Back to Journals » Vascular Health and Risk Management » Volume 19
Validation of CHA2DS2 VASc Score Predictability of Stroke and Systemic Embolization in a Middle Eastern Population with AF: The Jordan Atrial Fibrillation (JoFib) Study
Authors Ibdah R , Obeidat O, Khader Y , Al-Nusair J, Abusurrah O, Obeidat A, Obeidat A, Rawashdeh S , Alrabadi N , Obiedat AF, Alnadi NN, Hammoudeh A
Received 23 January 2023
Accepted for publication 31 March 2023
Published 24 April 2023 Volume 2023:19 Pages 255—264
DOI https://doi.org/10.2147/VHRM.S404575
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Daniel Duprez
Rasheed Ibdah,1,* Omar Obeidat,1,* Yousef Khader,2 Jowan Al-Nusair,1 Obada Abusurrah,1 Abedallah Obeidat,1 Ali Obeidat,1 Sukaina Rawashdeh,1 Nasr Alrabadi,3 Abdelrahman F Obiedat,4 Naseem N Alnadi,4 Ayman Hammoudeh4
1Division of Cardiology, Department of Internal Medicine, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 2Department of Community Medicine, Public Health and Family Medicine, Faculty of Medicine, Jordan University of Science & Technology, Irbid, 22110, Jordan; 3Department of Pharmacology, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 4Department of Cardiology, Istishari Hospital, Amman, Jordan
*These authors contributed equally to this work
Correspondence: Rasheed Ibdah, Division of Cardiology, Department of Internal Medicine, Jordan University for Science and Technology, Irbid, 22110, Jordan, Email [email protected]
Background and Purpose: CHA2DS2-VASc score is one of the most widely used scoring systems to assess the risk of systemic embolization and stroke in patients suffering from atrial fibrillation (Afib); furthermore, it is important in guiding their treatment. This study aimed to evaluate the predictivity of this score in the Jordanian population, build a deeper understanding of patients’ demographic and risk factors, and assess the usefulness of anticoagulation as a preventive measure.
Methods: A total of 2020 patients with Afib registered in the Jordanian Atrial Fibrillation (JoFib) registry were enrolled in this study. All patients were followed up for 1 year to assess their susceptibility to develop cerebrovascular accident (CVA) and systemic embolism (SE). The association between CHA2DS2-VASc score and risk of development of stroke or systemic embolization was analyzed based on bivariate and adjusted multivariate analyses. The ROC curve was used to assess the predictivity of the CHA2DS2-VASc score.
Results: The mean age of the study population was 67.8 years; 45.8% were males, and 81.8% were on anticoagulants. And, 71.8% had a CHA2DS2-VASc score of ≥ 3. During the follow-up period of 1 year; 69 developed new CVA (mean age, 72.8 years), and 9 developed SE. A total of 276 patients died; 18 patients died (6.5% out of all deceased)% from CVA. A moderate predictive power of the CHA2DS2-VASc score was demonstrated through ROC curve analysis with C statistics of 0.689 CI (0.634 to 0.744) for predicting the development of SE or CVA at 1 year.
Conclusion: CHA2DS2-VASc showed a moderate predictivity of stroke, SE, and all-cause mortality at 1 year. The study suggested disregarding gender differences in deciding to initiate anticoagulant therapy.
