Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Vagus Nerve Stimulation For Treatment Resistant Depression: Case Series Of Six Patients - Retrospective Efficacy And Safety Observation After One Year Follow Up
Authors Kucia K , Merk W , Zapalowicz K , Medrala T
Received 30 May 2019
Accepted for publication 15 August 2019
Published 25 November 2019 Volume 2019:15 Pages 3247—3254
DOI https://doi.org/10.2147/NDT.S217816
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Krzysztof Kucia,1 Wojciech Merk,1 Krzysztof Zapalowicz,2 Tomasz Medrala1
1Department of Psychiatry and Psychotherapy, School of Medicine in Katowice, Medical University of Silesia, GCM, Katowice 40-635, Poland; 2Neurosurgery Ward, GCM, Katowice 40-635, Poland
Correspondence: Krzysztof Kucia
Department of Psychiatry and Psychotherapy, School of Medicine in Katowice, Medical University of Silesia, GCM, ul. Ziolowa 45/47, Katowice 40-635, Poland
Tel +48 322059260
Email [email protected]
Objective: One year observation and evaluation of the VNS (vagus nerve stimulation) efficacy and safety for patients with treatment resistant depression in Polish conditions.
Methods: An open label, uncontrolled and one center retrospective study of VNS therapy was implemented with stable pharmacotherapy in 6 patients with treatment resistant depression (TRD). For the first 3 months, only VNS parameters were altered but the pharmacological treatment was unchanged and in the following 9 months, medication and VNS dosing parameters were altered according to the clinical state of the patients.
Results: The baseline 24-item Hamilton Depression Rating Scale (HAMD-24) score averaged 24. Both response (>50% reduction in baseline scores) and remission rates after 3 months of treatment were only 40%. After 1 year of VNS therapy, the response rates increased to 86%. Most frequent side-effects were voice alteration (86% at 3 months of stimulation) and headaches (40%).
Conclusion: VNS treatment was safe and effective in TRD patients and its efficacy increased with time. Efficacy ratings are similar to the previously reported studies using a congenial protocol.
Keywords: vagus nerve stimulation, treatment resistant depression, brain stimulation
Introduction
Depression is a common illness worldwide, with more than 300 million people affected of all ages.1 Severe intensity of depression may lead, in many cases, to life threatening suicidal behavior. In Poland, according to EZOP 2015 year epidemiological study,2 depression was the third most common mental disorder in terms of the prevalence and occurred in women (4.0%) twice as often than in males (1.9%). In an American study from 2012, mood and other behavioral health disorders were the most common diagnoses for Medicaid-covered and uninsured hospital stays in the United States – respectively 6.1% and 5.2% of stays.3
The group of antidepressant drugs with different mechanisms of action are widely used in treatment for major depressive disorder. According to 2018 Cipriani meta-analysis4 of 522 placebo-controlled and head-to-head trials of 21 antidepressants used for the acute treatment of adults comprising 116 477 participants, all drugs were significantly more effective then placebo.
However, together with psychotherapy and/or electroconvulsive therapy (ECT), these medications are not effective in all patients suffering from the depression.
In Sequenced Treatment Alternatives to Relieve Depression – STAR*D, the largest prospective clinical trial of treatment of major depressive disorder, funded by National Institutes of Health and completed in 2006, at the level 4, where only highly TRD patients were gathered after three failures, 33% of patients did not respond to the treatment despite adequate treatment.5 These results were coherent with previously presented data by Thase, where approximately 30% of patients with treatment-resistant depression (TRD) did not respond to any treatment.6
TRD is defined by lack of response or failure to fully respond or achieve remission after at least two proven antidepressant trials with adequate dosing and duration.8–10
In order to improve the response to antidepressant treatment, add-on augmentation has been developed, which is the combination of first-line antidepressive pharmacotherapy with a second treatment approach, either pharmacological, or neurostimulation techniques.11
Atypical antipsychotics and thyroid augmentation have been proven to have better antidepressive effect combined with first-line antidepressants in TRD.12
Neurostimulation options in TRD include electroconvulsive therapy (ECT), magnetic seizure therapy (MST), transcranial direct current atimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS), deep brain stimulation (DBS), cranial electrotherapy stimulation (CES), and vagus nerve stimulation (VNS). The most common therapy for TRD has been ECT, however its final clinical outcome is diminished through high relapse rates of up to 50%.13 rTMS response rates are low in patients resistant to ECT.14 DBS is a neurosurgical invasive option for TRD treatment that requires brain surgery, with limited clinical data available.15,16
Early clinical observations of mood improvement in first epilepsy treated patients with VNS, drew attention to its antidepressant action potential. Finally, after controlled studies in Europe and Canada in 2001–2005, the FDA approved VNS treatment in chronic depression and in TRD patients.17–20 Patients should have used 4 or more medications before VNS treatment and should be older than or at least 18 years old. Over 100 000 patients/year (psychiatric and neurological indications) have been treated in the whole world. Overall the number needed to treat (NNT) for VNS ranges from 4 to 10, which is clinically significant, considering the treatment resistant depression population.
