Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
Validation of the French version of the London Chest Activity of Daily Living scale and the Dyspnea-12 questionnaire
Authors Beaumont M, Couturaud F, Jego F, Pichon R, Le Ber C , Péran L, Rogé C, Renault D, Narayan S, Reychler G
Received 28 June 2017
Accepted for publication 17 November 2017
Published 30 April 2018 Volume 2018:13 Pages 1399—1405
DOI https://doi.org/10.2147/COPD.S145048
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Marc Beaumont,1,2 Francis Couturaud,3 Florence Jego,4 Romain Pichon,5 Catherine Le Ber,1 Loïc Péran,1 Christophe Rogé,6 David Renault,6 Swathi Narayan,7 Gregory Reychler8–10
1Pulmonary Rehabilitation Unit, Morlaix Hospital Centre, Morlaix, France; 2European University of Occidental Brittany, EA3878, Brest, France; 3Department of Internal Medicine and Chest Diseases, EA3878 (GETBO), CIC INSERM 0502, University Hospital of Brest, European University of Occidental Brittany, Brest, France; 4Clinical Research Unit, Morlaix Hospital Centre, Morlaix, France; 5Pulmonary Rehabilitation Unit, Rennes, France; 6Pulmonology Unit, Morlaix Hospital Centre, Morlaix, France; 7European University of Occidental Brittany, Brest, France; 8Institut de Recherche Expérimentale et Clinique (IREC), Pôle de Pneumologie, ORL & Dermatologie, Université Catholique de Louvain, Brussels, Belgium; 9Service de Pneumologie, Cliniques Universitaires Saint-Luc, Brussels, Belgium; 10Service de Médecine Physique et Réadaptation, Cliniques Universitaires Saint-Luc, Brussels, Belgium
Introduction: Eighty percent of COPD patients experience dyspnea during activities of daily life (ADL). To the best of our knowledge, the Modified Medical Research Council (MMRC) dyspnea scale is the only validated scale designed to quantify dyspnea during ADL available in the French language. Two other instruments are only available in English versions: the London Chest Activity of Daily Living (LCADL) scale that allows a specific evaluation of dyspnea during ADL and the Dyspnea-12 questionnaire that evaluates the affective (emotional) and sensory components of dyspnea in daily life. The aim of this study was to translate and validate French versions of both LCADL and Dyspnea-12 questionnaires and to determine the reliability of these versions for the evaluation of dyspnea in severe to very severe COPD patients.
Methods: Both translation and cultural adaptation were based on Beaton’s recommendations. Fifty consecutive patients completed the French version of LCADL and Dyspnea-12 and other questionnaires (MMRC, Saint George’s Respiratory Questionnaire [SGRQ], Hospital Anxiety and Depression [HAD]), at a 2-week interval. Internal consistency, validity, and reliability of LCADL and Dyspnea-12 were evaluated.
Results: The French version of LCADL and Dyspnea-12 demonstrated good internal consistency with Cronbach’s α of, respectively, 0.84 and 0.91. LCADL was correlated significantly with item activity of SGRQ (ρ=0.55, p<0.001), total score of SGRQ (ρ=0.63, p<0.001), item impact of SGRQ (ρ=0.57, p<0.001), and HAD-depression (HAD-D) (ρ=0.47, p=0.001); and Dyspnea-12 was correlated significantly with MMRC (ρ=0.39, p<0.001), HAD-anxiety (ρ=0.64, p<0.001), and HAD-D (ρ=0.64, p<0.001). The French version of LCADL and Dyspnea-12 demonstrated good test–retest reliability with, respectively, intraclass coefficient =0.84 (p<0.001) and 0.91 (p<0.001).
Conclusion: The French versions of LCADL and Dyspnea-12 questionnaires are promising tools to evaluate dyspnea in severe to very severe COPD patients.