Keywords: atrial fibrillation, Jordan, CHA2DS2-VASc, stroke, risk
Introduction
Atrial fibrillation (AFib) is the most common type of treated cardiac arrhythmia. In fact, the lifetime risk of developing AFib is 1 in 4 for men and women aged 40 years and older.1 This risk increases among individuals of European ancestry, obesity, hypertension, diabetes and ischemic heart disease.2,3 Moreover, Afib has shown to independently increase the risk of death. In a nationwide study, all-cause mortality in AFib patients was found to be four times higher than that in the general population.4 In addition, patients with non-valvular AFib have an annual risk of 5% for developing arterial thromboembolism, including stroke.4
In order to guide treatment and determine the need for anticoagulant prescription, various clinical risk-scoring systems have been implemented. The CHA2DS2-VASc score, an updated version of the CHADS2 score, is widely utilized to approximate the risk of stroke in patients with non-rheumatic AFib. The score is calculated by summing the points for congestive heart failure, hypertension, age ≥75 years, diabetes, prior stroke, vascular disease, age 65–74 years and sex (female) category.5,6 It is a simple and effective tool for either low-risk or high-risk patients, but it does not provide the same advantage for AFib patients at an intermediate risk of stroke.7 In AFib patients with a CHA2DS2-VASc score of 1, treatment should be individualized based on a balance between thromboembolic and bleeding risks.8
With regards to mortality, previous literature have shown that these scores can predict 1-year all-cause mortality in patients with systolic heart failure with or without AFib and that the CHA2DS2-VASc score is an independent risk factor for mortality.9,10 Moreover, even though anticoagulation is recommended for scores of 2 or greater, a previous study concluded a protective effect on mortality only for scores of 5 or greater.10
We have come to the realization that the effective performance of scoring systems has not been sufficiently evaluated in different regions, and we believe that regional differences in terms of genetic and environmental factors may influence the effectiveness of these scores. Therefore, in this study, we aimed to evaluate the predictive ability of the CHA2DS2-VASc score for stroke and thromboembolism risk stratification of AFib patients in Jordan. The results of this study should be considered in conjunction with our previously published paper.11
Methodology
This is a prospective cohort study that employed baseline data from the Jordanian Atrial Fibrillation (JoFib) registry, which is a multi-centric, prospective, non-interventional, observational study registry. A total of 2020 patients aged 18 and above were consecutively enrolled from 18 hospitals and 11 outpatient cardiology clinics starting from May 25, 2019 to October 25, 2020.
This study has been conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. This study protocol was reviewed and approved by the Medical Research Ethical Committee and the Institutional Review Board (IRB) at the University of Jordan, approval number 10/2021/415. In addition, it has been approved by all Institutional Review Board (IRB) of participating centers, for more information please refer to http://clinicatrials.gov (unique identifier number NCT03917992). Written informed consent was obtained from the patients prior to study commencement. Treatment decisions were left to the discretion of the treating cardiologist.
Data were collected using standardized data forms at the time of enrollment, and at one, 6, and 12 months after. Diagnosis of AFib was confirmed by 12-lead electrocardiogram (EKG), rhythm strip lasting ≥30 seconds, ≥1 episode of AFib on an ambulatory ECG monitor, or a past diagnosis by a treating cardiologist. Baseline data included demographic and clinical information, laboratory data, medical history as well as history of anticoagulant use. The CHA2DS2-VASc and HAS-BLED scores were calculated for each participant.
Descriptive statistics were performed using means and standard deviation (SD) to describe the continuous variables, and percentages were used to describe the categorical variables. Bivariate and adjusted multivariate analyses were employed to examine the association between CHADS2VASc score and the incidence of cerebrovascular accident or systemic embolism. Specifically, binary bivariate analysis was utilized to determine the odds ratio of CHADS2VASc score using the optimal cutoff value derived from the receiver operating characteristic curve (ROC), adjusted for anticoagulation use. Additionally, multivariate analysis was performed to control for other confounding variables, such as dyslipidemia, smoking, and BMI, that were not incorporated into the score. These statistical methods enabled us to investigate the independent contribution of the CHADS2VASc score to the development of cerebrovascular accident or systemic embolism while controlling for other important risk factors. Predictive value of the CHA2DS2-VASc score was calculated according to the ROC analysis. SPSS 21 was used for the statistical analyses.
Results
Sample Characteristics
Of the 2020 patients recruited in this study, 1904 (94.3%) were followed for 1 year. 925 (45.8%) of the patients were males and the mean age for the patients was 67.8, ranging from 18 to 106 years. Most of the patients were hypertensive (74.6%), and about 43.6% of the patients were diabetic. In addition, 26.3% of them were known cases of heart failure, and 9% had chronic kidney disease. Of all the patients, 310 (15.3%) had prior history of stroke, and 31 (1.5) had a prior history of systemic embolization (SE). A total of 1653 (81.8%) patients were on anticoagulants. The majority of patients had CHA2DS2-VASc score ≥3 (71.8%). During the follow-up period, 69 (3.4%) patients developed new cerebrovascular accidents (CVA), 9 (0.45%) patients developed SE, 63 (3.1%) patients had major bleeding, and 276 (13.7%) patients died. Among the deceased patients, 6.5% (n=18) had cerebrovascular accident listed as the cause of death. This corresponds to a mortality rate of 0.9% (n=18) within the study population. Demographic characteristics are summarized in Table 1.