Taking into consideration other neurostimulation methods, VNS has better scientific evidence for efficacy compared to MST, tDCS, and CES and for maintenance in long term treatment it is better even than the gold standard – maintenance ECT, and also less invasive.11
After the surgical implantation of the VNS device, it is telemetrically activated by a wand connected to a computer. All the adjustments of stimulus intensity parameters are non-invasive and are executed with the external telemetric wand. VNS settings are programmed to supply intermittent stimulation with a current of 0.25–3.0mA, a frequency of 20–50 Hz, a pulse width of 130–500ms, and a duty cycle.21
In general, the exact mechanism of action of VNS is still unknown. There is a hypothesis of the desynchronization of neuronal activity by the modulation of neurotransmitter release, hippocampal plasticity and anti-inflammation action.22 The long term VNS modulates the neurotransmission of norepinephrine, serotonin, and gamma-aminobutyric acid (GABA).24 Locus coeruleus (LC) activated by VNS is the main source of norepinephrine.25 LC lesions stop both the antiepileptic and antidepressant action of VNS.23
The excitatory projections from LC to the Dorsal Raphe Nucleus (DRN), which is the major source of serotonin in the brain,26 have been shown to activate the excitatory α1- adrenoreceptors on the cell bodies of the serotonergic neurons, thus increasing the activity of these neurons.27 VNS also induces c-fos activity, a nuclear protein expressed under high neuronal activity.29,30 Furmaga compared activity changes in the animal brain after VNS stimulation and that caused by sertraline and desimipramine31 by double labelling of ΔFosB and serotonin, and he found that sertraline had similar effect to VNS in DRN. The effects of VNS were more widespread than those of antidepressants.
In the hippocampal plasticity hypothesis of depression, chronic stress leads to atrophy and synaptic changes in limbic brain. VNS increases the expression of neurotrophic and growth factors – brain derived neurotrophic factor (BDNF) and basic fibroblast growth factor (bFGF),32 which promote the neuroplasticity, ie, the formation and differentiation of neurons, decreased in mood disorders. Another way antidepressants could increase BDNF efficacy is the activation by phosphorylation of its receptor: tropomyosin receptor kinase B (TrkB). Furmaga demonstrated that both long term and short term VNS in rats activated the TrkB receptors.31 Clinical studies have proven that therapeutic effects appear after several months of treatment and the plasticity hypothesis explains its mechanism. It requires weeks for newborn cells to become mature neurons with the functional network of synapses to rebuild the neuronal paths in frontal and limbic structure circuits.33
Method
The VNS treatment was evaluated in an open, unblinded, not sham controlled, one center trial conducted in Department of Psychiatry and Psychotherapy at Katowice Silesian Medical University. Every patient, before the device implantation, underwent baseline assessments. All the patients had their stimulation initiated on the second day after the implantation of device - their set of parameters is shown in Table 1. First changes of stimulation parameters were done if needed at the 5th week after the start of stimulation. During the first 3 months after the stimulation onset, no pharmacological treatment changes were made, only the stimulation parameters of VNS were set according to comfort and tolerability of patients every 2 weeks. The pharmacological profile of all patients before the implantation of the VNS device is shown in Table 2. At the follow-up period 3 months after VNS implantation, pharmacological medication changes together with stimulation parameter adjustments were performed at one month period visits.
 |
Table 1 For All Patients: Pulse Width 500 Mikroseconds, Stimulation Time 30 Seconds, Interval Between Stimulations 5 mins |
 |
Table 2 The Pharmacological Profile Of All Patients Before The Implantation Of The VNS Device |
Patients
Patients with TRD signed informed consent to undergo implantation of the VNS device at neurosurgery unit after qualification exam at psychiatry ward. The current major depressive episode had lasted more than 2 years and/or the patient had at least four major depressive episodes in his life. Age of the patients was between 63 and 56, 3 males and 2 women. All patients had experienced at least 2 unsuccessful adequate medication trials for the current major depressive episode with antidepressant drugs, evaluated by Antidepressant Resistance Rating score of ≥3 (30) and one patient had electroconvulsive therapy (ECT) for the current major depressive episode.