Keywords: COPD, dyspnea, evaluation, physiotherapy, quality of life
Introduction
COPD is characterized by a permanent and progressive obstruction of airways, inflammation, and systemic manifestations.1 During COPD progression, muscle, cardiovascular, psychological, nutritional, bone, or neurological impairments may appear.2 Breathlessness, the main symptom presented by COPD patients,3 results from bronchial and parenchyma alterations and peripheral muscle changes, all alterations that contribute dramatically to the disability of COPD patients.3,4
Activities of daily life (ADL) are defined as all movements performed every day by a person with the aim of taking care of himself/herself for participation in social life.5,6 COPD patients report important difficulties during ADL. In a study of Polatli et al,7 the vast majority of COPD patients interviewed mentioned a negative impact of their disease on their ADL. Garrod et al8 showed that ~80% of COPD patients interviewed experienced dyspnea during ADL. The most frequently altered ADL are walking and getting upstairs, followed by sexual activity, domestic tasks, and personal care.7,9,10 Dyspnea and fatigue are the main causes of these limitations, particularly in ADL involving upper limbs.4 To the best of our knowledge, the Modified Medical Research Council (MMRC) scale is the only validated scale in French language to evaluate the functional status of dyspnea in ADL.11 However, this scale is moderately sensitive to changes and explores a limited part of ADL (mainly walking). The London Chest Activity of Daily Living (LCADL) scale allows evaluating dyspnea in the current ADL performed by patients. The Dyspnea-12 questionnaire12 allows evaluating the affective (emotional) and sensory components of dyspnea. In COPD patients, Garrod et al and Yorke et al showed that LCADL8,13 and Dyspnea-12 were valid and reliable questionnaires.12,14 Moreover, LCADL was responsive to improvement following pulmonary rehabilitation. While LCADL was developed in English8 and was translated and validated in Portuguese and Spanish,15–17 there is no translated and validated version in French language. Similarly, Dyspnea-12 questionnaire is only validated in English.12
The aim of this study was thus to develop and to validate a French version of both LCADL and Dyspnea-12 questionnaires and to assess the reliability of these versions for the evaluation of dyspnea in severe and very severe COPD patients.
Methods
Ethics statement
The study was approved by the local ethic board (CPP Ouest 6 – CPP 886 – Soins courants; RCB: 2015-A00631-48) on June 2015 and registered (ClinicalTrials.gov identifier: NCT02555202). All patients provided written informed consent.
Procedure
Translation and cultural adaptation were based on Beaton’s recommendations.18 Initial translation was realized independently by two native French speakers (FC and MB), with permission to translate and use the questionnaires obtained from the authors of the original versions. A synthesis of the two translations was realized to end in a common version. The translation return (from French toward English) was performed by an independent English native speaker (SN), unaware of the original questionnaires, in order to check accuracy.
A version of each translation was tried and discussed with 10 patients to raise concerns about whether the sentences used were understood. Patients found them comprehensible completing both questionnaires and no patient hadany queries about the sentences used in these two translations. This final version was thus used in the present study (Supplementary materials).
Protocol
To test the validity and reliability of both questionnaires, we only used the instruments which were used for the development of each questionnaire.13,14,19
Validity
Internal consistency and validity of the French version of LCADL and Dyspnea-12 were estimated at the time COPD patients paid their annual visit to the outpatient clinic.
For LCADL, the primary end point was the correlation of LCADL with activity’s score of Saint George’s Respiratory Questionnaire (SGRQ), as it was used for the original validation of LCADL.8 Secondary end points were the correlation of LCADL with the total score of SGRQ, impact’s score of SGRQ, MMRC scale, Hospital Anxiety and Depression (HAD) scale – item anxiety (HAD-A) and item depression (HAD-D), as it was used for the original validation of LCADL.
For Dyspnea-12, the primary end point was the correlation of Dyspnea-12 with MMRC scale12 as it was used for the original validation of Dyspnea-12. Secondary end points were the comparison of Dyspnea-12 with HAD scale – item anxiety and item depression, FEV1, as it was used for the original validation of Dyspnea-12.