 |
Table 1 Demographic Characteristics of Study Sample and Follow-Up Outcomes |
New CVA or SE Patients’ Characteristics
Patients who experienced CVA were old with a mean age of 72.8 (p value = 0.000). The development of a new CVA or SE was significantly higher amongst diabetics, hypertensive patients and patients with prior stroke. However, gender, obesity, smoking and dyslipidemia were not statistically significantly higher in new stroke or SE patients as summarized in Table 2. The risk for developing CVA or SE was high in patients with CHA2DS2-VASc score of 4 or more in males and 5 or more in females with an odds ratio of 3.1 (1.761 to 5.457) after adjusting for dyslipidemia, smoking, and BMI. Also, the odds ratio was 4.337 (2.579 to 7.294) after adjusting for anticoagulant use only. No other factor was significantly related to the development of CVA or SE as summarized in Table 3. We found that anticoagulant use does not provide protection for development of CVA or SE, with an odds ratio of 1.031 CI (0.532 to 1.997), after adjusting for CHA2DS2-VASc score. However, its use protects from all causes of death and death from stroke with an odds ratio of 0.530 (0.387 to 0.726), 0.231 (0.087 to 0.613), respectively. ROC curve analysis demonstrated moderate predictive power of the CHA2DS2-VASc score with C statistics of 0.689 CI (0.634 to 0.744) in predicting the development of SE or CVA at 1 year, Figure 1. In addition, the C statistics for predicting CVA was 0.673 CI (0.615 to 0.732) and for predicting SE 0.774 CI (0.653 to 0.895) as summarized in Table 4. The optimum cut-off based on the sensitivity and specificity was 4 in males and 5 in females.
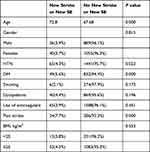 |
Table 2 Characteristics of Stroke Patients |
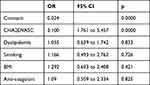 |
Table 3 Binary Logistic Regression Odd Ratio for Development of Stroke and SE |
 |
Table 4 ROC Curve Results |
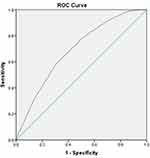 |
Figure 1 ROC cure for CHAD2VASC score and development of stroke or SE at 1 year. |
The risk of death from any cause was higher in patients with CHA2DS2-VASc score ≥4 in males and ≥5 in females, with an odds ratio of 2.585 CI (1.914 to 3.493), after adjusting for dyslipidemia, smoking, and BMI. Also, the odds ratio was 4.023 CI (3.055 to 5.296), after adjusting for anticoagulant use. The C-statistics in predicting all causes of death and death from stroke for CHA2DS2-VASc score was 0.698 CI (0.666 to 0.730) and 0.798 CI (0.697 to 0.899), respectively.
Discussion
Afib’s association with the development of CVA and SE and their high impact on patients’ lives demanded the development of a system to assess their risk. One of those scoring systems was CHADS2 score which assigned 1 point for each of the following: congestive heart failure (CHF), hypertension (HTN), age ≥75, diabetes (DM), and 2 points for previous stroke or transient ischemic attack (TIA); scores range from 0 to 6.12 Later on, the CHA2DS2-VASc score was introduced to be more inclusive in assessing risk factors than the CHADS2 score. It included the CHADS2 components plus the addition of vascular disease history, age 65–74, and sex category (female) with 1-point assigned to each. It also modified the score given to age ≥75 to be 2 points instead of 1. Many studies and guidelines worldwide support its usefulness in stratifying patients, diagnosed with afib, the risk for development of CVA and SE, and planning their management.5,13,14
This study on 2020 patients diagnosed with Afib who were recruited from multiple hospitals and outpatients’ clinics throughout Jordan, and were followed up for 1 year, suggests a moderate predictivity of CHADS2VASc score for the development of new stroke and SE. Also, it suggested a high predictivity for mortality of all causes and mortality from stroke and SE specifically. This result came in concordance with a meta-analysis that consisted of eight studies, the C statistics ranging from 0.64 to 0.79 (median 0.673), while in our study 0.689 95 CI (0.634 to 0.744).15 However, Inwu et al16 reported poor overall performance of the CHADS2VASc score in predicting stroke with C statistics of 0.52 (0.48–0.57), and for predicting death 0.60 (0.58–0.62). Furthermore, the performance of the score was higher in patients who were on oral anticoagulants in comparison with patients who were not.16 In this study, patients with CHA2DS2-VASc scores ≥2 in males or ≥3 in females had a greater risk of developing a stroke, having a relative risk of 9.394 (2.316 to 38.104), than patients with CHA2DS2-VASc scores <2 in males and <3 in females. This result came in concordance with a meta-analysis by Zhu et al,17 who reported a risk ratio of [RR]=5.15; 95% confidence interval [CI], 3.85–6.88 in patients with scores ≥2 than patients with score <2, independent of anticoagulant use.