None of the patients had history of suicide, atypical or psychotic depression, schizophrenia, schizo-affective disorder or delusional disorder, bipolar disorder or a secondary diagnosis of delirium, dementia, and other cognitive disorders as the health problems that might interfere with surgical and anesthetic procedures.
Stimulation was initiated the day following operation with the parameters presented in Table 1. The output current parameters were adjusted according to individual maximal tolerability at each study visit in order to get maximum efficacy of depressive symptoms' treatment but only frequency and intensity were changed. There were no changes of pulse width on stimulation timings throughout the whole observation period of 12 months. Maximum value for frequency was 30Hz in 3 individuals and for intensity was 1 mA in 2 patients.
VNS: Implantation
The implantable system made by Cyberonics Inc., Houston, USA, consisted of the generator VNS DemipulseTM Model 103 and two helicoidal electrodes with lead and connector pin to insert into the generator. The manufacturer also provided the external programing wand for telemetric adjustment of stimulation parameters. The operations were performed with the patients under general anesthesia, placed in supine position with the head slightly extended. Patients received prophylactic intravenous antibiotics preoperatively and for 6 days postoperatively. A transverse incision measuring 30–40 mm was made in left posterior triangle of the neck at the cricothyroid interval level. The lateral margin of sternocleidomastoid (SCM) muscle was retracted medially to visualize cervical neurovascular bundle. The left vagus nerve was exposed by blunt dissection, mobilized over a length of 30–40 mm and retracted superiorly with 2 latex loops. Second transverse incision, long enough for generator placement, was made in the left chest wall, a few centimeters below the clavicle. Between both incisions the subcutaneous tunnel was created using a tunnelling device and the lead was drawn from the neck incision to the chest incision. In the next step the electrodes were wrapped around the vagus nerve. To prevent unwanted traction or dislocation during patients’ neck movements, the lead was looped and placed in a latex collar, which in turn was attached to the internal surface of the SCM by a nonresorbable stitch. The connector pin of lead was connected to the generator and electrodiagnostic testing was performed to confirm a proper function of the system. Finally, the generator was placed in a pocket bluntly created by a surgeon between the pectoralis major and minor muscles and attached to the pectoralis fascia by a nonresorbable stitch. The wounds were closed in layers.
In all cases surgical procedures were noncomplicated with minimal blood loss. The healing was uneventful; all patients were pleased with cosmetic effect and tolerated their subcutaneously placed device well.
Concomitant Therapy
4 weeks prior to the planned VNS implantation and 12 weeks after, antidepressant medications were stable. At the follow-up period 3 months after VNS implantation, all necessary psychotropic treatment changes were performed, similar to the other non-psychiatric drugs (eg, antibiotics).
Outcome Measures
Baseline HAMD-24 score was compared to ratings after 3 months of VNS and after additional 3, 6, and 9 months to assess the depression severity. Response was defined as a ≥50% reduction in HAMD-24 score from the baseline and remission was determined as a HAMD-24 score ≤10.
Results
Recruitment
A total of 6 patients were observed in this study. Their epidemiologic and affective disorder features are summarized in Table 3. The unsuccessful current major depressive disorder number of treatments averaged 4,2 ±2,8. One patient had received electroconvulsive therapy (ECT) during the current major depressive episode. The baseline scores of depression scales – HAMD-24, MADRS certified a severe level of depression in all the observed patients.
 |
Table 3 Demographic And Clinical Characteristics Of Patients |
Stimulation Parameters
Most of the patients (60%) received stimulation with 30Hz frequency. The others' stimulation was reduced to 20Hz due to adverse events – especially voice alteration, in order to improve its tolerability. The output currents ranged from 0.25 to 1 mA - mean 0,5±0.25 mA during the first 3 months after VNS initiation and mean 0.65±0.35 mA during the follow up period. The pulse width of 500μs with stimulation on for 30 s and off for 5 mins remained unchanged throughout the whole time of observation.
