Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Vagal Nerve Stimulation in Epilepsy: Experiences of Participants with Cognitive Deficits
Authors Pipan E , Apostolou A, Bograkou M, Brooks P, Vigren P, Gauffin H
Received 10 December 2019
Accepted for publication 17 April 2020
Published 8 May 2020 Volume 2020:16 Pages 1181—1188
DOI https://doi.org/10.2147/NDT.S241716
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Eva Pipan,1 Alexandros Apostolou,2 Maria Bograkou,2 Petra Brooks,2 Patrick Vigren,2 Helena Gauffin2
1University Medical Centre Ljubljana, Ljubljana, Slovenia; 2Department of Neurology and Department of Clinical and Experimental Medicine, Linköping University, Linköping, Sweden
Correspondence: Eva Pipan
University Medical Centre Ljubljana, University of Ljubljana, Ljubljana, Slovenia
Email [email protected]
Introduction: The purpose of this study was to examine patients’ experiences of vagal nerve stimulation (VNS) with a special interest in patients with cognitive deficit (CD).
Materials and Methods: An open, retrospective study was conducted on 82 patients with pharmacoresistant epilepsy, who were treated with VNS for at least 10 months. Based on the inability to live independently, they were divided into two groups: patients with cognitive deficit (CD group) and patients without cognitive deficit (non-CD group). A specially designed questionnaire was used for semi-structured interviews about patients’ experiences of VNS treatment.
Results: Approximately one-third described a continuous reduction of seizure frequency of 50% or more and were regarded as responders. Fewer subjects in the CD group were responders than in the non-CD group. Approximately one-third of all subjects had no positive effect of VNS treatment. More CD patients described additional improvements and the most common were milder seizures and improved alertness. The most commonly reported adverse effect was hoarseness.
Discussion: VNS treatment in patients without CD had better effect on seizure frequency reduction than in patients with CD, but many patients with CD reported other benefits from the treatment.
Conclusion: Non-CD patients had higher seizure frequency reduction than CD patients during VNS treatment, but many CD patients described other benefits.
Keywords: epilepsy, vagus nerve stimulation, cognitive deficit, cognitive dysfunction
Introduction
Worldwide, 0, 5–1% of the population is diagnosed with epilepsy of which 20–30% have pharmacoresistant epilepsy1,2 Epilepsy is more common in patients with CD and in this group 10–20% are diagnosed with epilepsy.3–5 The percentage of patients with pharmacoresistant epilepsy in the CD group is significantly higher, around 45%6–8 Persons with CD often have additional disabilities, such as cerebral palsy, autism or psychiatric comorbidities.4,9 Because of the need for multiple medications for different health conditions, they are more burdened by adverse effects often compromising their cognition and behavior even further. The intractable seizures themselves are also compromising cognitive abilities, something of special significance in children, where the refractory epilepsy may disrupt a developmental period.10,11
These factors contribute to enhanced vulnerability of patients with epilepsy and CD, posing a unique challenge for medical management.
Evaluation of treatment efficacy and general well-being may be problematic due to varying degrees of cognitive and expressive abilities that may be further compromised by limited verbal ability.12 Those patients often need more anti-seizure medications (ASMs) to achieve desirable seizure control, something that is often not perceived.13–15 In addition, they appear to be more prone to adverse side effects from ASMs than those who are not cognitively challenged.4,16 It is desired to simplify the treatment regime as much as possible.17 Vagus nerve stimulation (VNS) is a non-pharmacological treatment option for patients with pharmaco-resistant epilepsy, who are not candidates for epilepsy surgery or who have undergone such surgery without sufficient seizure control. Studies indicate that VNS treatment is not as efficacious in reducing seizure frequency in CD patients as is in patients without any cognition problems.18,19 For 45% of patients with epilepsy and CD pharmacological treatment with ASMs is not sufficient to achieve seizure freedom and it is therefore important to examine if VNS treatment is an option from which this specific group of patients can benefit.8
The purpose of this study was to examine patients’ experiences of VNS treatment with a special focus on patients with CD.
