Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 13
Vaccination Status and Its Determinants Among Children Aged 12 to 23 Months in Mettu and Sinana Districts, Oromia Region, Ethiopia: A Comparative Cross Sectional Study
Authors Mebrate M , Workicho A, Alemu S , Gelan E
Received 11 July 2022
Accepted for publication 13 September 2022
Published 23 September 2022 Volume 2022:13 Pages 335—348
DOI https://doi.org/10.2147/PHMT.S380303
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Milkessa Mebrate,1 Abdulhalik Workicho,2 Soresa Alemu,1 Ebsa Gelan3
1Mettu Health Science College, Mettu, Ethiopia; 2Department of Epidemiology, College of Health Science, Jimma University, Jimma, Ethiopia; 3Departement of Statistics, College of Natural Science, Mettu University, Mettu, Ethiopia
Correspondence: Soresa Alemu, Mettu, Oromia, Ethiopia, Tel +251 917273506, Email [email protected]
Background: Globally, more than 19 million children have not received all of their vaccination benefits, resulting in an estimated one million deaths worldwide each year. Vaccine-preventable diseases are becoming more common in Ethiopia, despite the fact that official vaccination coverage is sufficient to develop herd immunity locally for some diseases such as measles. This mistrust of the official report prompted us to conduct a community survey and compare it to other areas where there have been no reports of vaccine-preventable disease.
Methods: A community-based comparative cross-sectional study was conducted from 20/01– 20/02/2021 in Sinana and Mettu districts. Probability proportional to estimate size was used to select 23 clusters. We recruited 228 from Mettu and 436 from Sinana by systematic random sampling. We used a structured questionnaire to collected data from mother–child pair using card and history. We conducted independent t-tests to test coverage differences between districts. We identified determinants of full vaccination status by multivariate logistic regression analysis after bivariate candidate selection.
Results: Fully vaccinated children accounted for 62.7% in Sinana and 91.6% in Mettu, demonstrating a significant coverage difference (p< 0.001). Being a resident of Mettu (AOR: 3.5, 95% CI [1.5, 6.9]), intended pregnancy (AOR 5.9, 95% CI [2.4, 11.3]), 4 or more antenatal care visits (AOR: 2.09, 95% CI [1.4, 3]), having postnatal care (AOR: 3.5, 95% CI [1.6, 7.9]), younger child age (AOR: 0.87, 95% CI [0.8, 0.9]), having up to three children (AOR 3, 95% CI [1.13, 8]) and good knowledge of vaccine schedule (AOR: 2.4, 95% CI [1.4, 4]) were associated positively with full vaccination status.
Conclusion: Full vaccination status was 91.6% in Mettu and 62.7% in Sinana district. Place of residence, ANC, PNC, pregnancy intention, child number, age of child and knowledge of vaccination schedule were significantly associated with vaccination status of the children.
Keywords: vaccination status, determinants, 12– 23 months children, Mettu district, Sinana district
Introduction
Vaccination of children has been recognized as one of the most cost-effective programs against vaccine-preventable diseases (VPD) because it reduces child mortality and morbidity worldwide.1 As a result, the World Health Organization recommends that all children receive one dose of BCG, three doses of OPV (oral polio vaccine) or IPV, three doses of Pentavalent (diphtheria, pertussis, tetanus, Hib (Hemophilus Influenza B Virus), and HepB (Hepatitis B Virus) vaccines, two doses of Rotavirus vaccine, three doses of PCV, and one dose of measles vaccine before their first birthday as routine EPI (Expanded Program of Immunization), which was later expanded to Ethiopia in 1980. In this way children are considered as being fully vaccinated if they have received all the recommended vaccines at the age of one year, incompletly vaccinated if they missed at least a single dose of vaccine and not vaccinated if they did not received even a single dose.2–6
According to a recent CDC (communicable disease control) report, there has been a significant reduction in pertussis by 91%, paralytic polio, diphtheria and smallpox by 100%, pneumococcus at age five years by 89%, rotavirus hospitalization at three years old by 51%, Hib, rubella, and measles by more than 99%, hepatitis A and B by 97% and 68%, respectively, and mumps by 96% from pre-vaccination era to 2017 as a result of increased vaccine coverage worldwide.6 This has also resulted in a significant decline in the mortality and morbidity of children under the age of five in Africa.7 However, the current failure to vaccinate has resulted in an estimated 1 million deaths per year from vaccine-preventable diseases worldwide. Nearly 30% of these deaths account for children under the age of five.8–10
The recurrent measles outbreaks in America, Europe, South East Asia, and Africa in 2017 and 2018; the endemic WPV1 (wild poliovirus) in Afghanistan and Pakistan; the CVDPV (circulating vaccine derived poliovirus) outbreak in many African countries; and uneradicated rubella indicated a gap in routine vaccination coverage at the national and subnational levels.11,12 The World Health Organization also reported that, during the outbreak from 2011 to 2016, a high proportion of laboratory-confirmed measles cases were in children unvaccinated for measles worldwide.13