Efficacy
Severity of depression assessed by the HAMD-24 mean scores decreased under VNS as shown in Figure 1. There is in the HAMD-24 course of scores through 12 months period of every rated patient.
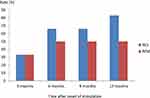
During the first 3 months the number of patients who responded to the treatment equaled those with remission. The rest of the patients needed an extra 3 months to pass the response criteria threshold – as shown in Figure 2, we could divide patients in 3 groups: 1) no response, 2) fluctuating response and 3) early remission. Only one patient did not achieve remission after 6 months. 2 patients could be assigned to the fluctuating response group and 2 patients to early remission.
 |
Figure 2 Response RES and remission rates REM (%), defined as reduction of >50% in the HAMD-24 score compared to baseline HAMD-24 score; remission was defined as a HAMD-24 score of <10%. |
Finally, after 12 months' observation, 83% (5/6) of patients reached criteria of response, but the number of remitted patients remained the same 50% (3/6).
Adverse Events
The adverse events recorded during the 12 months observation period after the VNS stimulation onset are summarized in Table 4. The most common adverse event during the first 3 months was voice alterations during 30 second VNS stimulation (80% of patients) and headache and pain complaints (40% of patients). After 12 months, 83% of patients still reported voice alterations only during the time of stimulation but no one suffered pain and headaches. According to the adverse event classification, these voice alterations were definitely related to the VNS and they were moderate, influencing the patients' quality of life as they had some problems in social relations resulting from the voice hoarseness. During 12 months observation period, there were no serious adverse events that might have resulted in patient hospitalization.
 |
Table 4 Adverse Events Recorded During The Observation Period After The VNS Stimulation Onset |
Discussion
Carreno and Frazer35 noticed in their review in 2017, that the evidence for efficacy of VNS in TRD is quite substantial, however more research is still needed before efficacy could be established conclusively.
Thus, we compared our results to the European34 and American multi-center VNS study17 as both studies had similar protocol in methodology and design. The size of our observed group was very small but it was quite homogenous as there were only patients with unipolar diagnosis by DSM V, and the same as in both studies, unsuccessful adequate medication trials for the current major depressive episode was 4,2 ± 2,8. The remission rate after 3 months was 33%, compared to 17% in the two studies and after 1 year of VNS therapy the response rate increased to 83%, compared to 53%. We observed the increase in remission rates after one year observation, compared to the first 3 months.
In a Sperling open-label case control study with a follow-up period up to 12 months, where the group consisted of 18 patients suffering from TRD, with the same VNS settings, a significant improvement of HAMD to a mean of 10.2 points was observed,36 as in our study – 9.6 points.
In the case control retrospective study published by Mueller,19 a group of 20 patients with TRD was treated with low-strength/high-frequency VNS (≤1,5 mA, 20 Hz) and high-strength/low-frequency (>1,5 mA, 15 Hz) VNS. Significant decrease in the HAMD was observed in patients who were treated with the low-strength/high-frequency stimulation parameters. The scores of the patients treated with high-strength/low-frequency combination did not change. 60% of our patients had low-strength/high-frequency stimulation with frequency 30Hz and 0.65±0.35 mA during the follow up period. The frequency was the first parameter to be increased when the patient demonstrated HAMD deterioration. Only the adverse events, like voice changes, were stopping us from using 30Hz stimulation in some patients.
In another open, uncontrolled European multi-center study with 28 chronic TRD unipolar patients, 35.7% met criteria for response after 12 months' treatment time.37
In the longest – 5 years and the largest (795 patients) naturalistic study of VNS efficacy in TRD by Aaronson, 5 year cumulative response rate was 67.6% with remission rate of 43.3%.38
Conclusion
After 1 year of VNS therapy, the response rates increased to 83%. Most frequent side-effects were voice alteration (83% at 3 months stimulation, decreased to 50% after 6 months) and headaches (33%). Thus, VNS treatment was safe and effective in TRD patients and its efficacy increased with time. Efficacy ratings were similar to the previously reported studies using a congenial protocol.
