Back to Journals » International Journal of General Medicine » Volume 16
Usefulness of Serum Translocator Protein as a Potential Predictive Biochemical Marker of Three-Month Cognitive Impairment After Acute Intracerebral Hemorrhage: A Prospective Observational Cohort Study
Authors Zhou J, Yang C, Xv Q, Wang L, Shen L, Lv Q
Received 3 September 2023
Accepted for publication 8 November 2023
Published 20 November 2023 Volume 2023:16 Pages 5389—5403
DOI https://doi.org/10.2147/IJGM.S438503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jing Zhou, Chunsong Yang, Qichen Xv, Liyun Wang, Liangjun Shen, Qingwei Lv
Department of Neurosurgery, Shengzhou People’s Hospital (the First Affiliated Hospital of Zhejiang University Shengzhou Branch), Shengzhou, Zhejiang, People’s Republic of China
Correspondence: Chunsong Yang, Department of Neurosurgery, Shengzhou People’s Hospital (the First Affiliated Hospital of Zhejiang University Shengzhou Branch), Shengzhou, Zhejiang, People’s Republic of China, Email [email protected]
Background: Translocator protein (TSPO) is a biomarker of neuroinflammation and brain injury. This study aimed to ascertain the potential of serum TSPO as a predictor of cognitive impairment after acute intracerebral hemorrhage (ICH).
Methods: In this prospective observational cohort study, 276 patients with supratentorial ICH were randomly assigned to two groups (184 patients in the study group and 92 in the validation group) in a 2:1 ratio. Serum TSPO levels were gauged at admission, and cognitive status was assessed using the Montreal Cognitive Assessment Scale (MoCA) post-stroke 3 months. A MoCA score of < 26 was considered indicative of cognitive impairment.
Results: Serum TSPO levels were inversely correlated with MoCA scores (ρ=− 0.592; P< 0.001). Multivariate linear regression analysis showed that serum TSPO levels were independently associated with MoCA scores (β, − 0.934; 95% confidence interval (CI), − 1.412--0.455; VIF, 1.473; P< 0.001). Serum TSPO levels were substantially higher in patients with cognitive impairment than in the remaining patients (median, 2.7 versus 1.6 ng/mL; P< 0.001). Serum TSPO levels were linearly correlated with the risk of cognitive impairment under a restricted cubic spline (P=0.325) and independently predicted cognitive impairment (odds ratio, 1.589; 95% CI, 1.139– 2.216; P=0.016). Subgroup analysis showed that the relationship between serum TSPO levels and cognitive impairment was not markedly influenced by other parameters, such as age, sex, drinking, smoking, hypertension, diabetes mellitus, body mass index, and dyslipidemia (all P for interaction > 0.05). The model, which contained serum TSPO, National Institutes of Health Stroke Scale scores and hematoma volume, performed well under the receiver operating characteristic curve, calibration curve and decision curve, and using the Hosmer–Lemeshow test. This model was validated in the validation group.
Conclusion: Serum TSPO level upon admission after ICH was independently associated with cognitive impairment, substantializing serum TSPO as a reliable predictor of post-ICH cognitive impairment.
Keywords: translocator protein, intracerebral hemorrhage, cognitive impairment, biomarkers
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a frequently encountered emergency disorder that represents devastating cerebrovascular disease.1 Cognitive impairment has been believably accepted as a very common complication among ICH survivors and its emergence severely affects the quality of life among such patients.2 A series of molecular reactions such as inflammation, oxidation, apoptosis, and necrosis contribute to the pathophysiological processes underlying cognitive impairment.3 Conventionally, severity scales such as the National Institutes of Health Stroke Scale (NIHSS) and hematoma volume are used to predict cognitive impairment in patients with ICH.4 Notably, many researchers have gradually concentrated on various biomarkers that may aid in the prediction of cognitive impairment during clinical work of ICH.5–7
Translocator protein (TSPO) is a transmembrane protein on the outer mitochondrial membrane that was initially recognized as a peripheral benzodiazepine receptor.8 This mitochondrial protein is involved in varieties of microglial inflammatory responses, such as cytokine secretion, proliferation, motility, and phagocytosis.9,10 Notably, TSPO expression in the brain has been identified as a marker of brain injury and neuroinflammation in numerous neurological diseases, including encephalitis, multiple sclerosis, stroke, and head trauma.11–13 Experimentally, TSPO ligands exert beneficial effects in counteracting neuroinflammation and neuronal damage under many neuropathological conditions such as ischemic stroke, ICH, and traumatic brain injury.14–16 Interestingly, circulating TSPO has emerged as a biochemical marker for assessing severity and predicting poor neurologic function in patients with ICH, head trauma, and stroke.17–19 Here, we enrolled a group of ICH survivors to investigate the predictive value of serum TSPO level for post-ICH cognitive impairment.
