Back to Journals » Infection and Drug Resistance » Volume 14
Utility of a Cell-Direct Polymerase Chain Reaction-Based Nucleic Acid Lateral Flow Immunoassay for Detection of Bacteria in Peripheral Blood Leukocytes of Suspected Sepsis Cases
Authors Imai H , Watanabe Y , Shimada D , Suzuki J, Endo S , Kaku M, Seki M
Received 20 October 2021
Accepted for publication 25 November 2021
Published 4 December 2021 Volume 2021:14 Pages 5137—5144
DOI https://doi.org/10.2147/IDR.S345361
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Haruka Imai,1 Yuji Watanabe,2 Daishi Shimada,1 Jun Suzuki,1 Shiro Endo,1 Mitsuo Kaku,1 Masafumi Seki1
1Division of Infectious Diseases and Infection Control, Tohoku Medical and Pharmaceutical University Hospital, Sendai City, Miyagi, Japan; 2Laboratory for Clinical Microbiology, Tohoku Medical and Pharmaceutical University Hospital, Sendai City, Miyagi, Japan
Correspondence: Masafumi Seki
Division of Infectious Diseases and Infection Control, Tohoku Medical and Pharmaceutical University, 1-15-1 Fukumuro, Miyagino-ku, Sendai City, Miyagi, 983-8536, Japan
Tel +81-22-983-1221
Fax +81-22-290-8959
Email [email protected]; [email protected]
Background: The detection of the pathogens in the blood is essential for the management of septic patients; however, conventional blood culture takes 2– 3 days. Therefore, rapid and convenient methods may be useful to aid clinical decision-making.
Methods: Blood samples with sepsis clinically diagnosed in cases that fulfilled the diagnostic criteria were used and analyzed the utility of a novel bacterial nucleic acid identification test using a cell-direct polymerase chain reaction (cdPCR)-based nucleic acid lateral flow immunoassay (NALFIA) which were named as “DiagnoSep” to detect representative bacteria in peripheral blood leukocytes in patients admitted to our hospital and compared the conventional blood culture results simultaneously taken from the patients.
Results: We analyzed the total 42 samples in the terms of this study and found 18 (42.8%) were positive on cdPCR-NALFIA, and 24 (57.1%) were positive on blood cultures. Although the positive rate was higher with blood cultures, 15 samples showed positive results from both blood cultures and cdPCR-NALFIA, and the identified bacteria agreed for 10 samples. Of the 18 cdPCR-NALFIA-positive cases, the results for 8 samples differed from the results of blood cultures; four of them had an implanted pacemaker or prosthetic joint and were positive for Staphylococcus aureus or Staphylococcus epidermidis on cdPCR-NALFIA.
Conclusion: Blood culture tests are probably the gold standard in identifying causative organisms in sepsis, but the rapid results from cdPCR-NALFIA simultaneously used with blood culture may make it an important auxiliary diagnostic tool for identifying infecting organisms and lead to the improvement of mortality of the septic patients, because these combined results provide the wide information on the possible pathogens in early phase.
Keywords: bacteremia, blood culture, rapid diagnosis
Background
Identification of the infecting organism is essential for the diagnosis and treatment of infections, but detection rates from different kinds of culture samples are not necessarily good.1 Particularly in sepsis patients, the detection rate from blood cultures is reportedly around 10–50%, although the detection of bacteria by blood culture takes 2–3 days. The reasons for such low detection rates include transience of the bacteremia or the fact that antibiotics have already been started at the time the sample is taken. Repeating two sets of blood cultures is always recommended, but various rapid tests that support the conventional culture methods have been devised.2
Recently, some novel pathogen-identifying systems based on genetic and proteomic methods, such as next-generation sequencing (NGS) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), have been developed.3–5 These methods could detect the pathogenic bacteria and fungi appropriately without blood cultures, but the machines are sometimes very large and expensive. In addition, recent differential expression analysis of transcriptomic data enables genome-wide analysis of gene expression changes associated with biological conditions of interest, including sepsis, and reported gene signatures for sepsis mortality may facilitate the development of reliable diagnosis and prognosis biomarkers for sepsis and acute respiratory distress syndrome.6,7
One of these, a novel test to identify bacterial nucleic acid in peripheral blood leukocytes (DiagnoSep) using a cell direct polymerase chain reaction and nucleic acid lateral flow immunoassay (cdPCR-NALFIA) has been applied clinically (Fuso Pharmaceutical Industries, Osaka, Japan).8 The combination of cell-direct PCR is a simple, rapid, and sensitive detection method for identifying seven bacterial species often isolated from blood cultures: Staphylococcus aureus (S aureus), Staphylococcus epidermidis (S epidermidis), Enterococcus faecalis (E faecalis), Escherichia coli (E coli), Klebsiella pneumoniae (K pneumoniae), Pseudomonas aeruginosa (P aeruginosa), and Enterobacter cloacae (E cloaca). The use of cdPCR-NALFIA makes it possible to detect the DNA of bacteria phagocytized by leukocytes. Furthermore, the assay can be directly applied to blood samples without cultivation, and results are obtained within 4–5 hours.