As Helge Mueller stated in the review of augmentation strategies for TRD,11 DBS or VNS should be strongly recommended as promising adjunctive options to ECT – the gold standard. VNS has strong scientific evidence for efficacy and safety, although first treatment symptoms are 3–12 months delayed and patience is needed.
Application of VNS in TRD is recommended in such national guidelines as: CANMAT 201639 and NICE 2012,7 but long-term naturalistic observational studies are needed for the future evaluation of VNS efficacy and safety in TRD.40
Disclosure
The authors report no conflict of interest in this work.
References
1. World Health Organisation. Depression. (2018) Available from: http://www.who.int/news-room/fact-sheets/detail/depression.
2. Kiejna A, Piotrowski P, Adamowski T, et al. The prevalence of common mental disorders in the population of adult poles by sex and age structure – an EZOP Poland study. Psychiatr Pol. 2015;49(1):15–27. doi:10.12740/PP/30811
3. Lopez-Gonzalez L, Pickens GT, Washington R, Weiss AJ. Characteristics of Medicaid and uninsured hospitalisations, 2012. In: HCUP Statistical Brief #183. Rockville, MD: Agency for Healthcare Research and Quality; 2014.
4. Cipriani A, Furukawa T, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi:10.1016/S0140-6736(17)32802-7
5. Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Spencer D, Fava M. The STAR*D study: treating depression in the real world. Cleve Clin J Med. 2008;75(1):57–66. doi:10.3949/ccjm.75.1.57
6. Thase ME, Rush JA. Treatment-resistant depression. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology. New York, NY: Raven; 2005.
7. Cleare A, Pariante CM, Young AH, et al.; Members of the Consensus M. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459–525. doi:10.1177/0269881115581093
8. Bschor T. Therapy-resistant depression. Expert Rev Neurother. 2010;10:77–86. doi:10.1586/ern.09.137
9. Wiles N, Thomas L, Abel A, et al. Clinical effectiveness and cost-effectiveness of cognitive behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care: the CoBalT randomised controlled trial. Health Technol Assess. 2014;18:1–167. doi:10.3310/hta18310
10. Holtzmann J, Richieri R, Saba G, et al. Quelle définition pour la dépression résistante? [How to define treatment-resistant depression?]. La Presse Médicale. 2016;45(3):323–328. doi:10.1016/j.lpm.2016.02.002
11. Müller H, Moeller S, Lam A, Lücke C, Braun N, Philipsen A. Vagus Nerve Stimulation (VNS) and other augmentation strategies for Therapy-Resistant Depression (TRD): review of the evidence and clinical advice for use. Front Neurosci. 2018;12(239). doi:10.3389/fnins.2018.00239
12. Fornaro M, Stubbs B, De Berardis D, et al. Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci. 2016;17:241. doi:10.3390/ijms17020241
13. Charlson F, Siskind D, Doi SA, McCallum E, Broome A, Lie DC. ECT effcacy and treatment course: a systematic review and metaanalysis of twice vs. thrice weekly schedules. J Affect Disord. 2012;138:1–8. doi:10.1016/j.jad.2011.03.039
14. Kedzior KK, Schuchinsky M, Gerkensmeier I, Loo C. Challenges in comparing the acute cognitive outcomes of high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) vs. electroconvulsive therapy (ECT) in major depression: a systematic review. J Psychiatr Res. 2017;91:14–17. doi:10.1016/j.jpsychires.2017.03.002
15. Schlaepfer TE, Lieb K. Deep brain stimulation for treatment of refractory depression. Lancet. 2005;366:1420–1422. doi:10.1016/S0140-6736(05)67582-4
16. Buhmann C, Huckhagel T, Engel K, et al. Adverse events in deep brain stimulation: a retrospective longterm analysis of neurological, psychiatric and other occurrences. PLoS One. 2017;12(7). doi:10.1371/journal.pone.0178984
17. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol Psychiatry. 2000;47:276–286. doi:10.1016/S0006-3223(99)00304-2
18. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatmentresistant depression: effcacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–728. doi:10.1016/S0893-133X(01)00271-8