Reliability
Reliability of the French version of LCADL and Dyspnea-12 was estimated by the method of test–retest over a period of 15 days. Patients filled in LCADL and Dyspnea-12 questionnaires and the other questionnaires (MMRC, SGRQ, and HAD) during outpatient clinic visits. Participants were provided with a copy of all questionnaires and were asked to fill them all out 15 days later at home and to mail them back.
LCADL scale
This 15-item, self-administered questionnaire has been developed by Garrod et al.19 It allows an evaluation of dyspnea in patients with COPD during daily activities divided into four components: self-care, domestic, physical, and leisure. Patients could score from 0: “I would not do anyway” to 5: “I need someone else to do this”. LCADL score is calculated by aggregating the points assigned to each question, with a higher score representing maximal disability.
Dyspnea-12
This 12-item self-administered questionnaire12,14 measures dyspnea severity in both its physical and affective components, independently from activity limitation. Patients score ranges from “none” (corresponding to score 0) to “severe” (score 3). Dyspnea-12 score is calculated by aggregating the points assigned to each question; the higher the score, the greater the severity.
SGRQ
SGRQ is a reliable measure of health status in COPD patients20 and has been validated in French language.21 It is sensitive to changes in health status over time22 and a minimal clinical important difference (MCID) has been proposed.23,24 SGRQ consists of 50 items with four scores: symptoms, activity, psychosocial impact, and a total score. The highest score at 100 represents the maximal negative impact of COPD on quality of life.
MMRC dyspnea scale
MMRC dyspnea scale is the first self-administered scale which assesses the impact of dyspnea on ADL.25 It consists of five grades increasing in severity of chronic respiratory disease11 from “I only get breathless with strenuous exercise” to “I am too breathless to leave the house or I am breathless when dressing or undressing.”
HAD scale
It is a validated self-administered scale used for assessing psychological distress. It was validated in French language.26 It consists of 14 items, seven for evaluating anxiety and seven for depression, with a score ranging from 0 to 21 for each domain. HAD-anxiety or HAD-depression score ≥1127,28 suggests significant anxiety or depression. A MCID has been proposed.29
Study population
All COPD patients attending the outpatient clinic for their annual follow-up in the pulmonology unit of Morlaix Hospital Centre were eligible for the study if they were diagnosed with severe or very severe COPD, according to Global initiative for Obstructive Lung Disease (GOLD) guideline’s criteria,30 and able to complete questionnaires in French language. Exclusion criteria were COPD exacerbation in the previous month or during the 15 days following inclusion, and absence of written informed consent. To assess stability of the disease, we questioned patients about acute exacerbation between test and retest.
Sample size
For the analysis of reliability, according to Walter et al,31 by considering an intraclass coefficient (ICC) of 0.8 as being acceptable, 46 subjects were required. Taking into account a 10% proportion of unreturned questionnaires, the total sample size was set to 50 patients.
Statistical analysis
A descriptive analysis was performed for demographic parameters and for questionnaires results. The internal consistency of LCADL and Dyspnea-12 was assessed using Cronbach’s α coefficient. The validity of LCADL and Dyspnea-12 was measured using Pearson’s or Spearman’s correlation between, respectively, the score of the French version of LCADL and item activity’s score of SGRQ, total score of SGRQ, item impact’s score of SGRQ, MMRC scale, and HAD scale; and the score of the French version of Dyspnea-12 and MMRC scale, HAD scale, and FEV1.
Floor and ceiling effects were verified if at least 15% of participants reached the lowest or the highest score, respectively. The test–retest reliability was evaluated using ICC for agreement and agreement was estimated using Bland–Altman method.
All tests were two-tailed, with a statistical significance level fixed at a p-value of 0.05. The data were computed using SPSS 20.0 (IBM Corporation, Armonk, NY, USA).