Materials and Methods
Study Design
A qualitative design was chosen to explore the experiences of treatment with VNS. Participants and their caregivers were interviewed according to a semi-structured questionnaire (Table 1). Data were collected through interviews with patients and their caregivers, conducted during their regular checkups for control and adjustment of the VNS. The interviews were carried out by a neurology specialist or by a trained epilepsy nurse specialized in VNS treatment.
 |
Table 1 Semi-Structured Questionnaire Designed for the Purposes of the Study |
The questionnaire consisted of eleven open-ended questions. Five of them were focusing on the period before the implantation of VNS (seizure frequency, seizure features, ASMs, other treatment of epilepsy, the ability to live independently) and the remaining six were focusing on the period after the implantation of VNS (complications after the implantation, experienced effect on seizure frequency, potential changes in features of seizures or behavior, as well as experienced negative effects). Demographics, previous medical history and stimulation parameters were retrospectively collected from medical records.
Ethics
This study was performed in accordance with the Helsinki declaration. The regional ethical committee of Linköping approved of the study (number 2015/206-31). Participants gave their verbal and written informed consent.
The Evaluable Population
We identified 122 patients with epilepsy who had received VNS treatment at Linköping University Hospital since 1997. Of those, 13 were deceased and 24 patients (6 with CD, 18 without CD) had VNS deactivated before the beginning of this study. At the start of the study 85 subjects were identified as receiving active VNS treatment for at least 10 months. Two patients chose not to participate and one patient was lost to follow-up. 82 patients were included in total.
Exclusion criteria were VNS treatment for less than 10 months and discontinuation of VNS treatment. The division into two study groups was made based on either already confirmed diagnosis of CD or the inability to live independently because of CD. The need for a caregiver or a living support for any extent of activities of daily living due to CD was evaluated for each participant. Participants were classified as having a CD if they met any of these two criteria (Table 2).
 |
Table 2 Inclusion Criteria for CD Group |
Patient Characteristics
This study included 82 patients with active VNS treatment. 45 met the criteria for CD group and the remaining 37 were assigned to non-CD group. Patient demographics and features are listed in Table 3. The division into responders and non-responders was made based on patients’ experience of the reduction of seizure frequency due to VNS treatment. Responders were defined as those who became seizure-free or had 50% or more seizure frequency reduction.
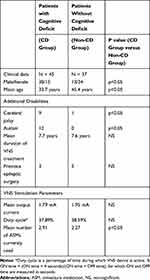 |
Table 3 Participants’ Characteristics and VNS Stimulation Parameters at the Time of Interview |
VNS Device and Stimulation Parameters
A programmable device ( Neurocybernetic Prosthesis – NPC, Cyberonics Inc., Texas, USA) was implanted at least 10 months prior to the beginning of the study. VNS stimulation was introduced gradually after the implantation and stimulation parameters (duty cycle and output current) were adjusted individually during checkups according to the patient’s tolerance and effects of stimulation. Initial stimulation parameters for all patients were output current 0.5 mA, pulse width 250 µs and ratio between ON and OFF time of the device 30 sec: 5 min (duty cycle 10%). Follow-up visits were usually initially scheduled every two weeks and later every three months. The aim of adjusting the parameters was to achieve the equilibrium between desirable effects of the stimulation and adverse side effects.
Statistics
For statistical analyses, the software program Statistical Package for Social Sciences version 22.0 was used. Frequencies were calculated and cross tabulations were carried out. Non-parametric analyses and Mann–Whitney U-test were used for statistical analyses. P-values less than 0.05 were considered significant.
Results
Eighty-two patients were interviewed according to the questionnaire and 45 of these had CD. Two participants have had the VNS device implanted during the 1990s while the others underwent implantation from the year of 2000 and onwards. Demographically the groups were not perfectly matched. The participants with CD included more men and were also younger and used more ASMs than the group without CD. The mean time of VNS treatment was 7, 64 years, std 4, 49. The minimum duration was 0, 85 years and the maximum was 20, 29 years. There was no statistically significant difference in duration of treatment between individuals with and without CD.
Effect on Seizure Frequency
Among all the participants, 31 out of 82 described a continuous and persisting reduction of seizure frequency of 50% or more, four of those achieving seizure freedom.
Fewer participants in the CD group were responders than in the non-CD group (Mann Whitney U-test p = 0.006). Numbers are shown in Table 4.