19. Kosel M, Schlaepfer TE. Beyond the treatment of epilepsy: new applications of vagus nerve stimulation in psychiatry. CNS Spectr. 2003;8:515–521. doi:10.1017/S1092852900018988
20. Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry. 2002;51:280–287. doi:10.1016/S0006-3223(01)01343-9
21. Cusin C, Dougherty DD. Somatic therapies for treatmentresistant depression: ECT, TMS, VNS, DBS. Biol Mood Anxiety Disord. 2012;2:14. doi:10.1186/2045-5380-2-14
22. Aalbers M, Vles J, Klinkenberg S, Hoogland G, Majoie M, Rijkers K. Animal models for vagus nerve stimulation in epilepsy. Exp Neurol. 2011;230:167–175. doi:10.1016/j.expneurol.2011.04.014
23. Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol. 2017;289:21–30. doi:10.1016/j.expneurol.2016.12.005
24. Ruffoli R, Giorgi FS, Pizzanelli C, Murri L, Paparelli A, Fornai F. The chemical neuroanatomy of vagus nerve stimulation. J Chem Neuroanat. 2011;42:288–296. doi:10.1016/j.jchemneu.2010.12.002
25. Takigawa M, Mogenson GJ. A study of inputs to antidromically identified neurons of the locus coeruleus. Brain Res. 1977;135:217–230. doi:10.1016/0006-8993(77)91027-7
26. Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59(6 Suppl. 4):S3–S14. doi:10.1212/WNL.59.6_suppl_4.S3
27. Grimonpreza A, Raedta R, Baekenb C, Boona P, Voncka K. The antidepressant mechanism of action of vagus nerve stimulation: evidence from preclinical studies. Neurosci Biobehav Rev. 2015;56:26–34. doi:10.1016/j.neubiorev.2015.06.019
28. Prudic J, Sackeim HA, Devanand DP. Medication resistance and clinical response to electroconvulsive therapy. Psychiatry Res. 1990;31:287–296. doi:10.1016/0165-1781(90)90098-P
29. Naritoku DK, Terry WJ, Helfert RH. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res. 1995;22:53–62. doi:10.1016/0920-1211(95)00035-9
30. Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by vagal nerve stimulation. Neuropsychopharmacology.;. 2008;33:1884–1895. doi:10.1038/sj.npp.1301570
31. Furmaga H, Carreno FR, Frazer A. Vagal nerve stimulation rapidlyactivates brain-derived neurotrophic factor receptor TrkB in rat brain. PLOSONE. 2012;7(5):e34844. doi:10.1371/journal.pone.0034844
32. Follesa P. Vagus nerve stimulation increases norepinephrineconcentration and the gene expression of BDNF and bFGF in the rat brain. BrainRes. 2007;1179:28–34.
33. Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi:10.1038/npp.2009.104
34. Schlaepfer T, Frick C, Zobel A, et al. Vagus nerve stimulation for depression: efficacy and safety in a European study. Psychol Med. 2008;38:61–661. doi:10.1017/S0033291707001924
35. Carreno F, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics. 2017;14:716–727. doi:10.1007/s13311-017-0537-8
36. Sperling W, Reulbach U, Kornhuber J. Clinical benefits and cost effectiveness of vagus nerve stimulation in a long-term treatment of patients with major depression. Pharmacopsychiatry. 2009;42(3):85–88. doi:10.1055/s-0028-1103294
37. Christmas D, Steele JD, Tolomeo S, Eljamel MS, Matthews K. Vagus nerve stimulation for chronic major depressive disorder: 12-month outcomes in highly treatment-refractory patients. J Affect Disord. 2013;150(3):1221–1225. doi:10.1016/j.jad.2013.05.080
38. Aaronson S, Sears P, Ruvuna F, et al. A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am J Psychiatry. 2017;174:7. doi:10.1176/appi.ajp.2017.16010034
39. Milev RV, Giacobbe P, Kennedy SH; Group CDW. et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 4. neurostimulation treatments. Can J Psychiatry. 2016;61(9):561–575. doi:10.1177/0706743716660033
40. Cimpianu CL, Strube W, Falkai P, Palm U, Hasan A. Vagus nerve stimulation in psychiatry: a systematic review of the available evidence. J Neural Transm. 2017;124:145–158. doi:10.1007/s00702-016-1642-2
41. Keller MB. Issues in treatment-resistant depression. J Clin Psychiatry. 2005;66(Suppl 8):5–12.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.