Materials and Methods
Study Design, Ethical Approval, and Participant Selection
Between January 2018 and July 2022, a prospective observational unicentral cohort study was performed to determine the relationship between serum TSPO levels and cognitive status among ICH survivors at the Shengzhou People’s Hospital. Patients with ICH were consecutively enrolled, and eligible survivors with ICH were randomly divided into two groups (study and validation groups) in a 2:1 ratio. The recruitment criteria were as follows: (1) primary supratentorial intraparenchymal hemorrhage; (2) age ≥ 18 years; (3) first-onset stroke; (4) conservative hematoma treatment; and (5) hospital admission within 24 h post-ICH. The exclusion criteria were as follows: (1) history of neuropsychiatric diseases, such as cognitive decline, dementia, psychosis, intracranial tumors, Parkinson’s disease, intracranial infection, and multiple sclerosis; (2) systemic diseases or severe diseases in other organs, such as malignancies, heart failure, liver cirrhosis, uremia, chronic obstructive pulmonary disease, and ascites; (3) specific conditions, such as death within 3 months after ICH, refusal to participate, pregnancy, missed visits, incomplete materials, and unavailable samples; and (4) other conditions affecting the scale assessment, such as aphasia, dysarthria, and visual or hearing impairment. This study was conducted in compliance with the guidelines of the Declaration of Helsinki and its amendments. The study protocol was approved by the Ethics Committee of the Shengzhou People’s Hospital (No. 2020-K-Y-007-01). Patients’ proxies provided written informed consent to participate in this study.
Data Collection and Cognitive Assessment
The registered demographic data included age, sex, educational duration, and body mass index (BMI). BMI was estimated using the following equation: body weight (kg) divided by height squared (m2). The recorded vascular risk factors were cigarette smoking, alcohol consumption, hypertension, diabetes mellitus, and dyslipidemia. Previously used drugs include statins, anticoagulants, and antiplatelet agents. The heart rate, respiratory rate, body temperature, blood oxygen saturation, and arterial blood pressure at admission were measured noninvasively. Baseline hematoma volume was calculated using the following equation:0.5×a×b×c. Hematoma locations were divided into superficial and deep sites. Hematoma with subarachnoid or intraventricular extension was observed. Neurological status was assessed using the NIHSS score. Cognitive status was assessed using the Montreal Cognitive Assessment Scale (MoCA) three months after ICH. A MoCA score of <26 was defined as cognitive impairment. If the schooling educational time was no longer than 12 years, the MoCA score was added by 1 point to correct the bias of schooling years.20
Sample Collection and Immune Analysis
Venous blood collected at admission was used to measure the TSPO levels. A 5-mL blood sample was acquired via antecubital venipuncture, promptly put into a gel-containing biochemistry tube, and then spun for 20 min at 3000 × g. Separated serum was decanted into an Eppendorf tube and preserved at − 80 ◦C freezer until assayed. About every three months, a batch of serum samples was melted, and TSPO levels were quantified using a human TSPO enzyme-linked immunosorbent assay kit (SEJ628Hu; Cloud-Clone Corp., USA) following the manufacturer’s instructions. The detection range varied from 0.312 to 20 ng/mL, detection sensitivity was 0.114 ng/mL, the intra-assay coefficient of variation was < 10%, and the inter-assay coefficient of variation was < 12%. All measurements were performed in duplicate by the same skilled personnel who were blinded to patient information. The two measurements were averaged for subsequent data analysis.