Ethiopia has achieved tetanus and wild poliovirus eradication, as well as a reduction in disease burden from vaccine-preventable diseases due to increased infant and maternal immunization. Aside from this success, one of the challenges is the discrepancy between direct survey data and administrative report data of vaccination coverage. For example, according to administrative data, national full vaccination coverage was 88% in 2019, whereas the EDHS (Ethiopian Demographic Health Survey) reported only 43%.14 The EPI coverage survey done in selected zones in Ethiopia also indicated the gap in an HMIS (Health Information Management System) report.15 In 2019, the EDHS key indicator for vaccination of children aged 12 to 23 months shows 72% DPT (diphtheria, pertussis and tetanus vaccine) 3/pentavalent coverage, while the official report shows more than 90%.16,17 Aside from this disparity, administrative coverage reports are inaccurate due to denominator error, as well as errors during recording and compilation.18 The other challenge is that, despite reported basic immunization coverage of more than 85%, the measles outbreak has recently become one of the country’s major health problems, particularly in the Oromia and Somali regional states.5
Since the Covid-19 pandemic entered Ethiopia in March 3, 2020, health service utilization had dramatically decreased, especially in urban dwellers. This decrease lasted no longer than six months after the entrance of the Covid-19 pandemic and no significant change was observed in utilization of the expanded program of immunization(EPI) specifically in rural parts of Ethiopia.19–21
Many studies have been conducted to investigate the determinants of low immunization uptake, and it has been discovered that disparities in access due to wealth, education, place of residence, differences in birth cohort, remote area, displacement, and densely populated urban areas are the major factors for vaccine coverage inequality, nationally and sub-nationally. Mother’s educational level, mother’s age at birth, birth order, residence, economic status, ANC (antinatal care) follow-up, and fear of vaccination have all been linked to immunization status in studies.22–25 However, some variables, such as child age, home-based postnatal care by health service outreach, and residence were not found in sufficient quantities in the literature, to our knowledge.
Even though the studies were useful in determining vaccination status and determinants, they used an unstandardized old model cluster survey sampling technique that leaves room for uncertainty in representativeness.18 We were interested in assessing vaccination status of children aged 12–23 months because of the importance of vaccination status assessment due to limited knowledge on true vaccination status of local areas where there is a VPD outbreak, discrepancy between official and survey data, and inaccuracy of official reports. In addition to information gaps, assessing vaccination status is important for increasing vaccination coverage and minimizing vaccination inequality, which is significant in reducing child morbidity and mortality. So, our aim was to assess vaccination status and its determinants among children aged 12–23 months in Mettu and Sinana districts and test the difference in coverage.
Materials and Methods
Study Area and Period
From January 20 to February 20/2021, we conducted research in the districts of Sinana and Mettu. We chose Sinana because a measles outbreak in the rural area had been reported to the zonal health office, and Mettu was chosen for comparability because no outbreak had been reported. Both areas are almost identical in terms of accessibility because the capital towns of Mettu for the Ilu Aba Bora zone and Robe for the Bale zone are within the districts.
Sinana is a Bale zone district in Oromia regional state in southeast Ethiopia. It is approximately 450 kilometers from Addis Ababa. There are two urban and 25 rural kebele in the district. According to the central statistical authority (CSA), the district’s population in 2020 was expected to be 168,015 people, with 82,327 (49%) females and 85,688 (51%) males. With an estimated 35,003 households, 4301 (2.56%) were urban and 163,714 (97.44%) were rural. The district currently has 24 health posts and six health centers. In 2020, the district’s estimated number of surviving infants at one year was 5494. Mettu is also one of the Ilu Aba Bora zone districts in southwest Ethiopia’s Oromia region. The district is located 600 kilometers from Addis Abeba. The district has 29 rural kebele and no urban kebele. The total population of Mettu district was estimated to be 88,054, with 43,147 (49%) females, and an average 18,345 households according to the CSA’s population projection in 2020. Recently the district had five health centers and 26 health posts. The number of surviving infants at one year was approximately 2,835 (3.217%).
Study Design
We conducted a community-based comparative cross-sectional study to assess vaccination status and its determinants among children aged 12–23 months in Sinana and Mettu district from January 2020 to February 2020/2021.
Population
Source population
All mother/caretaker–child (12–23 months) pairs living in Sinana and Mettu district.
Study population
Mother/caretaker–child (12–23 months) pairs living in randomly selected clusters during data collection.
Study Unit
Mother/caretaker–child pairs interviewed during data collection from the selected households or clusters.
Sample Size and Sampling Technique
Sample Size
Sample size was computed using Epi info software. We considered design effect of 2.5 by considering 1/6 intra-cluster coefficient (ICC) and an individual can interview 10 mother–child pairs (m) per day, DEFF = 1+ (m–1) ICC yields 2.5. We also considered two clusters and total population of children in both districts who survived to first birthday, 8330 (Mettu = 2835; Sinana = 5494). Proportion of 76.8% was taken from previous literature and total sample size computed was 664.26 Therefore, our sample size was 664 mother–child pairs living in Mettu and Sinana districts. By using “r”, the sample size for Sinana district was 436 and 228 for Mettu district.