The present study compared the impact of this method, particularly in terms of the bacterial detection rate, with the results of blood cultures and local culture methods in patients with sepsis.
Methods
Samples
For the period from December 2015 to September 2018, peripheral blood samples were taken simultaneously with blood cultures in cases of suspected sepsis in patients admitted to our hospital, Tohoku Medical and Pharmaceutical University Hospital (650 beds, Sendai City, Miyagi, Japan). For blood cultures, a commercially available bacterial detection system (BacT/ALERT, bioMérieux, Kobe, Japan) was used.
At the same time, samples were taken from sites presumed to be locations of infection, and the results were compared. Sampling locations other than blood were sputum in 6 cases, urine in 10 cases, feces in 2 cases, vaginal discharge in 1 case, synovial fluid in 1 case, and pleural fluid in 1 case (data not shown).
Sepsis was suspected according to the Sepsis-2 according to the 2012 international definition and diagnostic criteria, and new international definition Sepsis-3 diagnostic criteria published in 2016 were not used.2,9 The patients satisfied at least two of the following criteria: temperature ≥38°C or <36°C; heart rate ≥90 bpm; respiratory rate ≥20 bpm or PaCO2 <32 mmHg; white blood cell (WBC) count ≥12,000/µL or <4000/µL; and immature granulocyte percentage >10%.
Ethics Approval and Consent to Participate
This study was approved by the Committee for Clinical Scientific Research of Tohoku Medical and Pharmaceutical University Hospital on July 08 and October 09, 2015 (Nos. ID2015-2-008 and ID2015-2-025, as Trial cdPCR-NALFIA for sepsis 1 and 2, respectively: https://www.hosp.tohoku-mpu.ac.jp/department/infection.html).
This study was conducted in accordance with the Declaration of Helsinki, and all patients provided written, informed consent for use of their blood specimens, although blood culture samples were specifically isolated as part of the routine hospital laboratory procedure.
Detection of Bacterial Nucleic Acid in Peripheral Blood Leukocytes
DiagnoSep (Fuso Pharmaceutical Industry, Osaka, Japan) uses cdPCR-NALFIA, which is named after the procedure in which a PCR test is performed with WBCs that have been dried and fixed in tubes. A basic scientific evaluation of this method was provided in our earlier paper.8 In brief, these comprise the following five steps: preparing WBCs from the blood of a patient, coating a PCR tube with WBCs, increasing the membrane permeability of WBCs and bacteria by enzyme/surfactant treatment, amplifying bacterial DNA in neutrophils through bacterial specie-specific PCR, and evaluating the presence of PCR products using a nucleic acid lateral flow immunoassay.
cdPCR-NALFIA methods that can specifically detect each of the following seven bacterial species were applied: S. aureus, S. epidermidis, E. faecalis, E. coli, K. pneumoniae, P. aeruginosa, and E. cloacae. Thereafter, the presence of PCR products was evaluated using a bacterial species-specific nucleic acid lateral flow immunoassay for WBC samples. The results of the evaluation were the same as those of electrophoresis. If bacterial species-specific PCR products are present, red bands will appear within 20 minutes after chromatography is performed. The results are obtained within 4–5 hours from blood collection, although the conventional blood culture takes 2–3 days to identify the pathogenic bacteria.
