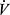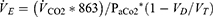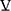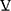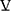Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Utility of Cardiopulmonary Exercise Testing in Chronic Obstructive Pulmonary Disease: A Review
Authors Behnia M , Sietsema KE
Received 28 July 2023
Accepted for publication 17 October 2023
Published 5 December 2023 Volume 2023:18 Pages 2895—2910
DOI https://doi.org/10.2147/COPD.S432841
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jill Ohar
Mehrdad Behnia,1 Kathy E Sietsema2
1Pulmonary and Critical Care, University of Central Florida, Orlando, FL, USA; 2The Lundquist Institute for Biomedical Innovation, Harbor-UCLA Medical Center, Torrance, CA, USA
Correspondence: Mehrdad Behnia, Pulmonary and Critical Care, University of Central Florida, PO Box 953814, Lake Mary, FL, 32749, USA, Tel +1 706-339-8634, Email [email protected]
Abstract: Chronic obstructive pulmonary disease (COPD) is a disease defined by airflow obstruction with a high morbidity and mortality and significant economic burden. Although pulmonary function testing is the cornerstone in diagnosis of COPD, it cannot fully characterize disease severity or cause of dyspnea because of disease heterogeneity and variable related and comorbid conditions affecting cardiac, vascular, and musculoskeletal systems. Cardiopulmonary exercise testing (CPET) is a valuable tool for assessing physical function in a wide range of clinical conditions, including COPD. Familiarity with measurements made during CPET and its potential to aid in clinical decision-making related to COPD can thus be useful to clinicians caring for this population. This review highlights pulmonary and extrapulmonary impairments that can contribute to exercise limitation in COPD. Key elements of CPET are identified with an emphasis on measurements most relevant to COPD. Finally, clinical applications of CPET demonstrated to be of value in the COPD setting are identified. These include quantifying functional capacity, differentiating among potential causes of symptoms and limitation, prognostication and risk assessment for operative procedures, and guiding exercise prescription
Keywords: dyspnea, obstructive lung disease, exercise intolerance, exercise limitation
Introduction and Overview
COPD is a disease of airflow limitation which causes shortness of breath and functional limitation.1,2 Pulmonary function testing (PFT), in particular, spirometry, is the cornerstone in diagnosis and follow-up of COPD. However, PFT has limitations. A patient with mild COPD based on spirometry can have dyspnea out of proportion to the test results. This often reflects the systemic nature of COPD,3 which can affect not only respiratory, but also cardiovascular and musculoskeletal systems, compounding symptoms and functional impairment. CPET can provide insight into mechanisms of dyspnea in COPD and may identify secondary or coexistent nonrespiratory processes such as heart failure, myopathy, or deconditioning which contribute to impairment. The test can also guide clinicians in different treatment options in COPD including prescribing exercise regimens to improve functional limitations and in assessment of treatment efficacy.
In this short review, we will briefly discuss pathophysiology of functional impairment in COPD with a focus on respiratory and cardiac limitations in the disease. We will then describe CPET and its uses in selected contexts for clinical decision-making.
Causes of Impairment in COPD
Pulmonary
COPD is commonly defined by identification of a postbronchodilator FEV1/FVC ratio less than 0.7, and disease severity classified from mild to very severe based on FEV1% of predicted.1 Additional variables including diffusion capacity for carbon monoxide (DLCO),3,4 total lung capacity (TLC), inspiratory capacity (IC), end expiratory lung volume (EELV), along with imaging studies, further distinguish broadly between subtypes of obstructive bronchitis versus parenchymal destruction (emphysema).
Airflow obstruction in COPD is regionally heterogenous, and this contributes to regional heterogeneity of ventilation to perfusion (V/Q) mismatching, which determines the efficiency of pulmonary gas exchange.5 Presence of low V/Q regions can compromise arterial oxygenation, whereas high V/Q regions result in elevation of total ventilation (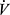 E) required to clear carbon dioxide for maintenance of a stable arterial CO2 pressure (PaCO2). COPD presents dual burdens of reduced breathing capacity due to impairment in lung mechanics, and elevated breathing requirements due to impairment of gas exchange efficiency.
E) required to clear carbon dioxide for maintenance of a stable arterial CO2 pressure (PaCO2). COPD presents dual burdens of reduced breathing capacity due to impairment in lung mechanics, and elevated breathing requirements due to impairment of gas exchange efficiency.
Hyperinflation and Operational Lung Volumes
As shown in Figure 1, tidal volumes (VT) at rest normally represent a small proportion of total lung capacity (TLC) with ample potential for increasing end inspiratory lung volume (EILV) and decreasing end expiratory lung volume (EELV) as needed.6 At rest, EELV is functional residual capacity (FRC), determined by passive equilibration of intra-alveolar and atmospheric pressures in the absence of respiratory effort. COPD, particularly emphysema, may be associated with hyperinflation, that is, elevated TLC and FRC due to high lung compliance. Regardless of whether or not there is resting hyperinflation, dynamic hyperinflation (DH) may occur when respiratory rate increases, such as during exercise, due to incomplete exhalation.6,7 As shown in Figure 1, this is marked by a transient rise in EELV above FRC and a shift of tidal breaths to higher absolute lung volumes.8 Hyperinflation has a number of important effects on breathing mechanics. It lengthens respiratory muscles, flattening diaphragm dome, thereby putting the muscles at a mechanical disadvantage. As the range of absolute lung volumes over which the tidal volume cycles moves to higher levels, tidal breathes approach the flatter, upper portion of the respiratory system compliance curve where higher pressures are needed to effect a given change in volume (Figure 1). With DH there is also an increase in effective intrathoracic pressure at the end of the incomplete exhalation (intrinsic positive end expiratory pressure or PEEP), which must be overcome by respiratory muscles at start of next inhalation.9 These effects are illustrated in the Campbell diagrams shown in Figure 2, and result in increased work of breathing for any given minute of ventilation (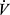 E).10 Because of V/Q mismatch,
E).10 Because of V/Q mismatch,  E may also be increased for any given metabolic rate, further amplifying the work of breathing for a given activity in COPD relative to normal. These effects are consistent with observations that progressively greater dyspnea and work of breathing in COPD are generally associated with the degree of impairment in DLCO,11 although prediction of exercise impairment from resting measures remains imperfect. Importantly, DH can occur even in the absence of resting hyperinflation when
E may also be increased for any given metabolic rate, further amplifying the work of breathing for a given activity in COPD relative to normal. These effects are consistent with observations that progressively greater dyspnea and work of breathing in COPD are generally associated with the degree of impairment in DLCO,11 although prediction of exercise impairment from resting measures remains imperfect. Importantly, DH can occur even in the absence of resting hyperinflation when  E and breathing frequency increase, and can occur even in individuals with relatively mild grades of COPD.12
E and breathing frequency increase, and can occur even in individuals with relatively mild grades of COPD.12
The pulmonary impairments in COPD thus include the primary disorder of impaired expiratory airflow, and the effects of this on reduced efficiency of pulmonary gas exchange, increased 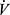 E requirements, reduced
E requirements, reduced  E capacity and increased work of breathing, together leading to dyspnea occurring at abnormally low levels of activity.