Results
Fifty consecutive patients met the inclusion criteria and completed the French version of LCADL and Dyspnea-12, and the others questionnaires (MMRC, SGRQ, and HAD) (Figure 1). Two patients were excluded because they had acute exacerbation in the 15 days following inclusion. Forty-eight patients completed the second set of questionnaires for the test–retest assessment. Demographic items, clinical data, and initial results of the questionnaires are reported in Table 1.
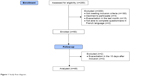  | Figure 1 Study flow diagram. |
LCADL
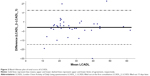
The French version of LCADL demonstrated good internal consistency (Cronbach’s α=0.84) and good test–retest reliability (ICC=0.84 [95% CI 0.72–0.91], p<0.001) (Figure 2).
LCADL score was significantly correlated with activity’s score of SGRQ (ρ=0.55, p<0.001), total score of SGRQ (ρ=0.63, p<0.001), impact’s score of SGRQ (ρ=0.57, p<0.001), HAD-D (ρ=0.47, p=0.001), but not with MMRC (ρ=0.28, p=0.05) nor with HAD-A (ρ=0.24, p=0.09).
Dyspnea-12
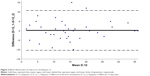
The French version of Dyspnea-12 demonstrated good internal consistency (Cronbach’s α=0.91) and good test–retest reliability (ICC=0.91 [95% CI 0.84–0.95], p<0.001) (Figure 3).
It was significantly correlated with MMRC (ρ=0.39, p<0.001), HAD-A (ρ=0.64, p<0.001), HAD-D (ρ=0.64, p<0.001), but not with FEV1 (ρ=−0.22, p=0.13). The analysis of the scores distribution in our patients’ population revealed an absence of ceiling and floor effects for LCADL and Dyspnea-12 with <3% of patients having the lowest or highest scores.
Discussion
This study shows good validity and internal consistency for both French versions of LCADL and Dyspnea-12. The test–retest reliability was verified for the translated versions of both questionnaires. These results contribute to a proper validation of our French version of these two instruments in severe or very severe COPD patients.
The results of the primary end points (SGRQ item activity for LCADL and MMRC for Dyspnea-12) obtained in our study are in agreement with the initial validation of both questionnaires LCADL and Dyspnea-12. For LCADL, the value of correlation with quality of life, assessed by means of the SGRQ, is lower than that observed in the original English validation. The fact that dyspnea is not the sole cause of alteration of quality of life in COPD can partly explain these findings.
For the secondary end points, some differences appeared in our study: LCADL was neither correlated with MMRC nor with HAD-A and Dyspnea-12 was not correlated with FEV1. As regards MMRC, the correlation failed to reach statistical significance, as was the case for the validation of the Spanish version.17 MMRC is widely used in clinical care but might be less discriminating than LCADL. Indeed, LCADL explores more situations than MMRC and that can explain the lack of correlation between these scales. As regards HAD, Garrod et al8 reported a significant correlation between LCADL and HAD-A but not with HAD-D. In our study, we found the opposite – a significant correlation between LCADL and HAD-D but not with HAD-A. Our patients had more severe disease according to GOLD and were younger than those studied by Garrod et al. Prevalence of depression increases with increasing COPD severity.32 Conversely, anxiety is more in older adults.33 These trends can explain differences between our results and those of Garrod et al.
For Dyspnea-12, we found no correlation with FEV1; in our study, we included only severe or very severe COPD patients and that can explain this absence of correlation. Earlier studies on this topic found conflicting results.34–38 Indeed, a lot of patients can present moderate COPD and very high disability. In contrast, patients with severe COPD can too exhibit moderate disability.