Results
Patients
A total of 42 samples were taken from 28 men and 14 women. The patients’ mean age was 74.8 years (range, 30–101 years). The clinical diagnosis in all 42 cases was suspected sepsis, and examined the results of blood culture tests and local culture tests. The final diagnoses are listed in Table 1. At the time of sample collection from each patient, the mean peripheral blood leukocyte count was 11,747/µL (range, 200–36,000/µL) (data not shown).
 |
Table 1 Patients’ Characteristics (n = 42) |
Positive Rates of cdPCR-NALFIA and Blood Cultures
Bacteria-positive rates in all patients are as follows: Eighteen samples (42.8%) were positive for bacteria on cdPCR-NALFIA, and 24 samples (57.1%) were positive on blood cultures. Fifteen samples (35.7%) were positive on both cdPCR-NALFIA and blood cultures, 3 samples (7.1%) were positive on cdPCR-NALFIA and negative on blood cultures, 9 samples (21.4%) were negative on cdPCR-NALFIA and positive on blood cultures, and 15 samples (35.7%) were negative on both cdPCR-NALFIA and blood cultures.
Results for each of the sepsis cases (42 samples) are shown in Table 2. Looking at individual cases, agreement on bacterial species detected from both blood cultures and cdPCR-NALFIA was total 10 samples, and seen in Cases 8, 12, 23, 26, 27, 30, 31, 34, 36, and 42. The species were E. coli in 5 cases, S. aureus in 4 cases, and K. pneumoniae in one case.
 |
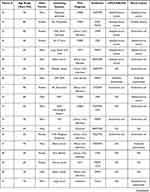 |
 |
Table 2 Clinical Background Characteristics and Microbiological Data of Each Patient |
Cases 21, 25, and 28 were positive for staphylococci (S. epidermidis, 1 case; S. aureus, 2 cases) on cdPCR-NALFIA, but positive for E. coli on blood cultures, showing a discrepancy in the results between the two tests. Case 22 was positive for S. aureus on cdPCR-NALFIA, but positive for E. faecalis on blood cultures. Case 24 was positive for S. aureus on cdPCR-NALFIA, but positive for Candida albicans on blood cultures. In Cases 22 and 24, both patients had undergone pacemaker implantation in the past.
In Cases 1, 18, and 19, positive results were obtained on cdPCR-NALFIA, but no bacteria were detected on blood cultures. In Case 18, the patient had previously undergone pacemaker implantation, and in Case 19, the patient had undergone artificial joint replacement. Both patients were positive for S. epidermidis on cdPCR-NALFIA.
Positive bacteria on blood cultures from Cases 11, 13, 14, 29, 32, 33, 37, and 41 were not detected on cdPCR-NALFIA in this study.
Discussion
Phagocytosis by neutrophils and macrophages plays an important role in the early defense responses of the body to infection, and innate immune responses are activated by factors such as the production of inflammatory cytokines and chemokines.2,9 Thus, there is a high likelihood that bacteria phagocytosed by neutrophils are pathogens that invoke an inflammatory response leading to sepsis.10 If these bacteria are detected and can be rapidly identified at the species level, such information would likely prove beneficial in deciding treatment strategies.3,11
The inflammatory biomarkers C-reactive protein (CRP) and procalcitonin (PCT) have been used in Japan with respect to SIRS/Sepsis, and these data also support the detection of infecting bacteria in blood.12,13 The recent analysis data using the gene expression profile of peripheral blood cells obtained within 24 h of pediatric ICU showed eight out of twenty novel genetic markers (SDC4, CLEC5A, TCN1, MS4A3, HCAR3, OLAH, PLCB1, and NLRP1) that help predict sepsis severity or mortality,14 but blood cultures require 2–3 days and are therefore impractical as a rapid test.11,15
In the present study, bacterial detection rates by cdPCR-NALFIA were examined in patients with suspected sepsis. Shimada et al reported similar results using the previous PCR and in situ hybridization kit (PCR-ISH, named HybriSep; Fuso Pharmaceutical Industries, Osaka, Japan), and the PCR-based assay was reported to provide a significantly higher bacterial detection rate than blood cultures in sepsis patients within 12 hours,16 and in the results of the present study using DiagnoSep, the bacterial detection rate was higher with blood cultures. Blood culture tests remain the gold standard for identifying the causative organism in sepsis, but results from blood cultures and cdPCR-NALFIA agreed in 10 patients in the present study, and the faster result of cdPCR-NALFIA may make this method important as an auxiliary diagnostic test for identifying infecting organisms. Furthermore, we previously reported that a rapid multiplex pathogen detection system (SeptiFast) complemented conventional culture-based methods.11 The results of DiagnoSep in this study were similar to these previous devices and also suggested some added diagnostic value for the timely detection of causative pathogens, particularly in antibiotic pre-treated patients.