E capacity and increased work of breathing, together leading to dyspnea occurring at abnormally low levels of activity.
 |
Figure 1 Rest and exercise spirograms, and placement of rest and exercise tidal breaths on the respiratory system compliance (Pressure – Volume) curve for a healthy individual (a and c) and an individual with chronic obstructive pulmonary disease (COPD) (b and d). End expiratory lung volume (EELV) decreases in the healthy individual during exercise, but increases in COPD, as reflected in increase, and decrease of inspiratory capacity (IC), respectively. The position of VT increases closer to total lung capacity (TLC) for the patient with COPD on the pressure volume curve (c, d) resulting in both a greater reduction in inspiratory reserve volume (IRV) and a larger ventilatory pressure (ΔP) requirement for a given change in volume (ΔV). RV, residual volume. Reprinted with permission of the American Thoracic Society. Copyright © 2023 American Thoracic Society. All rights reserved. O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180–184. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.7 |
 |
Figure 2 The Campbell diagram10 illustrating pleural pressure (Ppl), lung volume (VL), and work of breathing for a healthy individual (A) compared to one with expiratory airflow obstruction and dynamic hyperinflation (B). In both diagrams, volumes are on the vertical and pressure the horizontal axes. Dashed lines representing normal lung elastic recoil and dotted lines relaxed thoracic recoil; the intersection of these lines is relaxed volume (Vrel). The solid loops and counterclockwise arrows illustrate changes in pressure and volume of a tidal breath. The pressure required of inspiratory muscles throughout the breath (Pmus) is the distance between the dotted line and the inspiratory arm of the loop, illustrated by horizontal arrows. In (A), the tidal loop begins at functional residual capacity (FRC) which is Vrel. Shaded areas represent work of breathing, with blue the work against elastance of the lung and chest wall and yellow work against resistance to airflow. In (B), volumes are shifted upwards and end expiratory lung volume is higher than Vrel due to intrinsic positive end expiratory pressure (PEEPi). The tidal loop spans a greater distance on the pleural pressure axis; leftward excursion to greater negative Ppl results from higher airflow resistance and the corresponding increase in yellow area depicts an associated larger work of breathing. The rightward expansion of the loop results from PEEPi which obligates a greater Pmus at the initiation of breath (arrow) and adds work of breathing represented by the green shaded area. Reprinted from Loring SH, Garcia-Jacques M, Malhotra A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J Appl Physiol. 1985;107(1):309–314, Copyright © 2009 the American Physiological Society. Permission conveyed through Copyright Clearance Center, Inc.9 |
Extrapulmonary Limitations in COPD
Cardiovascular Limitation
Pulmonary vascular disease: It is known that exercise cardiac output is reduced in many individuals with COPD.13,14 Although the mechanisms involved are likely complex and heterogenous, the effects of lung disease on the pulmonary circulation clearly contribute to altered hemodynamics. Pulmonary capillary volume may be reduced, particularly in emphysema, due to loss of lung parenchyma, and vascular remodeling can be identified in pulmonary vessels across the spectrum of COPD.15 The latter is postulated to arise from alveolar hypoxia, exogenous exposures, and/or inflammation.16 In the majority of individuals with COPD, elevations in resting pulmonary artery pressure are correlated with other markers of disease severity, are mild to moderate in degree, and do not lead to overt right heart dysfunction.17 Exercise elevations in pulmonary pressures may nevertheless be excessive relative to healthy individuals.14 In a subset of patients, pulmonary hypertension is severe at rest and can lead to right heart dysfunction and failure. This group has more severe dyspnea and exercise limitation17 and greater risk of both COPD exacerbation and death,18 supporting the identification of it as a discrete pulmonary vascular phenotype.19
Work of breathing effects on systemic blood flow distribution: The work of breathing during exercise obligates increased blood flow from the systemic circulation to the muscles of respiration. At peak exercise in athletic young persons, reducing respiratory muscle work has been demonstrated to effectively augment blood flow to the exercising extremities.20 Such competition between respiratory muscles and exercising extremities for a finite cardiac output has been postulated as a cause of limitation in COPD due to the greatly exaggerated work of breathing.21 Changes in the distribution of systemic blood flow during exercise appear to differ in COPD compared to health,22,23 and the role of this in exercise limitation of patients remains incompletely defined.
Pressure volume interactions: Heart and lungs also interact through pressure-volume relationships in their shared location in the thorax.24 Consistent with this, a recent study25 demonstrated that during exercise at matched metabolic rates, COPD patients had greater systemic vascular resistance (SVR), lower stroke volume (SV), and lower cardiac output (CO) compared to controls, despite similar values at rest. These hemodynamic abnormalities correlated with severity of hyperinflation (lower IC/TLC) and with work of breathing (degree of negative intrathoracic pressure generated during inhalation) and exercise SV was positively correlated with %FEV1.25 While negative intrathoracic pressure swings during inhalation are thought to assist venous return to the heart in healthy persons, more negative pressures have been associated with lower stroke volume in COPD perhaps due to covariant factors.25 These findings support the concept that hyperinflation and intrathoracic pressures can negatively impact hemodynamic responses to exercise in COPD.25–28
Ventricular interdependence: Changes in right ventricular preload in the setting of COPD may affect left ventricular filling dynamics due to ventricular interdependence.29 Consistent with this, left ventricular diastolic dysfunction is demonstrated in patients with COPD, even with normal pulmonary artery pressures.30 It is also recognized that patients with heart failure, either with preserved31 or reduced32 ejection fraction who have comorbid COPD have worse functional class than those without lung disease.33 Hyperinflation has again been implicated in this as it has been associated with more left ventricular diastolic impairment and reduced performance on the six-minute walk test (6MWT). Reduction of hyperinflation with rigorous bronchodilator therapy is also reported to increase end diastolic volumes.34 Thus, cardiovascular function can be impaired in COPD due to a range of cardiopulmonary interactions, and in some cases also due to comorbid cardiovascular disease.
Skeletal muscle limitations: Both quantity and quality of skeletal muscle are altered in COPD.35 Some patients complain of limb fatigue as their primary limitation to exercise.36 The skeletal muscle dysfunction in COPD in many individuals may be partially attributed to deconditioning37 from avoidance of activity. However effects of systemic steroid therapy, systemic inflammation, or other features of chronic disease are also implicated.38 Among changes noted in skeletal muscles of COPD patients are a reduction in muscle mass and reduction in the proportion of type I, highly oxidative, fibers within muscle35 compared to age matched healthy individuals. The rate of increase of pulmonary oxygen uptake following the onset of exercise has also been demonstrated to be slower in COPD compared to normal, implying limitation either in central circulatory response to exercise or in skeletal muscle oxidative function. The latter is supported by experiments examining exercise by small muscle volumes, so not limited by central cardiovascular capacity, which have also demonstrated reductions in diffusive and convective oxygen delivery.39 Muscle health is a modifiable target for intervention in COPD, and exercise training arguably has more significant effect on function than other interventions.40,41
Summary of Impairments Affecting Exercise in COPD
Individuals with COPD have impaired lung function leading to reduced breathing capacity, increased breathing requirements due to V/Q mismatch, and exaggerated work of breathing and dyspnea for a given task. They may also have constraints on cardiovascular function related to the pulmonary circulation and/or cardiopulmonary interactions. Coexisting cardiovascular diseases are also commonly present due to shared risk factors. Skeletal muscle mass and function are frequently impaired and can be the site of exercise symptoms and limitation. Thus, not only is the basis of exercise limitation complicated in COPD, but also there is great potential for differences among individuals with respect to the factors of greatest importance. Functional testing which quantifies impairment and identifies factors most important to an individual’s limitations can add to the assessment of disease severity and enhance potential for personalizing clinical care.
Cardiopulmonary Exercise Testing (CPET)
Overview
Cardiopulmonary exercise testing combines measures of  E, pulmonary gas exchange (oxygen uptake,
E, pulmonary gas exchange (oxygen uptake,  O2 and carbon dioxide output,
O2 and carbon dioxide output,  CO2), and related variables, with measures of heart rate (HR) and blood pressure (BP) during a graded exercise stress. Commercial instruments are widely available which make measurements on a breath-by-breath basis and include software for data storage and analysis. Testing usually is done with a treadmill or cycle ergometer with gradual increase in exercise work rate until symptom-limitation. Findings are summarized in a set of variables that can be compared with reference values from healthy populations.42 Graphical displays of data such as those popularized by Wasserman et al and shown in Figures 3 and 4 are commonly used to facilitate analysis of ranges and interrelationships of these variables.43 Commonly measured variables derived from CPET are discussed below with an emphasis on their significance in COPD and summarized in Table 1.