Considering that not all houses have an eligible study population, we estimated number of houses that were visited per individual eligible child per cluster. The formula is:
NHH to find eligible child (first birthday) = 
N survived at first birthday per HH = 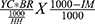
YC is age of eligible children in the cohort, BR is birth rate per 1000 population, HH is average household size and IM is infant mortality rate per household. Assuming every one survives after their first birthday and (YC = 1 year, BR = 36, IM = 43, HH = 4.8) (12), NHH to find eligible child is six households per eligible child. The total households visited to get 10 eligible children per data collector was 67 households by considering 10% non-response rate. The total households visited was 4383 (Sinana = 2778; Mettu = 1605). The sample size of the cluster/kebele was determined by dividing sample size of each district by number of mother–child pair interviewed from each kebele. Depending on number of data collectors and household size of each kebele, we assigned an average of 40 mother–child pairs per kebele for four data collectors in Sinana district and 20 mother–child pairs per kebele in Mettu district. Therefore, 11 kebele from Sinana district and 12 kebele from Mettu district were selected.
Sampling Technique
We used two stages of random sampling to choose a cluster/kebele and then a study unit from that cluster. To begin, the kebele was chosen using the probability proportion to estimate size (PPS) sampling technique. Sinana district has 25 rural kebeles, while Mettu district has 29 rural kebeles. We created a table frame in Excel that contains the total kebeles of each district as well as the households for each district separately. The total number of households were then computed. The final cumulative sum is the total number of households within the district divided by the number of clusters chosen from each district, which was K = 1488 for Mettu and 3182 for Sinana. The first cluster was selected randomly by using Excel “RANDOMBETWEEN (1,1488 for Mettu and 1,3182 for sinana)”, then we selected the other clusters consequently by (C2= C1 +K, C3= C2+k …) until all the sample clusters were selected. The selection of kebele was near below or equal to the subsequent cumulative households. To select the households we used systematic random sampling, which was done by using list of household head from the health post. We divided the total households within the selected kebele to households visited per kebele, which was 134 for Mettu and 268 for Sinana. This yielded sample size interval “k” that was different for each kebele. We selected random number by using random number table between 1 and k, which was “I” and the subsequent households to be visited to find eligible child was ith, [i+K]th, (i+2k)th …, [i + (n-1)k]th. The list of subsequent households’ names was visited using local coordinators who knew the households in each kebele.
Data Collection Procedure and Tools
A structured questionnaire administered by an interviewer was used to collect data from the mothers or caregivers. The questionnaires were created after a review of the literature. The questionnaire asked about the sociodemographic characteristics of the parents and children, maternal health care utilization, and knowledge of and attitude toward immunization-related factors. A total of eight data collectors and two supervisors were involved, with four data collectors and one supervisor assigned to each district. Degree-holder nurses were required for supervision, and diploma nurses were required for data collection. After identifying the eligible child at home, the data was collected. This was accomplished by visiting all of the households in the cluster. The data collectors were given the names of randomly selected households as well as assistance from a community member in identifying the households. The data was collected by observing EPI card and taking a history of vaccination from mother or caretaker. The presence of child was not mandatory where caretaker know very well about child vaccination history and where they were provided with a vaccination card.
Data-Processing and Analysis
First, we checked the collected data for completeness and consistency before creating a template with Epi data software. The data was then processed by entering it into the Epi data version 3.1 template and checking for double-entry or duplication. We then exported to SPSS version 21 software and cleaned it up by looking for errors and missing values. Data was screened for outliers, missed values, and extreme values. To describe sociodemographic characteristics and vaccination status, we used univariate analysis such as frequencies and measures of central tendencies. By binary logistic regression, bivariate analysis was used to select candidate variables at (p<0.25). Binary logistic regression was used for multivariate analysis. Explanatory variables that were independently associated with vaccination status were interpreted in terms of AOR at (p-value = 0.05, 95% confidence level). The Hosmer−Lemeshow good fit statistics were used for the multi-logistic regression assumptions. Independent sample t-test was used to test the coverage difference of all vaccinations between the districts. The overall fit of the final model was shown by −2log-likelihood statistics and tested by chi-square statistics. The Hosmer−Lemeshow good fit statistics were used to test whether the multi-logistic assumptions were met. The chi-square test was used to assess the coverage gap between Sinana and Mettu districts, as well as model fit. We interpreted the findings by comparing them to previous studies and summarized the data in frequencies, graphs, and dummy tables.