Notable results were obtained in Cases 18, 19, 22, and 24. Case 18 was that of a patient with a pacemaker, and the final diagnosis was pneumonia caused by K. pneumoniae, but cdPCR-NALFIA showed positivity for S. epidermidis. Case 19 was that of a patient after artificial joint replacement, and the final diagnosis was pseudogout, but cdPCR-NALFIA showed positivity for S. epidermidis. Case 22 involved a pacemaker lead infection from E. faecalis, but cdPCR-NALFIA showed positivity for S. aureus, suggesting the possibility of superinfection. Case 24 was another case of pacemaker lead infection, and the possibility of superinfection was suggested from Candida albicans positivity on blood cultures and S. aureus positivity on cdPCR-NALFIA. The results of these 4 cases suggest the possibility that, even when a sample is positive on cdPCR-NALFIA, a comprehensive determination based on blood culture results and local culture results is needed to diagnose sepsis. The blood samples for blood culture and cdPCR-NALFIA were generally taken using one set of needle and syringe as we described in the method section, however, in some cases in emergency situations, blood samples for cultures and cdPCR-NALFIA might be taken from the different sites separately in the process of 2 set blood collections. The 3 cases that were positive sample from cdPCR-NALFIA but negative in blood culture could be due to contamination because S. epidermidis and enterococcus are common contaminants. Further studies and reconfirmation of the results should be needed.
In 8 cases, samples were positive for bacteria only on blood cultures, even though the identified bacterial species were ones detectable by the cdPCR-NALFIA used, and Enterobacteriaceae were the causative bacteria in 6 of these cases. Whether the detection power of cdPCR-NALFIA for Enterobacteriaceae is inferior to blood cultures based on this finding cannot be determined with certainty based on the small sample size. In fact, of the 10 cases in which blood cultures and cdPCR-NALFIA agreed, Enterobacteriaceae were detected in 6 cases.
Being unaffected by antibiotics represents an advantage of cdPCR-NALFIA for detecting infecting organisms, and many sepsis patients were already receiving antibiotics when seen in this study, although confirmation of the utility of this advantage will require accumulation of a larger number of cases.1 cdPCR-NALFIA, like PCR-ISH, has a disadvantage in the limited number of bacterial species that can be identified, similar to the other PCR-based detection systems.15 However, identifiable species limited to the 7 species detectable by this cdPCR-NALFIA were isolated with high frequency on blood cultures. The present study included only one case of sepsis in which the pathogen was Candida albicans, which cannot be detected by this cdPCR-NALFIA.
Furthermore, compared with blood cultures, the rapid diagnosis with cdPCR-NALFIA, which takes only about 4–5 hours from the time of blood sample collection until results are obtained, is an advantage. However, we have some limitations in this study. At first, this is a single center study and samples size was small. The nominated terms were short period, such as only three years, and sepsis patients were suspected according to the former sepsis 2 criteria, and sepsis-3 criteria were not used. These may affect the relative low sensitivity of cdPCR-NALFIA and small size of our study. Furthermore, we did not perform the repeat cultures, however, cdPCR-NALFIA might be more sensitive in the finding of bacteria in the blood using by repeated culture medium as the samples. Some refinement or modification to the assay may also be needed before it can be used in clinical setting. Uncertainties remain regarding whether the use of cdPCR-NALFIA will lead to more optimal use of antibiotics, be applicable in clinical decision-making, and will contribute to improved infection treatment outcomes.1 Studies with larger amount of patients are needed and nationwide studies should be performed.
Conclusions
The utility of CDPCR-NALFIA was examined and its results were compared with those of the conventional blood culture method in suspected sepsis patients. Although the positive rate was slightly higher on blood cultures, the rapid results from cdPCR-NALFIA were appropriate and suggested that it will be a useful diagnostic tool for identifying and predicting infecting organisms in the clinical field, including emergency rooms and intensive care units. An additional advantage would represent that cdPCR-NALFIA may be unaffected by antibiotics. Simultaneous use of blood culture and cdPCR-NAFTALIA added some diagnostic value as an auxiliary diagnostic test for identifying infecting organisms, in particular to detect the DNA of bacteria phagocytized by leukocytes.