The results of this study have a clinical impact for French speaking COPD patients, which represent a large population. Dyspnea is the major symptom reported by COPD patients39,40 and the main reason for referral. Recent advances in the knowledge on the mechanisms of dyspnea41 highlight the interest of an evaluation that takes into account three domains:42 sensory or physical, affective, and impact.43 This complete evaluation gives more information and allows a better understanding of the causes and mechanisms of dyspnea, in the aim to treat it optimally. This is clearly mentioned in the American Thoracic Society statement about update on the mechanisms, assessment, and management of dyspnea.43
French validation of LCADL and Dyspnea-12 enables evaluating the impact of dyspnea and measuring sensory and affective components of dyspnea in daily living, respectively. With the recent validation of the multidimensional dyspnea profile,44 French respiratory caregivers and researchers can handle different tools enabling an indepth evaluation of dyspnea.
Our study had some limitations: first, we only included severe or very severe COPD patients from a single center to validate the Dyspnea-12 scale. Second, it could also be interesting to make use of the coefficient of determination (r2) that might be more useful to assess the degree of variation in one score, as is explained by the other.45 The choice of Pearson’s r in our study was driven for use for the original validation of Dyspnea-12.
In conclusion, the French versions of LCADL and Dyspnea-12 are valid and reproducible to evaluate dyspnea in severe or very severe COPD patients.
Acknowledgments
We wish to thank Society Orkyn for the financial support and especially Mrs Nadine Morel (Orkyn) for her help. We thank Miss Swathi Narayan for the translation of the questionnaires. We thank Pr Leroyer for help with the English language.
Disclosure
The authors report no conflicts of interest in this work.
References
Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. | ||
Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013;1(1):73–83. | ||
Ries AL. Impact of chronic obstructive pulmonary disease on quality of life: the role of dyspnea. Am J Med. 2006;119(10 Suppl 1):12–20. | ||
Jolley CJ, Moxham J. A physiological model of patient-reported breathlessness during daily activities in COPD. Eur Respir Rev. 2009;18(112):66–79. | ||
Blouin M. Dictionnaire de la réadaptation. Volume 2. Quebec: Gouvernement du Quebec; 1995. | ||
Velloso M, Jardim JR. Functionality of patients with chronic obstructive pulmonary disease: energy conservation techniques. J Bras Pneumol. 2006;32(6):580–586. | ||
Polatli M, Bilgin C, Şaylan B, et al. A cross sectional observational study on the influence of chronic obstructive pulmonary disease on activities of daily living: the COPD-life study. Tuberk Toraks. 2012;60(1):1–12. | ||
Garrod R, Bestall J, Paul E, Wedzicha J, Jones P. Development and validation of a standardized measure of activity of daily living in patients with severe COPD: the London chest activity of daily living scale (LCADL). Respir Med. 2000;94(6):589–596. | ||
Katz PP, Gregorich S, Eisner M, et al. Disability in valued life activities among individuals with COPD and other respiratory conditions. J Cardiopulm Rehabil Prev. 2010;30(2):126–136. | ||
Annegarn J, Meijer K, Passos VL, et al. Problematic activities of daily life are weakly associated with clinical characteristics in COPD. J Am Med Dir Assoc. 2012;13(3):284–290. | ||
Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. | ||
Yorke J, Moosavi SH, Shuldham C, Jones PW. Quantification of dyspnea using descriptors: development and initial testing of the Dyspnea-12. Thorax. 2010;65(1):21–26. | ||
Garrod R. Development and validation of a standardized measure of activity of daily living in patients with severe chronic obstructive pulmonary disease: the London chest activity of daily living scale. J Cardiopulm Rehabil. 2001;21(3):178. | ||
Yorke J, Swigris J, Russell AM, et al. Dyspnea-12 is a valid and reliable measure of breathlessness in patients with interstitial lung disease. Chest. 2011;139(1):159–164. | ||