CO2), and related variables, with measures of heart rate (HR) and blood pressure (BP) during a graded exercise stress. Commercial instruments are widely available which make measurements on a breath-by-breath basis and include software for data storage and analysis. Testing usually is done with a treadmill or cycle ergometer with gradual increase in exercise work rate until symptom-limitation. Findings are summarized in a set of variables that can be compared with reference values from healthy populations.42 Graphical displays of data such as those popularized by Wasserman et al and shown in Figures 3 and 4 are commonly used to facilitate analysis of ranges and interrelationships of these variables.43 Commonly measured variables derived from CPET are discussed below with an emphasis on their significance in COPD and summarized in Table 1.
 |
Table 1 Selected Variables Measured During CPET and Their Significance in Patients with COPD |
Peak  O2
O2
The highest  O2 measured at peak exercise (Figures 3A and 4A) is an estimate of maximal
O2 measured at peak exercise (Figures 3A and 4A) is an estimate of maximal 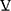 O2, which is the standard measure of exercise capacity. In most healthy young persons, maximal exercise is limited by oxygen delivery by the cardiovascular system,39 and not by the pulmonary system. Peak
O2, which is the standard measure of exercise capacity. In most healthy young persons, maximal exercise is limited by oxygen delivery by the cardiovascular system,39 and not by the pulmonary system. Peak 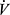 O2 therefore is a measure of cardiovascular fitness and capacity for increasing cardiac output (Q) in health, as implied in the Fick relationship:
O2 therefore is a measure of cardiovascular fitness and capacity for increasing cardiac output (Q) in health, as implied in the Fick relationship:
 |
Figure 4 Wasserman nine panel graphical display of CPET data from a test performed by a 50-year-old man with COPD, whose predicted peak exercise values are similar to those of the healthy subject shown in Figure 3. In this case, the work rate (WR) was increased by 15 W/min during the incremental phase. Data are displayed with same conventions described for Figure 3. Note findings that contrast with the healthy response including (A) and (C), peak values of both |
Where c(a-v)O2 is the arterial-venous oxygen content difference. From this it is apparent that maximal  O2 can be reduced by reduction of maximal Q, and also by factors limiting maximal c(a-v)O2 such as anemia, hypoxemia, or impaired oxygen utilization in the periphery. Clearly, however, peak
O2 can be reduced by reduction of maximal Q, and also by factors limiting maximal c(a-v)O2 such as anemia, hypoxemia, or impaired oxygen utilization in the periphery. Clearly, however, peak 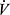 O2 can also be low if other limits are reached prior to attaining maximal Q. In COPD, peak
O2 can also be low if other limits are reached prior to attaining maximal Q. In COPD, peak 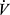 O2 is typically reduced, and often because of constraint due to ventilatory factors prior to attaining a maximal cardiovascular stress.
O2 is typically reduced, and often because of constraint due to ventilatory factors prior to attaining a maximal cardiovascular stress.
Anaerobic Threshold (AT)
The anaerobic threshold, or AT (variously called gas exchange threshold, ventilatory threshold, or other terms) is a noninvasive estimate of the lactate threshold (LT), which is the  O2 above which there is a metabolic acidosis and lactate accumulates in blood.44 Buffering of the acidosis by bicarbonate results in generation of CO2, which can be detected during CPET as an acceleration in
O2 above which there is a metabolic acidosis and lactate accumulates in blood.44 Buffering of the acidosis by bicarbonate results in generation of CO2, which can be detected during CPET as an acceleration in  CO2 relative to
CO2 relative to  O2 (Figures 3C and 4C), without the need to measure lactate. The AT is an important aspect of exercise function because it is related to tolerance for sustained exercise. Below AT exercise is tolerated for long periods of time without fatigue, whereas much above AT exercise is fatiguing within a short duration of time.44 Because there is typically a respiratory compensation in response to metabolic acidosis,
O2 (Figures 3C and 4C), without the need to measure lactate. The AT is an important aspect of exercise function because it is related to tolerance for sustained exercise. Below AT exercise is tolerated for long periods of time without fatigue, whereas much above AT exercise is fatiguing within a short duration of time.44 Because there is typically a respiratory compensation in response to metabolic acidosis, 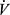 E requirements are amplified during exercise above AT, so that the lower the AT, the more quickly ventilatory limitation will occur in individuals with low breathing capacity due to COPD. Both peak
E requirements are amplified during exercise above AT, so that the lower the AT, the more quickly ventilatory limitation will occur in individuals with low breathing capacity due to COPD. Both peak  O2 and AT are affected by cardiovascular fitness. In COPD, because ventilatory factors may present an absolute limit to peak
O2 and AT are affected by cardiovascular fitness. In COPD, because ventilatory factors may present an absolute limit to peak 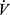 O2, it may be disproportionately reduced, and less amenable to exercise training, than the AT.
O2, it may be disproportionately reduced, and less amenable to exercise training, than the AT.
Oxygen Uptake Efficiency Slope (OUES)
An additional variable related to exercise  O2 is the slope of
O2 is the slope of 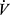 E relative to
E relative to 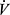 O2, linearized by expressing
O2, linearized by expressing 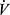 E in log10, and termed the oxygen uptake efficiency slope (OUES). The OUES has been found to correlate with peak
E in log10, and termed the oxygen uptake efficiency slope (OUES). The OUES has been found to correlate with peak 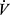 O2, but, importantly, is not dependent on the test being continued to maximal effort. It has been studied primarily in cardiovascular disease populations45 and less is reported regarding its use in COPD. One study reports that OUES is more likely to be reduced in heart failure than in COPD,46 suggesting that a low OUES in an individual with COPD may indicate presence of additional cardiovascular impairment. This is of interest because pulmonary and cardiac limitations often coexist, with COPD demonstrated in an estimated 40% of patients with heart failure,47 and heart failure in an estimated 20% of individuals with COPD.48
O2, but, importantly, is not dependent on the test being continued to maximal effort. It has been studied primarily in cardiovascular disease populations45 and less is reported regarding its use in COPD. One study reports that OUES is more likely to be reduced in heart failure than in COPD,46 suggesting that a low OUES in an individual with COPD may indicate presence of additional cardiovascular impairment. This is of interest because pulmonary and cardiac limitations often coexist, with COPD demonstrated in an estimated 40% of patients with heart failure,47 and heart failure in an estimated 20% of individuals with COPD.48
Heart Rate and O2 Pulse
Heart rate (HR) normally increases linearly relative to 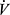 O2 and reaches a peak value that is dependent on age. Peak HR is usually low in COPD due to encountering ventilatory mechanical constraints prior to reaching central cardiovascular limits. Therefore, HR reserve or HRR (maximum predicted HR – peak exercise HR) is elevated (Figures 3B and 4B). The relationship between
O2 and reaches a peak value that is dependent on age. Peak HR is usually low in COPD due to encountering ventilatory mechanical constraints prior to reaching central cardiovascular limits. Therefore, HR reserve or HRR (maximum predicted HR – peak exercise HR) is elevated (Figures 3B and 4B). The relationship between  O2 and HR can be characterized as the ratio
O2 and HR can be characterized as the ratio 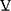 O2/HR, termed O2 pulse. From rearrangement of the Fick relationship, it is clear that this value is numerically the product of stroke volume (SV) and the C(a-v)O2:
O2/HR, termed O2 pulse. From rearrangement of the Fick relationship, it is clear that this value is numerically the product of stroke volume (SV) and the C(a-v)O2:
Much of the increase in SV during exercise occurs early in an incremental test, whereas C(a-v)O2 increases progressively up to peak effort. As a result, 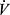 O2/HR normally increases nonlinearly and may asymptote towards the end of the test (Figures 3B and 4B). A low peak O2 pulse could therefore result in COPD from exercise ending due to ventilatory factors prior to attaining a maximal c(a-v)O2, and/or from impairment of stroke volume or c(a-v)O2 by the various mechanisms identified previously.49 Hypoxemia can reduce O2 pulse response by reduction of arterial O2 content, limiting potential for peripheral extraction. If peak exercise is improved with medical treatment and exercise training, peak HR and O2 pulse can also increase.
O2/HR normally increases nonlinearly and may asymptote towards the end of the test (Figures 3B and 4B). A low peak O2 pulse could therefore result in COPD from exercise ending due to ventilatory factors prior to attaining a maximal c(a-v)O2, and/or from impairment of stroke volume or c(a-v)O2 by the various mechanisms identified previously.49 Hypoxemia can reduce O2 pulse response by reduction of arterial O2 content, limiting potential for peripheral extraction. If peak exercise is improved with medical treatment and exercise training, peak HR and O2 pulse can also increase.