Data Quality Control
The questionnaire prepared in English was translated to Afan Oromo and another translator re-translated to English to check its consistency. Before the actual data collection, the questionnaire was tested on 5% of mothers of children whose age was between 12 and 23 months in Hurumu district, Ilu Aba Bora Zone. After pretesting, necessary amendments were made accordingly, including on ambiguities of the questions, wording, logic sequence, skipping order, and ethical issues. Both data collectors and supervisors were trained on the study instrument and data collection procedure before data collection time differently in each district. The training was more hands-on, with role-playing and questionnaire filling. To reduce interviewer bias, data collectors and supervisors were chosen from outside the study area. Supervisors and principal investigators provided ongoing follow-up and feedback on a daily basis during data collection. Following the completion of data collection, the principal investigator and supervisor checked for completeness and implemented corrective measures. Before analyzing the data, it was cleaned, coded, and screened.
Results
Sociodemographics
The response rate was 657 (98.9%) out of a total sample size of 664 mother/caregiver−child pairs, with 225 (34%) from Mettu and 432 (66%) from Sinana district. A total of 23 kebele were involved, with 11 (44%) from 25 rural kebeles in Sinana and 12 (41%) from 29 rural kebeles in Mettu. The overall mean age, median age, and standard deviation of mothers/caregivers were 26, 25, and 3 months for the corresponding children. The average age of participating children was 17 and 16 months in Mettu and Sinana districts, respectively, while it was 27 and 26 in Mettu and Sinana districts for mothers/caregivers. Majority of the mothers who participated 587 (89%) were married, 29 (4.4%) were divorced, 11 (1.7%) were widowed and 30 (4.6%) were single mothers. Regarding sex of children, 317 (48%) were male and 340 (52%) were female. Regarding educational level of mothers, 135 (20.55) were secondary and above, 391 (59.5%) were primary and 129 (19.6%) were illiterate (Table 1).
 |
Table 1 Sociodemographic Characteristics of Participants from Mettu and Sinana Districts |
Vaccination Status
From the total number of mother−child pair participants interviewed, 637 (96.9%) were ever vaccinated, with 477 (72.6%) fully vaccinated for the routine vaccination program of under one year targeted vaccines. The remaining 180/657 (27.4%) were either missing at least one dose; 160/657 (24.3%), or had never received a routine vaccine, 20/657 (3%) according to the country’s vaccination program. According to this finding, 637 (96.9%) of children received routine vaccinations, of which 370 (58%) were by card plus, 59 (9%), by card only, and 206 (33%), by history only. The overall dropout rate was 22%, and the dropout rate for penta was 0.95% (Figure 1).
 |
Figure 1 Level of individual vaccine coverage in both Mettu and Sinana districts, 2021. |
Vaccination Status Related to Sociodemographics
From 657 household heads 70 (10.6%) were female, out of which 57 (81.4%) of their children were fully vaccinated. Majority of the participating children were female, 340 (51.2%) (Table 2).
 |
Table 2 Sociodemographic Characteristics of Mother–Child Pair Participants Among Completly and Incompletly Vaccinated Children |
Vaccination Status Related to Health Care Utilization and Accessibility
We found institutional delivery of 564 (85.8%) from all participants, 507 (77%) of mothers attended ANC more than 2 times and only 62 (9%) were followed by health extension worker during their post-partum (Table 3).
 |
Table 3 Health Facility Utilization Related Factors in Completely and Incompletly Vaccinated Children |
Vaccination Status Related to Knowledge and Attitude of Mothers
From respondents, 498 (75.8%) of the interviewed mothers have an above-average positive attitude toward their children’s vaccination status, while the rest have a negative attitude. The majority of people are unaware of vaccine-preventable diseases, 611 (93%). However, 551 people (83.8%) remembered a vaccination schedule that was above the average (Table 4).
 |
Table 4 Attitude and Knowledge of Participating Mothers of Completely and Incompletely Vaccinated Participating Children |
Vaccination Status Comparison Between Mettu and Sinana Districts
By comparing the vaccination status of children in the districts, 206 (91.6%) of Mettu children were fully vaccinated while only 267 (62.7%) of Sinana children were fully vaccinated. As a result, the fully vaccinated status of children in Mettu was 31.5% higher than that of Sinana district. In inferential statistics by using independent sample t-test, the overall average coverage was significantly different with a mean difference of 1.442, CI (1.22, 1.67). The overall dropout rate was 8% for Mettu district and 30% for Sinana district. The dropout rate for Pentavalent was zero for Mettu while it was 1.4% for Sinana districts.