Acknowledgments
The authors are grateful to all doctors, clinical technicians, and students in the microbiological laboratory, whose sacrifice, efforts, devotion to patients and passion have made possible attractive report.
Funding
We performed this study as the collaborative research with Fuso Pharmaceutical Industry (Osaka, Japan), and received 1,500,000 yen as the scientific expenses, however, any other funding did not received from this company.
Disclosure
The authors report no conflicts of interest in this work.
References
1. van der Velden LB, Vos FJ, Mouton JW, Sturm PD. Clinical impact of preincubation of blood cultures at 37°C. J Clin Microbiol. 2011;49:275–280. doi:10.1128/JCM.00552-10
2. Singer M, Deutschman CS, Seymour CW, et al. The Third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–810. doi:10.1001/jama.2016.0287
3. Seki M, Gotoh K, Nakamura S, et al. Fatal sepsis caused by an unusual Klebsiella species that was misidentified by an automated identification system. J Med Microbiol. 2013;62:801–803. doi:10.1099/jmm.0.051334-0
4. Hariu M, Watanabe Y, Oikawa N, Seki M. Usefulness of matrix-assisted laser desorption ionization time-of-flight mass spectrometry to identify pathogens, including polymicrobial samples, directly from blood culture broths. Infect Drug Resist. 2017;10:115–120. doi:10.2147/IDR.S132931
5. Hariu M, Watanabe Y, Oikawa N, Manaka T, Seki M. Evaluation of blood culture broths with lysis buffer to directly identify specific pathogens by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry methods. Infect Drug Resist. 2018;11:1573–1579. doi:10.2147/IDR.S169197
6. Abbas M, El-Manzalawy Y. Machine learning based refined differential gene expression analysis of pediatric sepsis. BMC Med Genomics. 2020;13:122. doi:10.1186/s12920-020-00771-4
7. Grunwell JR, Rad MG, Stephenson ST, et al. Machine learning-based discovery of a gene expression signature in pediatric acute respiratory distress syndrome. Crit Care Explor. 2021;3:e0431. doi:10.1097/CCE.0000000000000431
8. Sugimoto N, Kubo S, Uehara H, Kaku M. Development and validation of a combined cell-direct polymerase chain reaction-based nucleic acid lateral flow assay for the rapid detection of bacterial pathogens associated with sepsis. J Jpn Assoc Infect Dis. 2017;91:752–758.
9. Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:7–60. doi:10.1007/s00134-007-0866-x
10. Yanagihara K, Fukuda Y, Seki M, et al. Effects of specific neutrophil elastase inhibitor, sivelestat sodium hydrate, in murine model of severe pneumococcal pneumonia. Exp Lung Res. 2007;33:71–80. doi:10.1080/01902140701198500
11. Yanagihara K, Kitagawa Y, Tomonaga M, et al. Evaluation of pathogen detection from clinical samples by real-time polymerase chain reaction using a sepsis pathogen DNA detection kit. Crit Care. 2010;14:R159. doi:10.1186/cc9234
12. Nishikawa H, Shirano M, Kasamatsu Y, et al. Comparative usefulness of inflammatory markers to indicate bacterial infection-analyzed according to blood culture results and related clinical factors. Diagn Microbiol Infect Dis. 2016;84:69–73. doi:10.1016/j.diagmicrobio.2015.09.015
13. Seki M, Watanabe A, Mikasa K, Kadota J, Kohno S. Revision of the severity rating and classification of hospital-acquired pneumonia in the Japanese Respiratory Society guidelines. Respirology. 2008;13:880–885. doi:10.1111/j.1440-1843.2008.01348.x
14. Banerjee S, Mohammed A, Wong HR, Palaniyar N, Kamaleswaran R. Machine learning identifies complicated sepsis course and subsequent mortality based on 20 genes in peripheral blood immune cells at 24 H Post-ICU admission. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.592303
15. Seki M, Takahashi H, Yamamoto N, et al. Polymerase chain reaction-based active surveillance of MRSA in emergency department patients. Infect Drug Resist. 2015;14:113–118. doi:10.2147/IDR.S80123
16. Shimada J, Hayahsi I, Inamatsu T, et al. Clinical trial of in-situ hybridization method for the rapid diagnosis of sepsis. J Infect Chemother. 1999;5:21–31. doi:10.1007/s101560050004
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