Adverse cardiopulmonary interactions, comorbid cardiovascular diseases, or skeletal muscle dysfunction associated with COPD can affect oxygen delivery and utilization even in submaximal range, resulting in abnormal  O2 responses during CPET. For example, these could include early plateau of O2 pulse and/or low AT due to limited stroke volume or impairment in oxygen delivery to muscle. A low AT is particularly important to recognize, as it contributes to ventilatory limitation by obligating a steeper
O2 responses during CPET. For example, these could include early plateau of O2 pulse and/or low AT due to limited stroke volume or impairment in oxygen delivery to muscle. A low AT is particularly important to recognize, as it contributes to ventilatory limitation by obligating a steeper 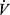 E increase, and it may be increased by exercise training, making it a practical target for intervention.
E increase, and it may be increased by exercise training, making it a practical target for intervention.
Ventilation and Breathing Reserve
Because most healthy people are limited by cardiovascular function prior to reaching limits of ventilation, there is typically a “breathing reserve” at the end of a CPET, calculated as the difference between peak 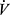 E and maximal voluntary ventilation (MVV) (Panel 9 of Figures 3 and 4).
E and maximal voluntary ventilation (MVV) (Panel 9 of Figures 3 and 4).
BR = MVV-Peak 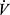 E or, expressed as a percent, BR = (MVV – peak
E or, expressed as a percent, BR = (MVV – peak  E)/ MVV
E)/ MVV
In healthy individuals BR is commonly 15–40% of MVV. Individuals with COPD typically have low BR due to a low MVV together with exaggerated  E requirements. In contrast, high BR is often present in cardiovascular disease due to limitation of exercise at a low level.50 In one analysis, the breathing reserve index (
E requirements. In contrast, high BR is often present in cardiovascular disease due to limitation of exercise at a low level.50 In one analysis, the breathing reserve index ( E/MVV) at AT discriminated between pulmonary mechanical limitation from cardiovascular limitation as cause of shortness of breath.51 It should be acknowledged that an MVV maneuver does not mimic the hyperpnea of exercise,52 and peak
E/MVV) at AT discriminated between pulmonary mechanical limitation from cardiovascular limitation as cause of shortness of breath.51 It should be acknowledged that an MVV maneuver does not mimic the hyperpnea of exercise,52 and peak 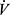 E may vary depending on the test protocol. Thus, BR may be best viewed as an approximation of ventilatory limitation.
E may vary depending on the test protocol. Thus, BR may be best viewed as an approximation of ventilatory limitation.
Exercise Breathing Mechanics
Ventilatory constraints of COPD during exercise in COPD can be characterized in more detail by analysis of expiratory flow limitation and changes in operational lung volumes. Respiratory flow rates are recorded continuously during CPET, so it is possible to record flow-volume loops of spontaneous breaths and compare them with a maximal flow volume loop measured before or after exercise (Figure 5). This has been used to identify the extent to which expiratory flow rates are at maximal limits during tidal breaths.53 To position measured flow rates correctly relative to lung volume, the IC is measured periodically during exercise, and, assuming the IC is performed maximally, a change in IC can be taken as a sign of a reciprocal change in EELV (end expiratory lung volume) (Figures 1 and 5). An increase in EELV thus indicates DH.
 |
Figure 5 Flow volume loops at rest and exercise. Tidal flow volume loops relative to maximal at rest (solid lines) and exercise (dashed lines) for a healthy individual (Normal, A) and an individual with obstructive lung disease (COPD, B). For Normal, tidal volume expands during exercise by both increase in end inspiratory volume and decrease in end expiratory volume. In COPD, the flow volume tracing of spontaneous breaths moves to the left as end expiratory volume increases due to dynamic hyperinflation. FRC, functional residual capacity. Reprinted with permission from Al Talag A, Wilcox P. Clinical physiology of chronic obstructive pulmonary disease. BCMJ. 2008;50(2):97–102.8 |
As has been identified, DH is an important factor in exercise intolerance in COPD. The increase in EELV brings tidal inhalations close to TLC. Although the VT and 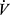 E at which this occurs varies with severity of disease, critically high values of VT/IC or low values of IRV have been identified at which subjective breathlessness increases dramatically. The significance of hyperinflation is demonstrated by studies in which exercise performance improves with reduction of hyperinflation, eg, with the use of bronchodilators.54,55 Dyspnea has been related to the work of breathing, which may also lead to respiratory muscle fatigue56 and, in some cases, exercise hypercapnia.13 The work of breathing and respiratory muscle fatigue are important to exercise impairment in COPD, but difficult to measure; it is not routinely available in clinical contexts. However, DH, which is often a critical precedent to their occurrence, can be detected during CPET by periodic measurement of IC, and dyspnea can be assessed by use of Borg scale or another instrument.57
E at which this occurs varies with severity of disease, critically high values of VT/IC or low values of IRV have been identified at which subjective breathlessness increases dramatically. The significance of hyperinflation is demonstrated by studies in which exercise performance improves with reduction of hyperinflation, eg, with the use of bronchodilators.54,55 Dyspnea has been related to the work of breathing, which may also lead to respiratory muscle fatigue56 and, in some cases, exercise hypercapnia.13 The work of breathing and respiratory muscle fatigue are important to exercise impairment in COPD, but difficult to measure; it is not routinely available in clinical contexts. However, DH, which is often a critical precedent to their occurrence, can be detected during CPET by periodic measurement of IC, and dyspnea can be assessed by use of Borg scale or another instrument.57
VD/VT and Related Variables
Throughout exercise,  E is closely related to
E is closely related to  CO2 such that arterial CO2 (PaCO2) is normally maintained very close to resting levels, at least below the AT. The determinants of
CO2 such that arterial CO2 (PaCO2) is normally maintained very close to resting levels, at least below the AT. The determinants of  E are represented in the formula:
E are represented in the formula:
From this it is apparent that 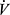 E is directly related to
E is directly related to  CO2, and inversely to the set point for PaCO2. In addition,
CO2, and inversely to the set point for PaCO2. In addition,  E is amplified by the degree of effective VD/VT, or dead space to tidal volume ratio.
E is amplified by the degree of effective VD/VT, or dead space to tidal volume ratio.
The VD/VT reflects the degree to which breath is distributed to unperfused or poorly perfused regions of the lung. It is calculated using the modified Bohr equation which compares concentrations of CO2 between arterial blood and expired breath:
VD/VT = (PaCO2 – PECO2) PECO2/ PaCO2, where PECO2 is mixed expired PCO2.
Even patients with relatively mild COPD may demonstrate an elevated VD/VT.58 The effect of this on breathing is to increase  E requirements both at rest and during exercise.
E requirements both at rest and during exercise.