Regarding the retention of card, the mother−child pairs who vaccinated at least a single vaccine accounted for 194 (86.6%) of 224 children of Mettu had EPI card, while 235 (56.9%) of 413 children from Sinana district had the vaccination card (Figure 2).
 |
Figure 2 Comparison of individual vaccine dose coverage of Mettu and Sinana districts, 2021. |
Factors Associated with Vaccination Status
In the binary logistic regression the majority of the factors were significantly associated with vaccination status being a factor for vaccination completion except child sex, mother’s occupation, marital status and attitude towards vaccination at p <0.25 significance and 95% confidence level. In multivariate analysis, factors related to child and family characteristics such as residence of Mettu (AOR: 3.5, 95% CI [1.7, 6.9]), intended pregnancy (AOR: 5.9, 95% CI [2.4, 11.3]), three or fewer children (AOR: 3, 95% CI [1.13, 8]), younger child age (AOR: 0.87, 95% CI [0.8, 0.9]) were significantly associated with full vaccination status. Health care utilization such as four or more ANC (AOR: 2, 95% CI [1.4, 3]) and PNC visited by health extension (AOR: 3.5, 95% CI [1.6, 7.9]) were also associated significantly with full vaccination status. From knowledge and attitude type variables, knowledge of mother on recalling vaccination schedule (AOR: 2.4, 95% CI [1.45, 4]) was associated with full vaccination status (Table 5). The multivariate data feed was significantly good (p <0.001) and the multivariate assumptions were insignificant (p = 0.63), which indicates that the multivariate assumptions were fulfilled.
 |
Table 5 Independent and Dependent Associated Variables with Vaccination Status of Participating Children |
Discussion
According to our findings, of the child participants,160 (24%) were partially, 20 (4.1%) were not at all and 477 (72.6%) were fully vaccinated. By merging partially and not at all vaccinated, the incomplete vaccination status was 180/657 (28.4%). Full vaccination status was 206/225 (91.6%) in Mettu district and 267/432 (62.7%) in Sinana district. On multivariate regression analysis, the odds of being fully vaccinated in Mettu resident children was 3.5 times higher than in Sinana district children, p <001. Children whose mothers had an intended pregnancy, had four or more antenatal follow-ups, had visited during postpartum to check health of resultant child, had good knowledge of vaccination schedule, and had three or fewer children had a higher chance of being fully vaccinated. The child’s age was also associated with full vaccination, with each one-month increase in child age from 12 to 23 months meaning a decreasing chance of being fully vaccinated by 13%.
Vaccination status refers to the number of vaccines received by a child in accordance with the county schedule. In this way, one-year-old children are the target population in Ethiopia for completing the full routine vaccines. However, equity in vaccination would be achieved if coverage increased similarly in different geographical areas; however, it is difficult to obtain the same vaccination status in different locations due to utilization and socioeconomic differences. This was also demonstrated in our findings between the Mettu and Sinana districts, with a mean coverage difference of 1.442 CI (1.22, 1.67). However, many studies have revealed that in lower- and middle-income African countries, the average full vaccination status ranges from 56% to 69%, with Chad having the lowest at 11% and Rwanda the maximum at 90%.23,27–29.
This is almost concordant with our findings, with a slight difference in total fully vaccinated status of both districts and similar to our findings in Sinana district. However, a community-based study conducted in another Ethiopian district revealed that the fully vaccinated status was 91.7%.30 This finding was high, but it was similar to our vaccination status finding in Mettu district. The observed similarities and differences could be due to social, economic, and cultural similarities and differences between locations, as well as the level of health care utilization and accessibility at the community level. A study in India found that the difference in vaccination coverage between states was caused by poverty, with children from middle-income states being more likely to be vaccinated than children from lower-income states.31 We cannot say about the poverty in our case since we did not assess level of poverty at district level. This could be due to accessibility of health facilities, which may be true in our finding that number of health posts in Mettu district is more than Sinana district despite the population of Mettu being lower than that of Sinana.32
In our study, children born from an intended pregnancy were more likely to be fully vaccinated than children born from an unintended pregnancy. Similarly, a study conducted in Debramarkos, Ethiopia, discovered that vaccination status was higher in an intended pregnancy than in an unintended pregnancy (AOR: 2.89 [1.17, 7.17]).30 The other concordant finding was that unintended pregnancy resultant newborns were more likely to receive inadequate vaccinations (OR: 1.25 [1, 1.56]).33 This could be because unintended pregnancy may be from unmarried mothers who are afraid of having a child without marriage because it is culturally taboo in both areas.
A previous study found that having a large number of children had a negative impact on vaccination status, which is what we discovered. In a study conducted in difficult-to-reach areas of Ethiopia, index children born to a mother with five or more children were less likely to be vaccinated.34 This was also demonstrated by the fact that children from mothers with more than three parities were less likely to be fully vaccinated than children from mothers with fewer than three parities.35 Children are less likely to be vaccinated as the number of children increases because mothers with fewer children are more likely motivated to engage in self-care.
The mothers’ attendance at the ANC follow-up was also found to be a determinant of vaccination status. ANC utilization increased the chance of having fully-vaccinated children (AOR: 1.64, CI [1.23, 2.18] and 1.36 CI [1.14, 1.62], respectively), compared to mothers who did not attend antenatal care, according to a study conducted in the Democratic Republic of the Congo and the southern part of Ethiopia.36,37 Attending four or more antenatal visits was found to be strongly associated with vaccination status in a multilevel analysis of the 2016 Ethiopian demographic health survey.38
Postnatal clinic utilization was a determinant of vaccination status. Children whose mothers received PNC from health extension workers were more likely to be fully vaccinated than children of mothers who did not receive PNC from health extension workers. Two studies conducted in north and northwest Ethiopia revealed findings consistent with ours.39,40 For both antenatal and postnatal care, the association may be due to increased health care utilization increasing the level of awareness of the benefits of vaccination, resulting in the child receiving full vaccination.