Among factors that were measured in this study DM, HTN, and previous stroke are suggested to be of high significance as risk factors for the development of new stroke, and SE. However, smoking, dyslipidemia, and obesity do not seem to impose a significant impact as risk factors for the development of new stroke or SE. These results should be interpreted with caution due to key limitations in assessing and measuring some of these factors.
Interestingly, the optimal statistical cut points this study suggests, CHA2DS2-VASc score of 4 for males and 5 for females, raises the question about the significance of the point assigned for the female gender in this scoring system. However, clinically the cut point is lower as guidelines recommend initiation of anticoagulation therapy for patients with a score of 1 or greater.18 This study suggested that the exclusion of the gender point and following the guideline of initiation of OAC therapy at a score of 1 or higher. Many previous studies suggested that the female gender is a risk modifier rather than a true independent risk factor, and it should be excluded in the decision to start OAC therapy, as the sex category risk component accentuates the stroke risk in females already eligible for OAC.18–22 However, Wagstaff et al22 recommend the continuation of considering the gender point in the decision to use anticoagulants.
Anticoagulant, which is one of the cornerstones in the management of Afib as a preventive measure for new stroke and SE, was assessed as an important subject in this study. The results suggest that the use of anticoagulants had a significant association with a decrease in mortality from all causes and mortality from new CVA and SE. However, in contrast to most previous studies, it does not support that the use of anticoagulants has a significant impact in reducing the development of new CVA or SE in the study population. These results however should be interpreted with caution. Based on the study population, most of the patients were on warfarin (39.4%) with 48.2% of the patients having INR less than 1.8, which does not reach the therapeutic level of INR 2–3, suggesting that they are pharmacologically not responsive and that could be the reason why anticoagulant did not seem to protect from a new stroke. This finding could be attributed to multiple factors such as a diet rich in vit K, and/or drug noncompliance.23 There was no statistically significant difference in the stroke or systemic embolization rate between patients on novel OAC and patients on warfarin (3.8%, vs 4.1%, p = 0.796). However, Banerjee et al24 reported a higher clinical benefit in patients on dabigatran, rivaroxaban, and apixaban than in patients on warfarin. It is noteworthy to mention that cardioembolic stroke is associated with the highest mortality rate and poorest prognosis among all causes of ischemic stroke.25,26 While our study did not demonstrate a significant reduction in stroke incidence with the use of anticoagulation, it did show a significant association with lower overall and cerebrovascular accident-related mortality rates. Based on these findings, it is possible to speculate that even if embolization occurs in anticoagulated patients, it may be less severe and associated with a better prognosis. Further studies on the use of anticoagulants to prevent new CVA and SE in AFIB patients are required to unravel more information.
There are several limitations of this study that warrant discussion. Observational studies inherently carry the risk of potential biases such as residual confounding, data collection inaccuracies, and patient recall bias. However, the recruitment of consecutive patients from various sectors of the local healthcare system helped mitigate these effects. Additionally, as the major cardiovascular events analyzed were hard endpoints such as death, stroke, and major bleeding, they were unlikely to be significantly influenced by recall issues. Although the number of patients lost to follow-up was lower in this study (5.7%) compared to other investigations conducted in Europe (>12%),27,28 it could still potentially affect the discriminatory ability of certain analyses and the generalizability of the results.
Conclusion
CHA2DS2-VASc score is a moderately accurate predictor of stroke, systemic embolization and all-cause mortality at 1 year. The female gender is not a risk factor for stroke or systemic embolization, consequently, gender should be disregarded before proceeding to start anticoagulation. Additional studies are warranted regarding the effect of novel anticoagulant and warfarin use in atrial fibrillation patients.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Ethics Approval and Consent to Participate
This study has been conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. This study protocol was reviewed and approved by the Medical Research Ethical Committee and the Institutional Review Board (IRB) at the University of Jordan, approval number 10/2021/415. In addition, it has been approved by all Institutional Review Board (IRB) of participating centers, for more information please refer to http://clinicatrials.gov (unique identifier number NCT03917992). Written informed consent was obtained from the patients prior to study commencement.