Quantifying PaCO2 and VD/VT requires analysis of arterial blood gases. However, barring significant deviations of PaCO2 from normal, relationship of  E to
E to  CO2 reflects the magnitude of the VD/VT, so
CO2 reflects the magnitude of the VD/VT, so 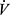 E/
E/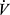 CO2 ratio or slope is often taken as a measure of efficiency of
CO2 ratio or slope is often taken as a measure of efficiency of  E for elimination of CO2. The relationship of
E for elimination of CO2. The relationship of  E to
E to 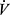 CO2 is commonly expressed either as the slope of Δ
CO2 is commonly expressed either as the slope of Δ E/Δ
E/Δ CO2, or as the nadir value of the
CO2, or as the nadir value of the 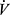 E/
E/ CO2 ratio (called the ventilatory equivalent for CO2), which occurs at or shortly after the AT. Normally,
CO2 ratio (called the ventilatory equivalent for CO2), which occurs at or shortly after the AT. Normally,  E is linearly related to
E is linearly related to  CO2 with a slope ranging between ~23 and 30 (Figures 3D and 4D). In addition to high VD/VT, the slope is also increased when there is reduced PaCO2 from hyperventilation. The Δ
CO2 with a slope ranging between ~23 and 30 (Figures 3D and 4D). In addition to high VD/VT, the slope is also increased when there is reduced PaCO2 from hyperventilation. The Δ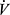 E/Δ
E/Δ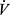 CO2 slope has become an important variable in CPET as it is a powerful prognostic marker in a number of cardiovascular conditions including chronic heart failure and pulmonary arterial hypertension.59,60 Patients with COPD who have pulmonary hypertension have a higher
CO2 slope has become an important variable in CPET as it is a powerful prognostic marker in a number of cardiovascular conditions including chronic heart failure and pulmonary arterial hypertension.59,60 Patients with COPD who have pulmonary hypertension have a higher  E/
E/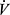 CO2 at nadir, and a higher
CO2 at nadir, and a higher  E/
E/ CO2 slope, and may also have lower oxygen saturation at rest and exercise than those without.61
CO2 slope, and may also have lower oxygen saturation at rest and exercise than those without.61
Paradoxically, in COPD the 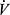 E/
E/ CO2 slope may actually become lower with greater disease severity due to ventilatory constraints leading to hypercapnia (Figure 6). As a result, the more useful variable for reflecting VD/VT in COPD may be the y-axis intercept of the
CO2 slope may actually become lower with greater disease severity due to ventilatory constraints leading to hypercapnia (Figure 6). As a result, the more useful variable for reflecting VD/VT in COPD may be the y-axis intercept of the 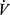 E to
E to  CO2 relationship (Figures 3F and 4F). The intercept is not constrained by dynamic mechanics (as the slope is) or by duration of the test (as the nadir is) and it also correlates better with exercise tolerance and dyspnea than slope and nadir across all stages of COPD.62
CO2 relationship (Figures 3F and 4F). The intercept is not constrained by dynamic mechanics (as the slope is) or by duration of the test (as the nadir is) and it also correlates better with exercise tolerance and dyspnea than slope and nadir across all stages of COPD.62
 |
Figure 6 Ventilatory efficiency ( |
Increased arterial to end tidal, or arterial to mixed expired PCO2 difference (P(a-ET) CO2), PECO2/PETCO2 ratio
At rest, PETCO2 is typically lower than PaCO2. This reflects dilution of the mean alveolar CO2 in exhalate by contributions from areas of relatively high V/Q. PETCO2 normally rises during exercise; however, due to elevation of venous PCO2 and steepening of alveolar phase of expired CO2 profile, such that it normally exceeds arterial value in mid-range of a CPET (Figure 3G). In COPD, P(a-ET) CO2 may instead remain relatively constant or may become more elevated50 due to effects of elevated dead space ventilation.
The PETCO2 may also be compared to the mixed expired CO2 (PECO2) as the PECO2/PETCO2 ratio. PETCO2 is determined predominantly by the most slowly emptying, low V/Q, units of lung, whereas in contrast, PECO2 is more influenced by better ventilated, fast time constant units. Because of increased heterogeneity of airway emptying in COPD, the PECO2/PETCO2 is lower than in other conditions associated with low PETCO2 including chronic heart failure and pulmonary vascular disease, indicating differences in pathophysiologic basis for V/Q mismatches in these conditions.63 Because the PECO2 can be derived from the ratio of  CO2 to
CO2 to  E, determination of PECO2/PETCO2 does not require blood gas analysis.
E, determination of PECO2/PETCO2 does not require blood gas analysis.
High P(A-A)O2 and Hypoxemia During Exercise
Hypoxemia may occur in COPD primarily due to V/Q mismatch or limitation of ventilatory response.64 With exercise, V/Q mismatch may improve in mild disease because of more even distribution of ventilation. However, in severe COPD, V/Q mismatch is typically worsened, and mechanical constraint may result in alveolar hypoventilation.50
In healthy subjects, PaO2 typically increases during exercise (except in some highly trained athletes) and although P(A-A) O2 increases, it generally remains under ~30 mmHg. In COPD, P(A-A) O2 may increase further and there may be significant hypoxemia. This is attributed primarily to V/Q mismatch, but diffusion limitation due to reduced transit time through pulmonary circulation may also play a role. Less commonly, shunting through a patent foramen ovale may occur with exercise due to elevated right sided pressures. Arterial oxygen desaturation may be detected noninvasively by pulse oximeter, although more subtle changes in PaO2 and P(A-A) O2 could require arterial blood gas measurement.50
Application of CPET in COPD
Functional Assessment
Exercise testing measures overall functional capacity, which is not precisely predicted by static pulmonary function testing. In addition to dynamic changes in breathing mechanics, exertional hypoxemia, and cardiopulmonary interactions,42,53 other coexisting conditions such as obesity, cardiovascular disease, or anemia may modify overall exercise function. Simple exercise performance tests such as the 6 minute walk test (6MWT) can reflect the sum effect of these factors but cannot provide as much insight as CPET on their nature and relative roles.65 Objective measures of functional capacity are of use to clinician and patient with respect to assessments of disability,66 expectations regarding work or living environments, or for prescription of exercise67 among other uses.
Prognosis and Risk Stratification
Exercise capacity is a predictor of survival in a wide range of clinical populations.68 Consistent with this, the 6MWT has been shown to be valuable in assessment of mortality in COPD.69,70 Combining 6MWT with FEV1, body mass index (BMI), and severity of dyspnea in the BODE index, provide even better prediction of overall mortality and mortality from respiratory causes in COPD.71 BODE index includes BMI, obstruction by FEV1%, dyspnea (by Modified Medical Research Council) scale, and exercise capacity by 6MWT. Peak  O2 is also a significant predictor of mortality in COPD72–74 and a number of studies suggest that it predicts mortality better than other diagnostic methods.48,65 In chronic heart failure, the prognostic significance of peak
O2 is also a significant predictor of mortality in COPD72–74 and a number of studies suggest that it predicts mortality better than other diagnostic methods.48,65 In chronic heart failure, the prognostic significance of peak 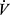 O2 is used in selection of advanced interventions such as heart transplant.75 Less has been reported regarding use of CPET in lung transplant assessments, although a low peak exercise work rate was among criteria identifying patients who would benefit from lung volume reduction surgery in the National Emphysema Treatment Trial.76
O2 is used in selection of advanced interventions such as heart transplant.75 Less has been reported regarding use of CPET in lung transplant assessments, although a low peak exercise work rate was among criteria identifying patients who would benefit from lung volume reduction surgery in the National Emphysema Treatment Trial.76
An important application of CPET for risk assessment is in consideration of major elective surgery. In COPD patients who are surgical candidates, CPET can screen for silent myocardial ischemia, and quantify cardiovascular reserve in terms of peak  O2. Among candidates for lung resection surgery, a peak
O2. Among candidates for lung resection surgery, a peak  O2 of <10 mL/kg/min has been reported to carry a 4-fold increase in cardiac and pulmonary complications compared with candidates with a peak
O2 of <10 mL/kg/min has been reported to carry a 4-fold increase in cardiac and pulmonary complications compared with candidates with a peak 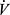 O2 of >20 mL/kg/min.77 CPET is thus part of guidelines for preoperative assessment prior to lung resection by both European Respiratory Society78 and American College of Chest Physicians.77 Although not specific to COPD, preoperative CPET is also recommended by some prior to major elective abdominal surgery in high risk individuals79 based on observations that AT is predictive of perioperative morbidity and mortality.77
O2 of >20 mL/kg/min.77 CPET is thus part of guidelines for preoperative assessment prior to lung resection by both European Respiratory Society78 and American College of Chest Physicians.77 Although not specific to COPD, preoperative CPET is also recommended by some prior to major elective abdominal surgery in high risk individuals79 based on observations that AT is predictive of perioperative morbidity and mortality.77
Identifying the Cause of Dyspnea in Individuals with COPD
Mechanisms of exercise limitation or symptoms are variable in COPD, and CPET can be helpful in identifying proximal cause of impairment and so target therapy.41,80 As an example, a low AT without evidence of ventilatory limitation predicts a high potential for improvement with conventional exercise training. In contrast, dynamic hyperinflation and ventilatory limitation indicate a need for rigorous treatment with bronchodilators or even support use of invasive interventions.41 Hyperinflation or arterial hypoxemia during exercise might alternatively guide choice of exercise training approach, such as use of interval training, discussed in the next section. Coexisting cardiovascular conditions such as exercise induced myocardial ischemia or claudication can also be identified for specific therapy.