An increase in child age was also negatively associated with vaccination status by decreasing chance of getting full vaccination by 13% ‘in every one-month increase in child age. The study conducted in Angola also revealed younger children were more likely to be fully vaccinated.41 The other study conducted in Ethiopia agreed with our finding indicating that the odds of full vaccination in a child early in its second year was 2.7 times higher than later in its second year (AOR: 2.7, 95% CI [1.36, 5.4]).42 This may be due to vaccine coverage increasing from time to time or respondents recalling recent vaccination status that corresponds to younger children more than older children.
According to our findings, children whose mothers had good knowledge of the routine vaccination schedule were more likely to be vaccinated thanc children of mothers with poor knowledge. Similarly, a study conducted in Niger revealed that children of mothers with good knowledge of vaccination schedule (AOR: 5.32, 95% CI [2.05, 13.82]) were more likely to be vaccinated.43 Knowing the vaccination schedule might help mothers to take their child on time. This may help to get children vaccinated, especially multi-dose vaccines. In contrast, mothers who have poor knowledge of vaccination schedule may miss the exact day when multi-dose vaccines are due and even the health care provider may be absent on that day, resulting in fewer vaccinated children.
Limitations of Study
Data for this finding were gathered from vaccination cards and interviews with mothers or caregivers. There may be uncertainty regarding participants who did not retain or provide a card from the health facility, resulting in information bias. We used the moderate type of ICC to calculate our sample size due to limited resources. Another limitation was that variables affecting children’s vaccination status were not considered from the perspective of fathers.
Strengths of the study
It is one of few studies conducted in Ethiopia to determine vaccination coverage difference between local districts. The sample technique that the authors used was the WHO 2015 manual community survey. The findings show the actual figure of vaccination coverage. Also the research was based on measles outbreak.
Conclusions
Generally, the full vaccination status of Mettu and Sinana district was 72.2%, which is low compared to district target coverage of 80%. The coverage differs between the districts: 91.6% in Mettu and 62.2% in Sinana. Place of residence, ANC, PNC, pregnancy intention, age of child, mothers’ knowledge of vaccination schedule and number of children the mother has determined vaccination status of the children age 12 to 23 months. Increasing health care utilization and preventing unwanted pregnancy can improve vaccination uptake.
The complete vaccination coverage of districts targeted nationally is above 80%, which is not attained by Sinana district. Therefore, we recommend Sinana district health office to increase the coverage to the target level.
Since some of the vaccine preventable diseases need high coverage to develop herd immunity like that of measles, which is above 95%, both districts should strengthen health facility utilization of mothers for ANC and PNC. We recommend that health extension workers of both districts provide home visit PNC and ANC to provide health education for all mothers. All health centers and health posts in the districts should increase mothers’ knowledge of routine vaccination schedules.
Lastly, we recommend that researchers do this type of study using conservative type of ICC and not depending only on official reports of vaccination coverage to indicate the real vaccination status of children. We also recommend that researchers conduct operational research on how to improve vaccination coverage.
Abbreviations
ANC, Antinatal Care; AOR, Adjusted Odds Ratio; BR, Birth Rate; CDC, Communicable Disease Control; CI, Confidence Interval; CMYP, Comprehensive Multiyear Plan; CVDPV, Circulating Vaccine Derived Poliovirus; DEFF, Design Effect; DPT, Diphtheria, Pertussis and Tetanus Vaccine; EDHS, Ethiopian Demographic Health Survey; EPI, Expanded Program of Immunization; GAVI, Global Alliance for Vaccine and Immunizations; GIVS, Global Immunization Vision and Strategy; GVAP, Global Vaccine Action Plan; Hep B, Hepatitis B Virus; Hib, Hemophilus Influenza B Virus; HMIS, Health Information Management System; ICC, Intra-Cluster Correlation Coefficient; IM, Infant Mortality; MCV, Measles Vaccine; MDG, Millennium Development Goal; MNT, Maternal and Neonatal Tetanus; MR, Mortality Ratio; NWPV1, New Wild Poliovirus; PCV, Pneumococcal Conjugated Vaccine; PNC, Postnatal Care; PPS, Probability Proportion to Size; RED, Reaching Every District; SOS, Sustainable Outreach Services; UNICEF, United Nation Children’s Fund; VPD, Vaccine Preventable Disease; WHO, World Health Organization; YC, Number of Years of eligible children in the cohort.
Data Sharing Statement
Data will be available upon request to the corresponding author.
