection, resulting in elevated NLR and MLR, the derived hematological indicators in the pneumonia group. Furthermore, the derived indicators NLR and MLR were superior to the original hematological inflammatory indicators in the ROC analysis. However, unlike the results of previous non-Omicron studies,40 other inflammatory indicators such as MLR, PLR and ELR were not statistically significant in the univariate analysis. Ultimately only NLR entered the model with statistical significance in multivariate logistic analysis.
Fibrinogen and D-dimer are traditional indicators for coagulation, while some studies have reported that they can also be indicators of inflammation rather than coagulation alone.41,42 Interestingly, there was no difference in D-dimer levels between the pneumonia group and the non-pneumonia group due to Omicron infection during the comparison of coagulation indicators in this study. In contrast, fibrinogen was significantly higher in the Omicron pneumonia group, and the derived indicators FLR and FDR were higher than their levels in the non-pneumonia group. Some studies have found that fibrinogen needs to be together with D-dimer for a more appropriate predictive role.43,44 Predictably, this study also found that FLR and FDR levels were significantly higher in the pneumonia group than in the non-pneumonia group and that the multifactorial analysis of FDR together with NLR was significant. Ultimately, the ROC analysis also confirmed that the combination of FDR and NLR was effective.
This study has several limitations. As a retrospective study, the study only involved symptomatic COVID-19 cases infected by Omicron, so the conclusions cannot be applied to asymptomatic Omicron cases. Only hematological indicators were discussed, and this study did not compare the symptoms of COVID-19 patients infected with Omicron. There was also no comparative analysis of specific CT manifestations of pulmonary involvement in the study. Undoubtedly NLR and FDR are traditional markers, but they are cheap, convenient, and a better choice. Moreover, due to the limited sample size, the protective effect of different vaccine statuses also requires further expansion of the sample size. The impact of different CT manifestations, symptoms, and vaccine status on the occurrence of pneumonia needs to be studied with a larger sample size.
Conclusion
In conclusion, the current study suggests that NLR and FDR can predict the presence of pneumonia in symptomatic COVID-19 patients infected with the SARS-CoV-2 Omicron variant.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author.
Ethical Approval and Consent to Participate
This study was authorized by the Ethics Commission for Clinical Research of Zhongda Hospital, affiliated to Southeast University. Informed consent was waived due to the nature of the study as a retrospective study. The study was conducted in accordance with the principles described in the Declaration of Helsinki and confidentiality of patients was guaranteed.
Acknowledgments
We thank the medical staff for their hard work in Lingang shelter hospital. Additionally, we want to express our gratitude to the heads of Lingang shelter hospital for their support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by a grant from Jiangsu Province Key research and development Program (Social Development) Special Project (BE2021734) and National Major Epidemic Treatment Base Construction Project (2019-320831-84-02-524538).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi:10.1056/NEJMoa2001316
2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi:10.1056/NEJMoa2001017
3. Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS-CoV-2 omicron variant in Southern Africa. Nature. 2022;603(7902):679–686. doi:10.1038/s41586-022-04411-y
4. Balint G, Voros-Horvath B, Szechenyi A. Omicron: increased transmissibility and decreased pathogenicity. Signal Transduct Target Ther. 2022;7(1):151. doi:10.1038/s41392-022-01009-8