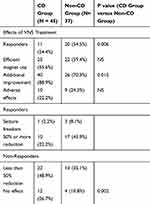 |
Table 4 Patients’ Experience of Seizure Frequency Reduction, Additional Improvements and Adverse Effects After Implantation of VNS |
Complications and Side Effects
At the time of the interview, 19 participants were experiencing side effects from the VNS treatment. However, before the initiation of the study 24 patients had the VNS deactivated due to side effects or lack of effect.
Common side-effects following implantation were hoarseness and discomfort. However as these were temporary they have been excluded from this report. Only persistent, continuous hoarseness was documented and it was the most commonly experienced side effect, present in eight subjects. Four subjects experienced problems with the implanted lead which led to reoperation. Two participants (2, 4%) contracted a surgical infection. Three participants experienced mild dyspnea on exertion, one experienced pain in the area of implantation, one was reported to exhibit negative behavioral changes. Two of the participants from CD group reported more than one side effect – loss of appetite in conjunction with hoarseness or problems with the implanted lead, respectively. Discontinuation of VNS treatment was not necessary for any of them.
Additional Improvements
Additional improvements were classified as all experienced improvements participants and their caregivers described as effects of the VNS treatment, other than seizure frequency reduction. They were divided into two categories: mitigating effect on seizure characteristics (less severe seizures, shorter seizures, shorter postictal period) and effect on general well-being (improved cognition, patients became more alert and energetic, less aggressive and calmer). Results are presented in Table 5.
 |
Table 5 Additional Improvements from VNS Treatment |
At least one additional improvement was reported by 67 of the participants, while 25 participants listed more than one. Most common improvements were less severe seizures, alertness and shorter postictal period. Additionally, 41 out of 45 participants in CD group described at least one additional beneficial effect whereas in non-CD group 26 out of 37 experienced one or two additional improvements (p>0,05).
Of those who were listed as non-responders based on the inadequate seizure frequency reduction, the majority experienced some other positive effect of VNS treatment.
Out of all 82 observed patients, 10 did not experience significant seizure frequency reduction or any other improvement from the VNS treatment. When adding 24 patients who had VNS deactivated, 34 out of 106 (approximately 32%) had no positive effect or experienced intolerable side effects and consequently had no beneficial effect of the VNS treatment.
Use of the Magnet
Forty-seven out of 82 patients experienced positive effects from the use of a magnet. Patients described different benefits: if there was an aura and the patient could predict the seizure onset, they could prevent it from happening by a swipe of the magnet; if the magnet was used during the seizure, it could shorten the seizure itself or shorten the postictal period. The biggest drawback observed was a lack of aura or other warning signs of the approaching seizure. Most patients in the CD group were not able to use magnet on their own and were dependent on their caregivers’ ability to recognize the oncoming seizure. The magnet had no effect on some participants despite sufficient compliance and early intervention.
VNS Stimulation Parameters
Output current at the time of interview showed a trend to be higher in the non-CD group, but it did not reach statistical significance (Mann–Whitney test, p = 0, 061).
In the non-CD group, there was a connection between output current and responders. Responders were treated with higher output current (p = 0, 009) than non-responders, but this connection could not be found in CD group. No significant correlation was found between duty cycle and effect of VNS, despite a trend that higher duty cycle was correlated with better effect.
Discussion
In this study, 38% of the subjects experienced a substantial effect of VNS on seizure frequency describing a decrease of number of seizures of at least 50%. Subjects without CD had better effect than subjects with CD, but in both groups many patients also described additional improvements.
People with CD are a very complex subgroup of epilepsy patients. Their diagnosis, management and treatment outcomes differ from those of a general epilepsy population and should be approached with special care, as suggested in guidelines.20 Prognosis for seizure control in this group is generally poorer and patients with CD are less likely to experience substantial seizure frequency reduction.21 Evidence of efficacy of VNS treatment is scarce, especially in adults. These studies all examine seizure frequency reduction but no other effects of VNS treatment. There is one meta-analysis of seven small studies on VNS in children which showed that VNS was less effective in CD than in non CD.22 Wheeler et al examined VNS treatment in a mixed group, with both children and adults, and also found better outcome in the non-CD group,23 which is in concordance with the result of the present study.