Ethics Approval and Consent to Participate
This study was conducted according to the principles of the Declaration of Helsinki.44 Ethical clearance was obtained from Jimma University, Institute of Health, Institutional Review Board (IRB) research committee offering to review the ethics of research design and objectives. Official letter was written to Bale and Ilu Aba Bora zonal health office and then to Sinana and Matu districts and to selected kebele administrators. After explaining the study objectives, each study participant provided written informed consent. Respondents participated on a voluntary basis. Participants were informed that the information to be collected was anonymous; those who were unhappy during the interview were asked to leave, and only those who were willing were interviewed. Throughout the research process, response confidentiality was maintained. Respondents’ privacy and cultural norms were respected.
Acknowledgments
We would like to extend our thanks to Jimma University for providing a conducive environment that helped us to conduct this study. We are also very glad to forward our special thanks for the unlimited assistance of hospital and health center managers. Our sincere thanks also go to data collectors, supervisors and the study participants for their contribution to this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received for this particular study.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Jenner E, Figure S, Figure S, Book VF. Vaccine Fact Book. Vaccine Fact; 2012:4–10.
2. Steve LW, Steve Sapirie RS, George Stroh AW. Immunization coverage cluster survey – reference manual, CH-1211. Geneva 27, Switzerland; 2005. Available from: www.who.int/vaccines-documents/.
3. Coiteux J, Febach H, DeBolt C, Zahir J, Phillip Wiltzius KG. Glossary of immunization and public health terms. Washington State Depart Health. 2020;711:1–33.
4. World Health Oraganization. Expanded Programme on Immunization (EPI) Fact Sheet. World Health Oraganization; 2017.
5. Ababa A. Ethiopia national expanded programme on immunization comprehensive multi- multi - year plan 2016–2020 federal ministry of health, Addis Ababa; 2020.
6. CDC. National notifiable diseases surveillance system, 2017 annual tables of infectious disease data. Atlanta, GA: CDC Division of Health Informatics and Surveillance; 2018. Available from: www.cdc.gov/nndss/infectious-tables.html.
7. Secretariat, Gvap WHO Press WHO. Global Vaccine Action Plan, 20 Avenue Appia, 1211. Geneva 27, Switzerland; 2016. Availabe from: www.who.int.
8. World Health Organization. ‘Vaccination coverage’, global health observatory data. World Health Organization. Available from: www.who.int/gho/immunization/en/.
9. World Health Organization. ‘Immunization coverage’. World Health Organization; 2018. Available from: www.who.int/mediacentre/factsheets/fs378/en/.
10. UNICEF. United nations children’s fund, ‘child survival: under-five mortality’; 2018. Available from: https://data.unicef.org/topic/child-survival/under-five-mortality/.
11. UNICEF. Every child survives and thrives global annual results report 2018; 2018.
12. Strategic Advisory Group of Experts on Immunization. 2015 assessment report of the global vaccine action plan. Igarss. 2014;2014(1):1–5.
13. Sheet F. World Health Organization R Office for South East A. Expanded Programme on Immunization (EPI); 2017.
14. Second M, Effective N, Coun A, et al. Major achievements/success immunization in Ethiopia major challenges on immunization program; 2019:628.
15. FMoH. Extended Program on Immunization (EPI) coverage in selected Ethiopian zones: a baseline survey for L10K ’ s routine immunization improvement initiative. JSI Res Train Inst. 2015;2015:1–51.
16. World Health Organization. Ethiopia: WHO and UNICEF estimates of immunization coverage: 2018 revision Ethiopia: WHO and UNICEF estimates of immunization coverage: 2018 revision; 2020:1–26.
17. Ethiopia Mini Demographic and Health Survey. Ethiopian Public Health Institute (EPHI) [Ethiopia] and ICF:Key Indicators. Rockville, Maryland UE and I; 2019. Available from: www.DHSprogram.com.
18. World Health Organization. World health organization Harvard national model united nations; 2012:1194–1198.
19. Bekele C, Bekele D, Hunegnaw B, et al. Impact of COVID-19 pandemic on utilization of facility-based essential maternal and child health Services in North Shewa Zone, Ethiopia. BMJ Open. 2022;555:0–16.
20. Shuka Z, Mebratie A, Alemu G, Rieger M, Bedi AS. Use of healthcare services during the COVID-19 pandemic in urban Ethiopia: evidence from retrospective health facility survey data. BMJ Open. 2022;12(2):1–5. doi:10.1136/bmjopen-2021-056745
21. Carter ED, Zimmerman L, Qian J, Roberton T, Seme A, Shiferaw S. Impact of the early stages of the COVID-19 pandemic on coverage of reproductive, Maternal, and Newborn Health Interventions in Ethiopia: a Natural Experiment. Front Public Heal. 2022;10(2):1–6.
22. Andrianos A, Kelly K. Childhood immunization. J Pediatr Nurs. 1994;9(2):136.
23. Tefera YA, Wagner AL, Mekonen EB, Carlson BF, Boulton ML. Predictors and barriers to full vaccination among children in Ethiopia. Vaccines. 2018;6(2):1–11. doi:10.3390/vaccines6020022
24. Mukungwa T. Factors associated with full immunization coverage amongst children aged 12 –23 months in Zimbabwe. Etude la Popul Africaine. 2015;29(2):1761–1774.