Pitta F, Probst VS, Kovelis D, et al. Validação da versão em português da escala London Chest Activity of Daily Living (LCADL) em doentes com doença pulmonar obstrutiva crónica. Rev Port Pneumol. 2008;14(1):27–47. | ||
Carpes MF, Mayer AF, Simon KM, Jardim JR, Garrod R. Versão brasileira da escala London Chest Activity of Daily Living para uso em pacientes com doença pulmonar obstrutiva crônica. J Bras Pneumol. 2008;34(3):143–151. | ||
Vilaro J, Gimeno E, Sanchez N, et al. Actividades de la vida diaria en pacientes con enfermedad pulmonar obstructiva crónica: validación de la traducción española y análisis comparativo de 2 cuestionarios. Med Clin (Barc). 2007;129(9):326–332. | ||
Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186–3191. | ||
Garrod R, Paul EA, Wedzicha JA. An evaluation of the reliability and sensitivity of the London chest activity of daily living scale (LCADL). Respir Med. 2002;96(9):725–730. | ||
Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl 2):25–31. | ||
Bouchet C, Guillemin F, Original A. Validation du questionnaire St Georges pour mesurer la qualité de vie chez les insuffisants respiratoires chroniques. Rev Mal Respir. 1996;13(1):43–46. | ||
Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. | ||
Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79. | ||
Welling JBA, Hartman JE, Ten Hacken NHT, Klooster K, Slebos D-J. The minimal important difference for the St George’s Respiratory Questionnaire in patients with severe COPD. Eur Respir J. 2015;46(6):1598–1604. | ||
Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. | ||
Lepine JP, Godchau M, Brun P. Anxiety and depression in inpatients. Lancet. 1985;326(8469–8470):1425–1426. | ||
SPLF. Recommandation pour la pratique clinique. Prise en charge de la BPCO, mise à jour 2009. Rev Mal Respir. 2010;27(5):522–548. | ||
Snaith RP. The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes. 2003;1:29. | ||
Puhan MA, Frey M, Büchi S, Schünemann HJ. The minimal important difference of the Hospital Anxiety and Depression Scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6:46. | ||
Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. | ||
Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med. 1998;17(1):101–110. | ||
Lacasse Y, Rousseau L, Maltais F. Prevalence of depressive symptoms and depression in patients with severe oxygen-dependent chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2001;21(2):80–86. | ||
Deb A, Sambamoorthi U. Depression treatment patterns among adults with chronic obstructive pulmonary disease and depression. Curr Med Res Opin. 2017;33(2):201–208. | ||
Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. | ||
Lareau SC, Meek PM, Roos P. Development and testing of the modified version of the Pulmonary Functional Status and Dyspnea Questionnaire (PFSDQ-M). Heart Lung. 1998;27(3):159–168. | ||
Belza B, Steele B, Hunziker J, Lakshminaryan S, Holt L, Buchner D. Correlates of physical activity in chronic obstructive pulmonary disease. Nurs Res. 2001;50(4):195–202. | ||
Bendstrup KE, Ingemann Jensen J, Holm S, Bengtsson B. Out-patient rehabilitation improves activities of daily living, quality of life and exercise tolerance in chronic obstructive pulmonary disease. Eur Respir J. 1997;10(12):2801–2806. | ||
Garcia-Aymerich J, Félez MA, Escarrabill J, et al. Physical activity and its determinants in severe chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2004;36(10):1667–1673. | ||
Sergysels R. Question 3-1. L’évaluation fonctionnelle de repos. Rev Mal Respir. 2005;22:20–23. | ||
Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150(8):1685–1689. | ||
Dangers L, Morelot-Panzini C, Schmidt M, Demoule A. Mécanismes neurophysiologiques de la dyspnée: de la perception à la clinique. Réanimation. 2014;23(4):392–401. | ||
Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009;167(1):53–60. | ||
Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–453. | ||
Banzett RB, O’Donnell CR, Guilfoyle TE, et al. Multidimensional dyspnea profile: an instrument for clinical and laboratory research. Eur Respir J. 2015;45(6):1681–1691. | ||
Williams MT, John D, Frith P. Comparison of the Dyspnoea-12 and multidimensional dyspnoea profile in people with COPD. Eur Respir J. 2017;49(3):1600773. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.