Exercise Prescription in COPD
Pulmonary rehabilitation is an effective nonpharmacological tool in treatment of patients with COPD and exercise training is a critical component of rehabilitation.41 Exercise prescriptions may be generated empirically or aided by low complexity exercise tests like the 6MWT, but measurement of gas exchange and ECG monitoring provide more comprehensive information. An initial prerehabilitation CPET can screen for adverse reactions to exercise, such as ischemic ECG changes or hypertensive response, that should be addressed prior to unmonitored training. It also facilitates titration of oxygen prescription for use during training and daily activities. Exercise desaturation has been found to be more pronounced during treadmill walking than during cycling exercise in individuals with COPD, so the former is preferable for this purpose.81,82
CPET is also helpful for selecting a training work rate. Typical exercise prescriptions call for exercise at the training work rate for a targeted duration of 30–60 min, three or more days per week.83 Ideally this exercise should be intermediate between AT and peak  O2. This is important because training at a work rate below AT is likely to have minimal benefit, and one too high may be unrealistic for the targeted exercise durations. The AT is not readily predictable in COPD; it may be low in patients who are deconditioned or have reduced muscle mass or may be an unusually high percentage of peak exercise capacity in patients who are active; so, it is best determined from CPET. Simple measures like heart rate or perceived exertion can then be used as guides to titrate work rate upward as tolerance improves.
O2. This is important because training at a work rate below AT is likely to have minimal benefit, and one too high may be unrealistic for the targeted exercise durations. The AT is not readily predictable in COPD; it may be low in patients who are deconditioned or have reduced muscle mass or may be an unusually high percentage of peak exercise capacity in patients who are active; so, it is best determined from CPET. Simple measures like heart rate or perceived exertion can then be used as guides to titrate work rate upward as tolerance improves.
A growing trend in both pulmonary and cardiac rehabilitation programs is use of interval training which consists of short bursts of near maximal exercise, but for limited periods of time (eg, 1–3 min), alternating with low level exercise or rest.84 This may be uniquely useful for COPD as it allows more intensive training of muscles while avoiding limitation due to breathing mechanics or symptoms, due to lag in increase of  E and other responses within the short duration of the activity.85
E and other responses within the short duration of the activity.85
Effects of training include a delay in onset of lactic acidosis which reduces  E requirement over a range of work rates around AT.86 Breathing pattern might also be modified with decrease in breathing frequency which may sometimes mitigate DH. Training induced increases in peak
E requirement over a range of work rates around AT.86 Breathing pattern might also be modified with decrease in breathing frequency which may sometimes mitigate DH. Training induced increases in peak 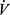 O2 may be modest in COPD due to persistent ventilatory limits.50 Importantly, however, functional benefit of even small increments in peak
O2 may be modest in COPD due to persistent ventilatory limits.50 Importantly, however, functional benefit of even small increments in peak 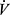 O2 and AT can be substantial. This can be demonstrated by comparison of endurance time for a very high intensity constant work rate performed before and after training. For this, exercise is performed at high a percent (eg, 70%) of the individual’s peak work rate where endurance time is limited to 4–10 min.87 Because there is a hyperbolic relationship between endurance time and work rate in this range of exercise,88 even small changes in aerobic parameters of exercise can result in substantial increases in endurance time over the asymptotic range of the relationship. Endurance testing has been a valuable tool in clinical trials for this reason89 although is not generally used in clinical settings.
O2 and AT can be substantial. This can be demonstrated by comparison of endurance time for a very high intensity constant work rate performed before and after training. For this, exercise is performed at high a percent (eg, 70%) of the individual’s peak work rate where endurance time is limited to 4–10 min.87 Because there is a hyperbolic relationship between endurance time and work rate in this range of exercise,88 even small changes in aerobic parameters of exercise can result in substantial increases in endurance time over the asymptotic range of the relationship. Endurance testing has been a valuable tool in clinical trials for this reason89 although is not generally used in clinical settings.
Conclusion
COPD is a heterogenous condition in which physical function may be impaired through multiple mechanisms. CPET can be uniquely valuable in evaluation and management of individuals with COPD. It provides an objective measure of exercise capacity, which has direct applications in risk stratification. It can be useful in assessing the cause of symptoms which are not always attributable to respiratory factors alone, so can guide recommendations regarding therapeutic interventions, including planning and prescription of exercise training. A robust body of literature demonstrating utility of CPET in patient care supports its use in a broader range of patients and clinical practice settings.
Abbreviations
AT, anaerobic threshold; BP, blood pressure; BR, breathing reserve; C(a-v)O2, arteriovenous oxygen content difference; CO, cardiac output; DH, dynamic hyperinflation; DLCO, diffusion capacity for carbon monoxide; EELV, end expiratory lung volume; EILV, end inspiratory lung volume; FEV1, forced expiratory volume at 1 second; FRC, functional residual capacity; FVC, forced vital capacity; fb, breathing frequency; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HCO3, serum bicarbonate; HR, heart rate; IC, inspiratory capacity; IRV, inspiratory reserve volume; LT, lactate threshold; OUES, oxygen uptake efficiency slope; PAO2, alveolar oxygen pressure; PaCO2, arterial CO2 pressure; PaO2, arterial oxygen pressure; PEEP, positive end expiratory pressure; PECO2, mixed expired CO2 pressure; PETCO2, end tidal CO2 pressure; PETO2, end tidal oxygen pressure; PFT, pulmonary function test; RER, respiratory exchange ratio; SpO2, arterial oxygen saturation; SV, stroke volume; TLC, total lung capacity; 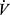 CO2, Carbon dioxide output; VD /VT, dead space to tidal volume ratio;
CO2, Carbon dioxide output; VD /VT, dead space to tidal volume ratio; 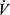 E, minute ventilation;
E, minute ventilation;  E/
E/ 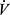 CO2, ventilatory equivalent for CO2;
CO2, ventilatory equivalent for CO2;  O2, Oxygen uptake; VT, tidal volume; V/Q, ventilation-perfusion; WR, work rate; 6MWT, 6-minute walk test.
O2, Oxygen uptake; VT, tidal volume; V/Q, ventilation-perfusion; WR, work rate; 6MWT, 6-minute walk test.
Disclosure
Dr Kathy E Sietsema reports royalties for “Wasserman & Whipp’s Princilpes of Exercise Testing and Interpretation; 6th ed.” from Wolters Kluwer. The authors report no other conflicts of interest in this work.
References
1. Global initiative for chronic obstructive lung disease; 2019. Available from: https://goldcopd.org/gold-reports/.
2. Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, et al. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2016;20(1):11–23. doi:10.5588/ijtld.15.0472
3. Behnia M, Wheatley C, Avolio A, et al. Influence of resting lung diffusion on exercise capacity in patients with COPD. BMC Pulm Med. 2017;17(1):117. doi:10.1186/s12890-017-0454-y
4. Behnia M, Wheatley C, Avolio A, et al. Alveolar-–capillary reserve during exercise in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:3115–3122. doi:10.2147/COPD.S142523
5. Petersson J, Glenny RW. Gas exchange and ventilation-perfusion relationships in the lung. Eur Respir J. 2014;44(4):1023–1041. doi:10.1183/09031936.00037014
6. O’Donnell DE, Milne KM, James MD, et al. Dyspnea in COPD: new Mechanistic Insights and Management Implications. Adv Ther. 2020;37(1):41–60. doi:10.1007/s12325-019-01128-9