Andriola et al have reported the highest seizure reduction in CD with 68% reaching 50% seizure reduction in a series of patients.24 There are fewer studies on how to comprehensively assess treatment outcomes beyond the seizure frequency reduction for patients with CD. Even though seizure frequency reduction seems to be the most important aspect of treatment outcome and the most accessible and easily objectified, it might not be the optimal outcome measure for this group of patients. Other aspects that contribute to quality of life (QoL) such as pharmacological side effects, additional disabilities, cognitive and behavioral problems, might be more prominent in patients with CD. In a study by Huf et al at a nursing home, most CD patients with VNS treatment did not have a prominent seizure when frequency reduction improved QoL measures and decrease in number of ASMs.19 VNS is a treatment option for intractable seizures pharmacological treatment only is not sufficient, especially because patients with CD are often not eligible for epilepsy surgery.25,26 It does not have pharmacological interaction with antiepileptic drugs or other medication, which is especially desirable in polypharmacy-burdened patients with CD. Furthermore, it does not require supervised administration and after the implantation surgery there is no additional compliance needed.27 However, surgery with risk for complications is required and the treatment is expensive. It is also required to follow-up on a regular basis.
According to this study, approximately one-third of the patients underwent the implantation of VNS and further follow-up in vain, since they reported no beneficial effects of treatment. Those who already had the device deactivated, had to undergo one more surgical procedure to have the device removed. VNS treatment will imply future surgery since the battery will be replaced after some years.28,29 Complications occur in 8, 6% of VNS surgery so risk for future complications must also be regarded when choosing VNS treatment.30 One more difficulty we were facing was also a decision to change the battery when expired in patients who did not show very clear positive effects of VNS. This subjects patients to additional surgeries and ads up to an even higher cost of treatment. Difficulties arise also with adjustment of the stimulation parameters. Finding the highest tolerable values of duty cycle and output current poses a unique challenge in the CD group. The staff responsible for adjustment of the parameters has generally shown to be more reluctant to increase the stimulation parameters in patients with CD even though the desired efficacy of treatment had not been reached.
The majority of patients in CD group did not experience reduction in seizure frequency prominent enough to be listed as responders. Their main, and in some cases only benefit from VNS, was either positive effect on seizure characteristics or on general well-being. Due to limited options of quantifying the efficacy of treatment, it is difficult to estimate if the benefits are worth the risk of potential complications and side effects. It should be further studied whether those additional improvements are important enough to justify VNS treatment. Due to potential limited verbal ability in this group of patients, it is more problematic to evaluate treatment effects and there is a higher risk of overlooking the adverse side effects, especially if the patient is not able to point them out on their own.12 Furthermore, patients with CD are not always able to express their consent to the treatment regime and some may have difficulties understanding the treatment. VNS adjustments lead to increased number of hospital visits which can cause patients even further distress. Lower effect on seizure frequency of the VNS observed in patients with CD compared to the general epilepsy population might be due to more complex conditions, as with different genetic or acquired brain diseases, less accurate patient history, but also general inability to express the potential benefits of the treatment. This study has several limitations. CD is a very broad term and studies focusing on VNS efficacy in patients with CD use numerous different denominations and classifications, thus, results are not always comparable. This leads to confusion in the field of determining actual benefits of VNS in this group. Ideally, benefits of VNS would be determined with objective, quantifiable measures, such as standardized QoL questionnaires. However, the latter are not validated for people with intellectual disability. The QoL measure tools that are suitable for people with CD are, on the other hand, not validated for determining the treatment outcomes in epilepsy.31,32
A possible bias is that many interviews were performed by the nurse or physician who was responsible for the VNS treatment and therefore the participants may have felt obliged to give positive comments on the treatment. The data in this study were collected retrospectively and for most patients the follow-up interval was long. Mean duration of VNS treatment was more than 7 years. Therefore, the data is less reliable, since participants could have difficulties to remember and might tend to under- or overestimate the efficacy of treatment. Part of the observed positive clinical effects was possibly achieved by drug treatment alterations during the follow-up. Many of patients in the CD group were unable to comprehend the questions or were unable to give a sufficient verbal reply.
Thus, caregivers or trustees were questioned or at least helped them answering to some extent. This has to be taken into consideration when evaluating the results since there is a potential bias in comparison of general well-being results. Patients might benefit from continuous collection of patient-related outcome measures (PROMs), which could help objectify the assessment of treatment outcomes.33
The VNS patients are a selected group who already have tried and not benefited from many other treatment options. Patients with CD often have a complex disease and seizures that are very difficult to treat. It is possible that a slight improvement can have a big impact on the quality of life of these patients but it must be further investigated if these benefits are justified by the high cost of the treatment.