Acknowledgement
The authors would like to thank the participating physicians who enrolled patients for the study (in alphabetical order): Abdallah Al-Kasasbeh, AbdelrahmanObiedat, Abdullah Bani-Melhim, Abdul Nasser Almohamed, Ahmad Abdelsattar, Ahmad Abu Awwad, Ahmad Sbaih, Ahmad Tamari, AlaaAbabneh, Alanoud Hamada, Alia Khamis, Ali Abu-Romman, Ali Shakhatreh, Amer Asia, Amin Ibdah, AmrAdas, AssemNammas, AwsMukhtar, AyadAl Qudsi, Basil Abdeen, Dalia Rawashdeh, EidAbu- Eid, Eyadeh Madanat, Fahd Al-Karmi, Farah Al-Sayyed, Farah Halaweh, Fatima Al Nadi, Hana Al- Raie, HaneenShawer, HaninSaleh, Hanna Makhamreh, HazemAbu Asbeh, HishamAmin, Ibrahim Abbadi, Ibrahim Jarrad, Islam Abu Sedo, Islam Obeidat, Ismail Hamam, Kais Bilbeisi, Lama Khader, LeenSharqawi, Liza Shaban, LubabaQabbaa, LubnaHaikal, LubabaQabbaah, Mahmoud Izraiq, Marwan Nemri, Marwan Al Sultan, Mo’athAlfawar’a, Mohammad Abdah, Mohammad Afeef, Mohammad Al-Jawabreh, Mohammad Ali Jarrah, Mohammad Hani, Mohammad Kraishan, MohannadAbabneh, MohamadJarrah, Mohammad Jabary, MunirZaqqa, Mustafa Jammal, MyassarAmr, Noor Al-Badaineh, Obada Mansour, OdaiKhasawneh, OdayAbu Ajameyyeh, Omar Abu Slieh, Omar Obeidat, Omar Salaymeh, OmranAbukhalaf, Osama Oudeh, QusaiAbu Ajameyyeh, RaedAwaisheh, Rami Bataineh, Rashid Abdah, Sa’ad Al-Muhaisen, Saed Al Ghamri, Safa’ Amro, Said Meri, SalehGhamdi, SukainaRawashdeh, Sara Al-Thunibat, Sara Younis, Sharif Adwan, SulafaSaffarini, Tamer Salhab, TawfiqBarqawi, YahyaIsmail, Yousef Khader, ZaidDabash, Zeina Abu-Orabi, ZainabSalahat, ZainahMatani, ZakariaShkoukani, and Zakariya Al-Hasanat.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding or sponsorship was received for this report or publication of this article.
Disclosure
The authors declare that they have no competing interests.
References
1. Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–1046. PMID: 15313941. doi:10.1161/01.CIR.0000140263.20897.42
2. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi:10.1161/CIR.0000000000000659
3. Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–844. PMID: 8114238. doi:10.1001/jama.1994.03510350050036
4. Lee E, Choi EK, Han KD, et al. Mortality and causes of death in patients with atrial fibrillation: a nationwide population-based study. PLoS One. 2018;13(12):e0209687. PMID: 30586468; PMCID: PMC6306259. doi:10.1371/journal.pone.0209687
5. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi:10.1378/chest.09-1584
6. Camm AJ, Kirchhof P, Lip GYH, et al. Guidelines for the management of atrial fibrillation: the task force for the management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. doi:10.1093/eurheartj/ehq278
7. van Doorn S, Debray TPA, Kaasenbrood F, et al. Predictive performance of the CHA2DS2-VASc rule in atrial fibrillation: a systematic review and meta-analysis. J Thromb Haemost. 2017;15:1065–1077. doi:10.1111/jth.13690
8. Sulzgruber P, Wassmann S, Semb AG, et al. Oral anticoagulation in patients with non-valvular atrial fibrillation and a CHA2DS2-VASc score of 1: a current opinion of the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy and European Society of Cardiology Council on Stroke. Eur Heart J Cardiovasc Pharmacother. 2019;5:171–180. doi:10.1093/ehjcvp/pvz016
9. Chen YL, Cheng CL, Huang JL, et al.; TSOC-HFrEF Registry investigators and committee. Mortality prediction using CHADS2/CHA2DS2-VASc/R2CHADS2 scores in systolic heart failure patients with or without atrial fibrillation. Medicine. 2017;96(43):e8338. PMID: 29069008; PMCID: PMC5671841. doi:10.1097/MD.0000000000008338
10. Harb S, Hussein AAH, Saliba WIS, et al. P5142 Effect of anticoagulation on mortality by CHADSVASC score in patients with atrial fibrillation: comparison to patients without atrial fibrillation. Eur Heart J. 2018;39(suppl_1):15.