7. O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180–184. doi:10.1513/pats.200508-093DO
8. Al Talag A, Wilcox P. Clinical physiology of chronic obstructive pulmonary disease. BCMJ. 2008;50(2):97–102.
9. Loring SH, Garcia-Jacques M, Malhotra A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J Appl Physiol. 1985;107(1):309–314. doi:10.1152/japplphysiol.00008.2009
10. Campbell EJ. Volume-pressure diagram of the lungs and transmural pressure of the airways. J Appl Physiol. 1959;14(1):153–154. doi:10.1152/jappl.1959.14.1.153
11. Elbehairy AF, O’Donnell CD, Abd Elhameed A, et al. Low resting diffusion capacity, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. J Appl Physiol. 1985;127(4):1107–1116. doi:10.1152/japplphysiol.00341.2019
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770–777. doi:10.1164/ajrccm.164.5.2012122
13. Guenette JA, Chin RC, Cheng S, et al. Mechanisms of exercise intolerance in global initiative for chronic obstructive lung disease grade 1 COPD. Eur Respir J. 2014;44(5):1177–1187. doi:10.1183/09031936.00034714
14. Sassmann T, Douschan P, Foris V, et al. Abnormal pulmonary hemodynamics during exercise is associated with exercise capacity in COPD. Respir Res. 2022;23(1):331. doi:10.1186/s12931-022-02238-9
15. Blanco I, Tura-Ceide O, Peinado V, et al. Updated Perspectives on Pulmonary Hypertension in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:1315–1324. doi:10.2147/COPD.S211841
16. Wright JL, Levy RD, Churg A. Pulmonary hypertension in chronic obstructive pulmonary disease: current theories of pathogenesis and their implications for treatment. Thorax. 2005;60(7):605–609. doi:10.1136/thx.2005.042994
17. Torres-Castro R, Gimeno-Santos E, Vilaró J, et al. Effect of pulmonary hypertension on exercise tolerance in patients with COPD: a prognostic systematic review and meta-analysis. Eur Respir Rev. 2021;30(160):200321. doi:10.1183/16000617.0321-2020
18. Vizza CD, Hoeper MM, Huscher D, et al. Pulmonary hypertension in patients with COPD: results from the comparative, prospective registry of newly initiated therapies for pulmonary hypertension (COMPERA). Chest. 2021;160(2):678–689. doi:10.1016/j.chest.2021.02.012
19. Kovacs G, Agusti A, Barberà JA, et al. Pulmonary vascular involvement in chronic obstructive pulmonary disease. Is there a pulmonary vascular phenotype? Am J Respir Crit Care Med. 2018;198(8):1000–1011. doi:10.1164/rccm.201801-0095PP
20. Harms CA, Babcock MA, McClaran SR, et al. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1985;82(5):1573–1583. doi:10.1152/jappl.1997.82.5.1573
21. Aliverti A, Macklem PT, How and why exercise is impaired in COPD. Respiration, 2001. 68(3): p. 229–239.
22. Louvaris Z, Rodrigues A, Dacha S, et al. High-intensity exercise impairs extradiaphragmatic respiratory muscle perfusion in patients with COPD. J Appl Physiol. 1985;130(2):325–341. doi:10.1152/japplphysiol.00659.2020
23. Vogiatzis I, Louvaris Z, Wagner PD. Respiratory and locomotor muscle blood flow during exercise in health and chronic obstructive pulmonary disease. Exp Physiol. 2020;105(12):1990–1996. doi:10.1113/EP088104
24. Cheyne WS, Harper MI, Gelinas JC, et al. Mechanical cardiopulmonary interactions during exercise in health and disease. J Appl Physiol. 1985;128(5):1271–1279. doi:10.1152/japplphysiol.00339.2019
25. Smith JR, Johnson BD, Olson TP. Impaired central hemodynamics in chronic obstructive pulmonary disease during submaximal exercise. J Appl Physiol. 1985;127(3):691–697. doi:10.1152/japplphysiol.00877.2018
26. Bogaard HJ, Dekker BM, Arntzen BW, et al. The haemodynamic response to exercise in chronic obstructive pulmonary disease: assessment by impedance cardiography. Eur Respir J. 1998;12(2):374–379. doi:10.1183/09031936.98.12020374
27. Stark-Leyva KN, Beck KC, Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol. 1985;96(5):1920–1927. doi:10.1152/japplphysiol.00756.2003
28. Stewart RI, Lewis CM. Cardiac output during exercise in patients with COPD. Chest. 1986;89(2):199–205. doi:10.1378/chest.89.2.199
29. Naeije R, Badagliacca R. The overloaded right heart and ventricular interdependence. Cardiovasc Res. 2017;113(12):1474–1485. doi:10.1093/cvr/cvx160
30. Funk G-C, Lang I, Schenk P, et al. Left ventricular diastolic dysfunction in patients with COPD in the presence and absence of elevated pulmonary arterial pressure. Chest. 2008;133(6):1354–1359. doi:10.1378/chest.07-2685
31. Mooney L, Hawkins NM, Jhund PS, et al. Impact of chronic obstructive pulmonary disease in patients with heart failure with preserved ejection fraction: insights from PARAGON-HF. J Am Heart Assoc. 2021;10(23):e021494. doi:10.1161/JAHA.121.021494
32. Dos Santos PB, Simões RP, Goulart CDL, et al. Eccentric left ventricular hypertrophy and left and right cardiac function in chronic heart failure with or without coexisting COPD: impact on exercise performance. Int J Chron Obstruct Pulmon Dis. 2021;16:203–214. doi:10.2147/COPD.S285812
33. Urban MH, Mayr AK, Schmidt I, et al. Effects of dynamic hyperinflation on left ventricular diastolic function in healthy subjects — a randomized controlled crossover trial. Front Med Lausanne. 2021;8:659108. doi:10.3389/fmed.2021.659108
34. Hohlfeld JM, Vogel-Claussen J, Biller H, et al. Effect of lung deflation with indacaterol plus glycopyrronium on ventricular filling in patients with hyperinflation and COPD (CLAIM): a double-blind, randomised, crossover, placebo-controlled, single-centre trial. Lancet Respir Med. 2018;6(5):368–378. doi:10.1016/S2213-2600(18)30054-7
35. Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):p. e15–62. doi:10.1164/rccm.201402-0373ST
36. Deschenes D, Pepin V, Saey D, et al. Locus of symptom limitation and exercise response to bronchodilation in chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2008;28(3):208–214. doi:10.1097/01.HCR.0000320074.73846.3b
37. Wagner PD. Skeletal muscles in chronic obstructive pulmonary disease: deconditioning, or myopathy? Respirology. 2006;11(6):681–686. doi:10.1111/j.1440-1843.2006.00939.x
38. Marillier M, Bernard A-C, Vergès S, et al. Locomotor muscles in COPD: the rationale for rehabilitative exercise training. Front Physiol. 2019;10:1590. doi:10.3389/fphys.2019.01590
39. Broxterman RM, Hoff J, Wagner PD, et al. Determinants of the diminished exercise capacity in patients with chronic obstructive pulmonary disease: looking beyond the lungs. J Physiol. 2020;598(3):599–610. doi:10.1113/JP279135
40. Troosters T, Casaburi R, Gosselink R, et al. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(1):19–38. doi:10.1164/rccm.200408-1109SO
41. Wouters EF, Posthuma R, Koopman M, et al. An update on pulmonary rehabilitation techniques for patients with chronic obstructive pulmonary disease. Expert Rev Respir Med. 2020;14(2):149–161. doi:10.1080/17476348.2020.1700796
42. Sietsema KE, Sue DY, Stringer WW, et al. Wasserman and Whipp’s Principles of Exercise Testing and Interpretation.
43. Wasserman K, Hansen JE, Sue DY, et al. Principles of Exercise Testing and Interpretation.
44. Poole DC, Rossiter HB, Brooks GA, et al. The anaerobic threshold: 50+ years of controversy. J Physiol. 2021;599(3):737–767. doi:10.1113/JP279963
45. Barron AJ, Medlow KI, Giannoni A, et al. Reduced confounding by impaired ventilatory function with oxygen uptake efficiency slope and VE/VCO2 slope rather than peak oxygen consumption to assess exercise physiology in suspected heart failure. Congest Heart Fail. 2010;16(6):259–264. doi:10.1111/j.1751-7133.2010.00183.x
46. Barron A, Francis DP, Mayet J, et al. Oxygen uptake efficiency slope and breathing reserve, not anaerobic threshold, discriminate between patients with cardiovascular disease over chronic obstructive pulmonary disease. JACC Heart Fail. 2016;4(4):252–261. doi:10.1016/j.jchf.2015.11.003
47. Mascarenhas J, Lourenço P, Lopes R, et al. Chronic obstructive pulmonary disease in heart failure. Prevalence, therapeutic and prognostic implications. Am Heart J. 2008;155(3):521–525. doi:10.1016/j.ahj.2007.10.040
48. Rutten FH, Cramer M-JM, Lammers J-WJ, et al. Heart failure and chronic obstructive pulmonary disease: an ignored combination? Eur J Heart Fail. 2006;8(7):706–711. doi:10.1016/j.ejheart.2006.01.010
49. Chuang M-L, Lin I-F, Huang S-F, et al. Patterns of oxygen pulse curve in response to incremental exercise in patients with chronic obstructive pulmonary disease – an observational study. Sci Rep. 2017;7(1):10929. doi:10.1038/s41598-017-11189-x