Conclusions
Approximately one-third of all patients described a reduction of seizure frequency of at least 50% seizure frequency while one-third of the patients had no effect of VNS treatment.
In addition to seizure frequency reduction, participants described other improvements of VNS treatment, i.e. milder or shorter seizures, alertness or beneficial effect of the magnet. Individuals with CD had lower effect on seizure frequency, but the majority of participants in CD group experienced at least one benefit from treatment, either reduction in seizures or additional improvements. Results of this study suggest that seizure frequency reduction should not be the only measure for VNS therapy successfulness and that patients with CD are likely to benefit from the treatment, at least to some extent. The work-up preceding treatment with VNS has to consider the vulnerability of patients with CD. Severe CD might be considered a contraindication for this kind of treatment, as well as poor caregiver support. It is important to create an algorithm for determining when to stop the treatment if it is not efficient enough. Further studies are needed to determine the overall effect of VNS on QoL of patients with CD.
Acknowledgments
We thank all the participants, their families and their caregivers for taking part in the interviews.
Disclosure
Helena Gauffin has received grants to travel to congresses from UBC-pharma, EISAI AB, Cyberonics, Galaxo Smith Kline, Mundipharma and LivaNova. Mrs Petra Brooks reports personal expenses covered by Cyberonics for lecture in Copenhagen 3–5 years ago. The authors report no other conflicts of interest in this work.
References
1. Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16(2):165–170. doi:10.1097/00019052-200304000-00008
2. Murray MI, Halpern MT, Leppik IE. Cost of refractory epilepsy in adults in the USA. Epilepsy Res. 1996;23(2):139–148. doi:10.1016/0920-1211(95)00090-9
3. Bowley C, Kerr M. Epilepsy and intellectual disability. J Intellect Disabil Res. 2000;44(5):529–543. doi:10.1046/j.1365-2788.2000.00270.x
4. Mattson RH. The role of the old and the new antiepileptic drugs in special populations: mental and multiple handicaps. Epilepsia. 1996;37(Suppl s6):45–53. doi:10.1111/j.1528-1157.1996.tb06039.x
5. Lhatoo SD, Sander JW. The epidemiology of epilepsy and learning disability. Epilepsia. 2001;42(Suppl 1):6–9. doi:10.1046/j.1528-1157.2001.00502.x
6. Jansen FE, Sadleir LG, Harkin LA, et al. Severe myoclonic epilepsy of infancy (Dravet syndrome): recognition and diagnosis in adults. Neurology. 2006;67(12):2224–2226. doi:10.1212/01.wnl.0000249312.73155.7d
7. Arzimanoglou A, French J, Blume WT, et al. Lennox-Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol. 2009;8(1):82–93. doi:10.1016/S1474-4422(08)70292-8
8. Steffenburg U, Hedstrom A, Lindroth A, Wiklund LM, Hagberg G, Kyllerman M. Intractable epilepsy in a population-based series of mentally retarded children. Epilepsia. 1998;39(7):767–775. doi:10.1111/j.1528-1157.1998.tb01163.x
9. Smiley E, Cooper SA, Finlayson J, et al. Incidence and predictors of mental ill-health in adults with intellectual disabilities: prospective study. Br J Psychiatry. 2007;191(4):313–319. doi:10.1192/bjp.bp.106.031104
10. Wheless JW, Maggio V. Vagus nerve stimulation therapy in patients younger than 18 years. Neurology. 2002;59(Issue 6, Supplement 4):S21–S25. doi:10.1212/WNL.59.6_suppl_4.S21
11. Shields WD. Management of epilepsy in mentally retarded children using the newer antiepileptic drugs, vagus nerve stimulation, and surgery. J Child Neurol. 2004;19(Suppl 1S):58–64. doi:10.1177/088307380401900107
12. Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia. 1998;39(7):677–686. doi:10.1111/j.1528-1157.1998.tb01151.x