25. World Health Organization U. WHO, UNICEF, World Bank. State of the World’s Vaccines and Immunization.
26. Legesse E, Dechasa W. An assessment of child immunization coverage and its determinants in Sinana District, Southeast?; 2015:1–14.
27. Clara M, Restrepoméndez MC, Barros JD, et al. Inequalities in full immunization coverage: trends in low- and middle-income countries. Bull World Health Organ. 2016;94:794–805. doi:10.2471/BLT.15.162172
28. Xeuatvongsa A, Hachiya M, Miyano S, Mizoue T, Kitamura T. Determination of factors affecting the vaccination status of children aged 12–35 months in Lao People’s Democratic Republic. Heliyon. 2017;3(3):e00265. doi:10.1016/j.heliyon.2017.e00265
29. Adedire EB, Ajayi I, Fawole OI, et al. Immunisation coverage and its determinants among children aged 12–23 months in Atakumosa-west district, Osun State Nigeria: a cross-sectional study. BMC Public Health. 2016;16(1):1–8. doi:10.1186/s12889-016-3531-x
30. Gualu T, Dilie A. Vaccination coverage and associated factors among children aged 12–23 Months in Debre Markos Town, Amhara Regional State, Ethiopia. Adv Public Heal. 2017;2017:1–6. doi:10.1155/2017/5352847
31. Shrivastwa N, Wagner AL, Boulton ML. Analysis of state-specific differences in childhood vaccination coverage in rural India. Vaccines. 2019;7(1):1–11. doi:10.3390/vaccines7010024
32. Vyas P, Kim D, Understanding Spatial AA. Contextual factors influencing intraregional differences in child vaccination coverage in Bangladesh. Asia-Pacific J Public Heal. 2019;31(1):51–60. doi:10.1177/1010539518813604
33. Singh A, Singh A, Thapa S. Adverse consequences of unintended pregnancy for maternal and child health in Nepal. Asia-Pacific J Public Heal. 2015;27(2):NP1481–NP1491. doi:10.1177/1010539513498769
34. Yadita ZS, Ayehubizu LM. Full immunization coverage and associated factors among children aged 12–23 months in Somali Region, Eastern Ethiopia. PLoS One. 2021;16:1–7. doi:10.1371/journal.pone.0260258
35. Awasthi A, Pandey CM, Singh U, Kumar S, Singh TB. ScienceDirect Maternal determinants of immunization status of children aged 12 – 23 months in urban slums of Varanasi, India. Clin Epidemiol Glob Heal. 2015;3(3):110–116. doi:10.1016/j.cegh.2014.07.004
36. Acharya P, Kismul H, Mapatano MA, Hatløy A. Individual- and community-level determinants of child immunization in the democratic Republic of Congo: a multilevel analysis. PLoS One. 2018;13(8):1–17. doi:10.1371/journal.pone.0202742
37. Hailu S, Astatkie A, Johansson KA, Lindtjørn B. Low immunization coverage in Wonago district, southern Ethiopia: a community-based cross-sectional study. PLoS One. 2019;14(7):1–18. doi:10.1371/journal.pone.0220144
38. Kinfe Y, Gebre H, Bekele A. Factors associated with full immunization of children 12–23 months of age in Ethiopia: a multilevel analysis using 2016 Ethiopia Demographic and Health Survey. PLoS One. 2019;14(11):1–14. doi:10.1371/journal.pone.0225639
39. Mekonnen ZA, Mekonnen ZA, Gelaye KA, Were MC, Tilahun B. Timely completion of vaccination and its determinants among children in northwest, Ethiopia: a multilevel analysis. BMC Public Health. 2020;20(1):1–13. doi:10.1186/s12889-020-08935-8
40. Aregawi HG, Gebrehiwot TG, Abebe YG, Meles KG, Wuneh AD. Determinants of defaulting from completion of child immunization in Laelay Adiabo District, Tigray Region, Northern Ethiopia: a case-control study. PLoS One. 2017;12(9):1–13. doi:10.1371/journal.pone.0185533
41. de Oliveira MFS, Martinez EZ, Rocha JSY. Factors associated with vaccination coverage in children <5 years in Angola. Rev Saude Publica. 2014;48(6):906–915. doi:10.1590/S0034-8910.2014048005284
42. Jimma MS, GebreEyesus FA, Chanie ES, Delelegn MW. Full vaccination coverage and associated factors among 12-to-23-Month Children at Assosa Town, Western Ethiopia, 2020. Pediatr Heal Med Ther. 2021;12(279):279–288.
43. Kunieda MK, Manzo ML, Shibanuma A, Jimba M. Rapidly modifiable factors associated with full vaccination status among children in Niamey, Niger: a cross-sectional, random cluster household survey. PLoS One. 2021;16:1–12.
44. World Health Organization. World medical association declaration of Helsinki. Bull World Health Organ. 2001;79(4):373.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