5. Harrigan SP, Wilton J, Chong M, et al. Clinical severity of omicron SARS-CoV-2 variant relative to delta in British Columbia, Canada: a retrospective analysis of whole genome sequenced cases. Clin Infect Dis. 2022. doi:10.1093/cid/ciac705
6. Ito N, Kitahara Y, Miwata K, Okimoto M, Takafuta T. Comparison of COVID-19 pneumonia during the SARS-CoV-2 omicron wave and the previous non-omicron wave in a single facility. Respir Investig. 2022;60(6):772–778. doi:10.1016/j.resinv.2022.08.001
7. Jassat W, Abdool KS, Mudara C, et al. Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: a retrospective observational study. Lancet Glob Health. 2022;10(7):e961–e969. doi:10.1016/S2214-109X(22)00114-0
8. Hu Y, Zhan C, Chen C, Ai T, Xia L, Adrish M. Chest CT findings related to mortality of patients with COVID-19: a retrospective case-series study. PLoS One. 2020;15(8):e0237302. doi:10.1371/journal.pone.0237302
9. Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 Pneumonia. Invest Radiol. 2020;55(6):327–331. doi:10.1097/RLI.0000000000000672
10. Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of Coronavirus Disease (COVID-19) Pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214(5):1072–1077. doi:10.2214/AJR.20.22976
11. Leonard-Lorant I, Severac F, Bilbault P, et al. Normal chest CT in 1091 symptomatic patients with confirmed Covid-19: frequency, characteristics and outcome. Eur Radiol. 2021;31(7):5172–5177. doi:10.1007/s00330-020-07593-z
12. Komurcuoglu B, Susam S, Batum O, et al. Correlation between chest CT severity scores and clinical and biochemical parameters of COVID-19 pneumonia. Clin Respir J. 2022;16(7):497–503. doi:10.1111/crj.13515
13. Nakajima K, Kato H, Yamashiro T, et al. COVID-19 pneumonia: infection control protocol inside computed tomography suites. Jpn J Radiol. 2020;38(5):391–393. doi:10.1007/s11604-020-00948-y
14. Kinikar AA, Vartak S, Dawre R, et al. Clinical profile and outcome of hospitalized confirmed cases of omicron variant of SARS-CoV-2 among children in Pune, India. Cureus. 2022;14(4):e24629. doi:10.7759/cureus.24629
15. Tong X, Huang Z, Zhang X, et al. Old age is an independent risk factor for Pneumonia development in patients with SARS-CoV-2 omicron variant infection and a history of inactivated vaccine injection. Infect Drug Resist. 2022;15:5567–5573. doi:10.2147/IDR.S380005
16. Murillo-Zamora E, Trujillo X, Huerta M, et al. First-generation BNT162b2 and AZD1222 vaccines protect from COVID-19 pneumonia during the omicron variant emergence. Public Health. 2022;207:105–107. doi:10.1016/j.puhe.2022.04.001
17. Agrawal U, Bedston S, Mccowan C, et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. Lancet. 2022;400(10360):1305–1320. doi:10.1016/S0140-6736(22)01656-7
18. Suzuki K, Ichikawa T, Suzuki S, Tanino Y, Kakinoki Y. Clinical characteristics of the severe acute respiratory syndrome coronavirus 2 omicron variant compared with the delta variant: a retrospective case-control study of 318 outpatients from a single sight institute in Japan. Peerj. 2022;10:e13762. doi:10.7717/peerj.13762
19. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med. 2022;386(19):1804–1816. doi:10.1056/NEJMoa2200797
20. Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327(7):639–651. doi:10.1001/jama.2022.0470
21. Bjork J, Bonander C, Moghaddassi M, et al. COVID-19 vaccine effectiveness against severe disease from SARS-CoV-2 omicron BA.1 and BA.2 subvariants - surveillance results from southern Sweden, December 2021 to March 2022. Euro Surveill. 2022;27(18):2200322. doi:10.2807/1560-7917.ES.2022.27.18.2200322
22. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi:10.1093/cid/ciaa248
23. Abrishami A, Eslami V, Arab-Ahmadi M, Alahyari S, Azhideh A, Sanei-Taheri M. Prognostic value of inflammatory biomarkers for predicting the extent of lung involvement and final clinical outcome in patients with COVID-19. J Res Med Sci. 2021;26:115. doi:10.4103/jrms.JRMS_1160_20