13. Doran Z, Shankar R, Keezer MR, et al. Managing anti-epileptic drug treatment in adult patients with intellectual disability: a serious conundrum. Eur J Neurol. 2016;23(7):1152–1157. doi: 10.1111/ene.13016
14. Kwok H, Cheung PW. Co-morbidity of psychiatric disorder and medical illness in people with intellectual disabilities. Curr Opin Psychiatry. 2007;20(5):443–449. doi:10.1097/YCO.0b013e3282ab9941
15. O’Dwyer M, Peklar J, Mulryan N, McCallion P, McCarron M, Henman MC. Prevalence and patterns of anti-epileptic medication prescribing in the treatment of epilepsy in older adults with intellectual disabilities. J Intellect Disabil Res. 2018;62(3):245–261. doi: 10.1111/jir.12461
16. Brodtkorb E, Sand T, Strandjord RE. Neuroleptic and antiepileptic treatment in the mentally retarded. Seizure. 1993;2(3):205–211. doi:10.1016/S1059-1311(05)80129-3
17. Mirza W, Credeur L, Penry JK. Results of antiepileptic drug reduction in patients with multiple handicaps and epilepsy. Drug Invest. 1993;5(6):320–326. doi:10.1007/BF03259239
18. Lund C, Kostov H, Blomskjøld B, Nakken KO. Efficacy and tolerability of long-term treatment with vagus nerve stimulation in adolescents and adults with refractory epilepsy and learning disabilities. Seizure. 2011;20(1):34–37. doi:10.1016/j.seizure.2010.10.002
19. Huf RL, Mamelak A, Kneedy-Cayem K. Vagus nerve stimulation therapy: 2-year prospective open-label study of 40 subjects with refractory epilepsy and low IQ who are living in long-term care facilities. Epilepsy Behav. 2005;6(3):417–423. doi:10.1016/j.yebeh.2005.01.009
20. Kerr M, Scheepers M, Arvio M, et al. Consensus guidelines into the management of epilepsy in adults with an intellectual disability. J Intellect Disabil Res. 2009;53(8):687–694. doi:10.1111/j.1365-2788.2009.01182.x
21. Rodin EA, Rhodes R, Velarde N. The prognosis for patients with epilepsy. J Occup Environ Med. 1965;7(11):560–563. doi:10.1097/00043764-196511000-00003
22. Sourbron J, Klinkenberg S, Kessels A, Schelhaas HJ, Lagae L, Majoie M. Vagus nerve stimulation in children: a focus on intellectual disability. Eur J Paediatr Neurol. 2017;21(3):427–440. doi:10.1016/j.ejpn.2017.01.011
23. Wheeler M, De Herdt V, Vonck K, et al. Efficacy of vagus nerve stimulation for refractory epilepsy among patient subgroups: a re-analysis using the Engel classification. Seizure. 2011;20(4):331–335. doi:10.1016/j.seizure.2011.01.002
24. Andriola MR, Vitale SA. Vagus nerve stimulation in the developmentally disabled. Epilepsy Behav. 2001;2(2):129–134. doi:10.1006/ebeh.2001.0160
25. Engel J. Principles of epilepsy surgery. Treat Epilepsy. 1996.
26. Rausch R. Factors affecting neuropsychological and psychosocial outcome of epilepsy surgery. Epilepsy Surg. 1991;487–493.
27. Gates J, Huf R, Frost M. Vagus nerve stimulation for patients in residential treatment facilities. Epilepsy Behav. 2001;2(6):563–567. doi:10.1006/ebeh.2001.0286
28. Couch JD, Gilman AM, Doyle WK. Long-term expectations of vagus nerve stimulation: a look at battery replacement and revision surgery. Neurosurgery. 2016;78(1):42–46. doi:10.1227/NEU.0000000000000985
29. Wheless JW, Gienapp AJ, Ryvlin P. Vagus nerve stimulation (VNS) therapy update. Epilepsy Behav. 2018;88:2–10. doi:10.1016/j.yebeh.2018.06.032
30. Revesz D, Rydenhag B, Ben-Menachem E. Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J Neurosurg Pediatr. 2016;18(1):97–104. doi:10.3171/2016.1.PEDS15534
31. Cummins RA. Assessing quality of life. In: Quality of Life for People with Disabilities: Models, Research and Practice. Stanley Thornes Ltd.Vol. 2. 1997:116–150.
32. Kerr P, Espie CA. Learning disability and epilepsy. I, towards common outcome measures. Seizure. 1997;6(5):331–336. doi:10.1016/S1059-1311(97)80032-5
33. Deshpande PR, L. Sudeepthi B, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2(4):137–144. doi:10.4103/2229-3485.86879
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