50. Wasserman K, Hansen JE, Sue DY, et al. Principles of Exercise Testing and Interpretaion.
51. Medoff BD, Oelberg DA, Kanarek DJ, et al. Breathing reserve at the lactate threshold to differentiate a pulmonary mechanical from cardiovascular limit to exercise. Chest. 1998;113(4):913–918. doi:10.1378/chest.113.4.913
52. Klas JV, Dempsey JA. Voluntary versus reflex regulation of maximal exercise flow: volume loops. Am Rev Respir Dis. 1989;139(1):150–156. doi:10.1164/ajrccm/139.1.150
53. Johnson BD, Weisman IM. Clinical exercise testing. In: Crapo GJ, Karlinsky J, King TE, editors. Baum’s Textbook of Pulmonary Diseases. Lippincott, Williams, Wilkins: Philadelphia; 2004:55–78.
54. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832–840. doi:10.1183/09031936.04.00116004
55. O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542–549. doi:10.1164/ajrccm.160.2.9901038
56. Faisal A, Alghamdi BJ, Ciavaglia CE, et al. Common mechanisms of dyspnea in chronic interstitial and obstructive lung disorders. Am J Respir Crit Care Med. 2016;193(3):299–309. doi:10.1164/rccm.201504-0841OC
57. Borg GA. Psychosocial basis of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81. doi:10.1249/00005768-198205000-00012
58. Elbehairy AF, Ciavaglia CE, Webb KA, et al. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2015;191(12):1384–1394. doi:10.1164/rccm.201501-0157OC
59. Sue DY. Excess ventilation during exercise and prognosis in chronic heart failure. Am J Respir Crit Care Med. 2011;183(10):1302–1310. doi:10.1164/rccm.201006-0965CI
60. Teopompi E, Tzani P, Aiello M, et al. Excess ventilation and ventilatory constraints during exercise in patients with chronic obstructive pulmonary disease. Respir Physiol Neurobiol. 2014;197:9–14. doi:10.1016/j.resp.2014.03.002
61. Holverda S, Bogaard HJ, Groepenhoff H, et al. Cardiopulmonary exercise test characteristics in patients with chronic obstructive pulmonary disease and associated pulmonary hypertension. Respiration. 2008;76(2):160–167. doi:10.1159/000110207
62. Neder JA, Arbex FF, Alencar MCN, et al. Exercise ventilatory inefficiency in mild to end-stage COPD. Eur Respir J. 2015;45(2):377–387. doi:10.1183/09031936.00135514
63. Hansen JE, Ulubay G, Chow BF, et al. Mixed-expired and end-tidal CO2 distinguish between ventilation and perfusion defects during exercise testing in patients with lung and heart diseases. Chest. 2007;132(3):977–983. doi:10.1378/chest.07-0619
64. Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199–208. doi:10.2147/COPD.S10611
65. Brown CD, Benditt JO, Sciurba FC, et al. Exercise testing in severe emphysema: association with quality of life and lung function. COPD. 2008;5(2):117–124. doi:10.1080/15412550801941265
66. Aguilaniu B, Gonzalez-Bermejo J, Regnault A, et al. Disability related to COPD tool (DIRECT): towards an assessment of COPD-related disability in routine practice. Int J Chron Obstruct Pulmon Dis. 2011;6:387–398. doi:10.2147/COPD.S20007
67. Benzo RP, Paramesh S, Patel SA, et al. Optimal protocol selection for cardiopulmonary exercise testing in severe COPD. Chest. 2007;132(5):1500–1505. doi:10.1378/chest.07-0732
68. Morris CK, Ueshima K, Kawaguchi T, et al. The prognostic value of exercise capacity: a review of the literature. Am Heart J. 1991;122(5):1423–1431. doi:10.1016/0002-8703(91)90586-7
69. Pinto-Plata VM, Cote C, Cabral H, et al. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J. 2004;23(1):28–33. doi:10.1183/09031936.03.00034603
70. Gerardi DA, Lovett L, Benoit-Connors ML, et al. Variables related to increased mortality following out-patient pulmonary rehabilitation. Eur Respir J. 1996;9(3):431–435. doi:10.1183/09031936.96.09030431
71. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa021322
72. Hiraga T, Maekura R, Okuda Y, et al. Prognostic predictors for survival in patients with COPD using cardiopulmonary exercise testing. Clin Physiol Funct Imaging. 2003;23(6):324–331. doi:10.1046/j.1475-0961.2003.00514.x
73. Oga T, Nishimura K, Tsukino M, et al. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med. 2003;167(4):544–549. doi:10.1164/rccm.200206-583OC
74. Yoshimura K, Maekura R, Hiraga T, et al. Identification of three exercise-induced mortality risk factors in patients with COPD. COPD. 2014;11(6):615–626. doi:10.3109/15412555.2014.898038
75. Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: international society for heart and lung transplantation guidelines for the care of cardiac transplant candidates--2006. J Heart Lung Transplant. 2006;25(9):1024–1042. doi:10.1016/j.healun.2006.06.008
76. Martinez FJ, Foster G, Curtis JL, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173(12):1326–1334. doi:10.1164/rccm.200510-1677OC
77. Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e166S–e190S. doi:10.1378/chest.12-2395
78. Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J. 2009;34(1):17–41. doi:10.1183/09031936.00184308
79. Levett DZH, Jack S, Swart M, et al. Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth. 2018;120(3):484–500. doi:10.1016/j.bja.2017.10.020
80. Stickland MK, Neder JA, Guenette JA, et al. Using Cardiopulmonary Exercise Testing to Understand Dyspnea and Exercise Intolerance in Respiratory Disease. Chest. 2022;161(6):1505–1516. doi:10.1016/j.chest.2022.01.021
81. Hsia D, Casaburi R, Pradhan A, et al. Physiological responses to linear treadmill and cycle ergometer exercise in COPD. Eur Respir J. 2009;34(3):605–615. doi:10.1183/09031936.00069408
82. Mahler DA, Gifford AH, Waterman LA, et al. Mechanism of greater oxygen desaturation during walking compared with cycling in patients with COPD. Chest. 2011;140(2):351–358. doi:10.1378/chest.10-2415
83. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):p. e13–64. doi:10.1164/rccm.201309-1634ST
84. Gao M, Huang Y, Wang Q, et al. Correction to: effects of high-intensity interval training on pulmonary function and exercise capacity in individuals with chronic obstructive pulmonary disease: a meta-analysis and systematic review. Adv Ther. 2022;39(7):3424. doi:10.1007/s12325-022-02175-5
85. Vogiatzis I, Nanas S, Roussos C. Interval training as an alternative modality to continuous exercise in patients with COPD. Eur Respir J. 2002;20(1):12–19. doi:10.1183/09031936.02.01152001
86. Casaburi R, Patessio A, Ioli F, et al. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis. 1991;143(1):9–18. doi:10.1164/ajrccm/143.1.9
87. Gloeckl R, Marinov B, Pitta F. Practical recommendations for exercise training in patients with COPD. Eur Respir Rev. 2013;22(128):178–186. doi:10.1183/09059180.00000513
88. Poole DC, Burnley M, Vanhatalo A, et al. Critical power: an important fatigue threshold in exercise physiology. Med Sci Sports Exerc. 2016;48(11):2320–2334. doi:10.1249/MSS.0000000000000939
89. Casaburi R, Merrill DD, Harding G, et al. A conceptual framework for use of increased endurance time during constant work rate cycle ergometry as a patient-focused meaningful outcome in COPD clinical trials. Chronic Obstr Pulm Dis. 2022;9(2):252–265. doi:10.15326/jcopdf.2021.0258
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.