24. Boncuoglu E, Coskun M, Kiymet E, et al. Can laboratory findings predict pulmonary involvement in children with COVID-19 infection? Pediatr Pulmonol. 2021;56(8):2489–2494. doi:10.1002/ppul.25452
25. Wang Q, Cheng J, Shang J, et al. Clinical value of laboratory indicators for predicting disease progression and death in patients with COVID-19: a retrospective cohort study. BMJ Open. 2021;11(10):e043790. doi:10.1136/bmjopen-2020-043790
26. Seyit M, Avci E, Nar R, et al. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Am J Emerg Med. 2021;45:569. doi:10.1016/j.ajem.2020.12.069
27. Yu GQ, Zhang Q, Wang RC, Jiang SQ. Predictive value of neutrophil-to-lymphocyte ratio and other inflammatory indicators in estimating clinical severity of coronavirus disease. World J Emerg Med. 2021;12(1):79–80. doi:10.5847/wjem.j.1920-8642.2021.01.014
28. Ye W, Chen G, Li X, et al. Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19. Respir Res. 2020;21(1):169. doi:10.1186/s12931-020-01428-7
29. Wang L, Yang L, Bai L, Huang Z, Peng Y. Association between D-dimer level and chest CT severity score in patients with SARS-COV-2 pneumonia. Sci Rep. 2021;11(1):11636. doi:10.1038/s41598-021-91150-1
30. Yao Y, Cao J, Wang Q, et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. 2020;8:49. doi:10.1186/s40560-020-00466-z
31. Ozen M, Yilmaz A, Cakmak V, et al. D-dimer as a potential biomarker for disease severity in COVID-19. Am J Emerg Med. 2021;40:55–59. doi:10.1016/j.ajem.2020.12.023
32. Hu X, Xing Y, Jia J, et al. Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19. Sci Total Environ. 2020;728:138812. doi:10.1016/j.scitotenv.2020.138812
33. Benoni R, Campagna I, Panunzi S, et al. Estimating COVID-19 recovery time in a cohort of Italian healthcare workers who underwent surveillance swab testing. Public Health. 2021;196:52–58. doi:10.1016/j.puhe.2021.05.014
34. Sun J, Zheng Q, Madhira V, et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2022;182(2):153–162. doi:10.1001/jamainternmed.2021.7024
35. Zhang X, Wu S, Wu B, et al. SARS-CoV-2 omicron strain exhibits potent capabilities for immune evasion and viral entrance. Signal Transduct Target Ther. 2021;6(1):430. doi:10.1038/s41392-021-00852-5
36. Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi:10.1056/NEJMoa2119451
37. Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387(1):21–34. doi:10.1056/NEJMoa2203965
38. Fors M, Ballaz S, Ramirez H, et al. Sex-dependent performance of the neutrophil-to-lymphocyte, monocyte-to-lymphocyte, platelet-to-lymphocyte and mean platelet volume-to-platelet ratios in discriminating COVID-19 severity. Front Cardiovasc Med. 2022;9:822556. doi:10.3389/fcvm.2022.822556
39. Vafadar ME, Teimouri A, Rezaee R, et al. Increased age, neutrophil-to-lymphocyte ratio (NLR) and white blood cells count are associated with higher COVID-19 mortality. Am J Emerg Med. 2021;40:11–14. doi:10.1016/j.ajem.2020.12.003
40. Damar CT, Torun A, Cakirca G, Portakal RD. Role of NLR, PLR, ELR and CLR in differentiating COVID-19 patients with and without pneumonia. Int J Clin Pract. 2021;75(11):e14781. doi:10.1111/ijcp.14781
41. Shorr AF, Thomas SJ, Alkins SA, Fitzpatrick TM, Ling GS. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest. 2002;121(4):1262–1268. doi:10.1378/chest.121.4.1262
42. Borowiec A, Dabrowski R, Kowalik I, et al. Elevated levels of d-dimer are associated with inflammation and disease activity rather than risk of venous thromboembolism in patients with granulomatosis with polyangiitis in long term observation. Adv Med Sci. 2020;65(1):97–101. doi:10.1016/j.advms.2019.12.007
43. Hayiroglu MI, Cinar T, Tekkesin AI. Fibrinogen and D-dimer variances and anticoagulation recommendations in Covid-19: current literature review. Rev Assoc Med Bras. 2020;66(6):842–848. doi:10.1590/1806-9282.66.6.842
44. Murat S, Murat B, Dural M, Mert GO, Cavusoglu Y. Prognostic value of D-dimer/fibrinogen ratio on in-hospital outcomes of patients with heart failure and COVID-19. Biomark Med. 2021;15(16):1519–1528. doi:10.2217/bmm-2021-0341
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