11. Hammoudeh A, Khader Y, Tabbalat R, et al. One-year clinical outcome in middle Eastern patients with Atrial fibrillation: the Jordan Atrial fibrillation (JoFib) Study. Int J Vasc Med. 2022;2022:4240999. doi:10.1155/2022/4240999
12. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi:10.1001/jama.285.22.2864
13. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. doi:10.1093/eurheartj/ehr488
14. Olesen JB, Gislason GH, Torp-Pedersen C, Lip GY. Atrial fibrillation and vascular disease--A bad combination. Clin Cardiol. 2012;35(Suppl1):15–20. PMID: 22246947; PMCID: PMC6652656. doi:10.1002/clc.20955
15. Chen JY, Zhang AD, Lu HY, Guo J, Wang FF, Li ZC. CHADS2 versus CHA2DS2-VASc score in assessing the stroke and thromboembolism risk stratification in patients with atrial fibrillation: a systematic review and meta-analysis. J Geriatr Cardiol. 2013;10:258–266. doi:10.3969/j.issn.1671-5411.2013.03.004
16. Yu I, Song T-J, Kim BJ. CHADS2, CHA2DS2-VASc, ATRIA, and Essen stroke risk scores in stroke with atrial fibrillation. Medicine. 2021;100(3):e24000. doi:10.1097/MD.0000000000024000
17. Zhu WG, Xiong QM, Hong K. Meta-analysis of CHADS2 versus CHA2DS2-VASc for predicting stroke and thromboembolism in atrial fibrillation patients independent of anticoagulation. Tex Heart Inst J. 2015;42(1):6–15. PMID: 25873792; PMCID: PMC4378047. doi:10.14503/THIJ-14-4353
18. Oladiran O, Nwosu I. Stroke risk stratification in atrial fibrillation: a review of common risk factors. J Community Hosp Intern Med Perspect. 2019;9(2):113–120. PMID: 31044042; PMCID: PMC6484493. doi:10.1080/20009666.2019.1593781
19. Nielsen PB, Skjøth F, Overvad TF, Larsen TB, Lip GY. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: should we use a CHA2DS2-VA score rather than CHA2DS2-VASc? Circulation. 2018;137(8):832–840. doi:10.1161/CIRCULATIONAHA.117.029081
20. Mikkelsen AP, Lindhardsen J, Lip GY, Gislason GH, Torp-Pedersen C, Olesen JB. Female sex as a risk factor for stroke in atrial fibrillation: a nationwide cohort study. J Thromb Haemost. 2012;10(9):1745–1751. PMID: 22805071. doi:10.1111/j.1538-7836.2012.04853.x
21. John Camm A, Savelieva I. Female gender as a risk factor for stroke associated with atrial fibrillation. Eur Heart J. 2017;38(19):1480–1484. doi:10.1093/eurheartj/ehx103
22. Wagstaff AJ, Overvad TF, Lip GYH, Lane DA. Is female sex a risk factor for stroke and thromboembolism in patients with atrial fibrillation? A systematic review and meta-analysis. QJM. 2014;107(12):955–967. doi:10.1093/qjmed/hcu054
23. Waldo AL, Becker RC, Tapson VF, Colgan KJ; NABOR Steering Committee. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol. 2005;46(9):1729–1736. doi:10.1016/j.jacc.2005.06.077
24. Banerjee A, Lane DA, Torp-Pedersen C, Lip GYH. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, and apixaban) versus no treatment in a “real world” atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb Haemost. 2012;107:584–589. doi:10.1160/TH11-11-0784
25. Arboix A, Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev. 2010;6(3):150–161. PMID: 21804774; PMCID: PMC2994107. doi:10.2174/157340310791658730
26. Arboix A, Oliveres M, Massons J, Pujades R, García-Eroles L. Early differentiation of cardioembolic from atherothrombotic cerebral infarction: a multivariate analysis. Eur J Neurol. 1999;6:677–683. doi:10.1046/j.1468-1331.1999.660677.x
27. Boriani G, Proietti M, Laroche C, Fauchier L, Marin F, Nabauer M. Association between antithrombotic treatment and outcomes at 1-year follow-up in patients with atrial fibrillation: the EORP-AF general long-term registry. Europace. 2019;21(7):1013–1022. PMID: 30904925. doi:10.1093/europace/euz032
28. De Caterina R, Kim YH, Koretsune Y, et al. Safety and effectiveness of edoxaban in atrial fibrillation patients in routine clinical practice: one-year follow-up from the global noninterventional ETNA-AF program. J Clin Med. 2021;10(4):573. doi:10.3390/jcm10040573
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