Statistical Analysis
The SPSS 23.0 software (SPSS Inc., Chicago, IL, USA), R 3.5.1, software (https://www.r-project.org), and GraphPad Prism 7.01 (GraphPad Software Inc., San Diego, California, USA) were used for statistical analysis and figure formation. MedCalc 20.1 (MedCalc Software, Mariakerke, Belgium) was in use for estimation of sample size. In the current study, there should be a least sample size at 38 and 49 for bivariate correlation analysis and intergroup comparison respectively. The least number of cognitive impairment cases should be 51 for multivariate analysis. Hence, the sample size was enough big for statistical analysis in this study. The Kolmogorov–Smirnov test was done for testing normal distribution of measurement data. Measurement data conforming to a normal distribution are shown as the mean (standard deviation, SD), and intergroup comparisons were performed using the independent sample t-test. Those with non-normal distributions are presented as medians (lower-upper quartiles), and intergroup comparisons were performed using the Mann–Whitney U-test. Enumeration data were reported as counts (proportions), and the chi-squared test or Fisher’s exact test was used for intergroup comparisons. Bivariate correlations between the MoCA scores and other variables were analyzed using Spearman’s rank correlation coefficient. Using MoCA scores as the dependent variable, a multivariate linear regression model was configured to ascertain the independent correlated factors. Correlations are reported as beta (β) (95% confidence interval, 95% CI) and VIF values. The area under the receiver operating characteristic (ROC) curve (AUC) was estimated and an optimal value was chosen using the Youden method. Using the restricted cubic spline, the linear correlation between serum TSPO levels and risk of cognitive impairment was determined. The multivariate logistic regression model was constructed to explore the factors that independently influenced cognitive impairment. Associations are presented as odds ratios (OR) (95% CI). Subgroup analysis was performed for age, sex, drinking, smoking, diabetes mellitus, dyslipidemia, and hypertension using the stratified logistic regression model. Interactions between the subgroups were tested using the likelihood ratio test. The prediction model, in which the independent factors of cognitive impairment were incorporated, was described using the nomogram. The prediction efficiency of the model was assessed using the ROC curve, its prediction fit was determined using the Hosmer-Lemeshow goodness of fit statistics and calibration curve analysis, and its clinical effectiveness was investigated using the decision curve analysis. To assess the predictive efficiency of serum TSPO levels and the combination model, AUCs were compared between the study and validation groups. Differences were considered statistically significant at two-sided P < 0.05.
Results
Patient Recruitment and Baseline Characteristics
During the study period, an aggregate of 436 patients with ICH were consecutively enrolled in accordance with the prespecified inclusion criteria; based on the presented exclusion criteria, 160 patients were excluded from this study; and finally, 276 patients were appropriate for further clinical investigation (Figure 1). The demographic, clinical, radiological, and biochemical data are presented in Table 1. The study group included 184 patients and the validation group comprised 92 patients. There were no significant differences in the baseline data between the study and validation groups (all P>0.05; Table 2).
 |
Table 1 Basic Data of Patients with Acute Intracerebral Hemorrhage |
 |
Table 2 Basic Data Between Study Group and Validation Group Among Patients with Acute Intracerebral Hemorrhage |
Admission Serum TSPO and MoCA Scores 3 Months After ICH
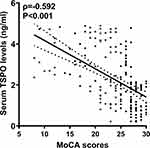
As depicted in Figure 2, serum TSPO levels were highly inversely correlated with the MoCA scores (P<0.001). As shown in Table 3, the MoCA scores at three months post-stroke were intimately correlated with age, intraventricular hemorrhage, NIHSS scores, hematoma volume, blood glucose levels, serum C-reactive protein levels, and serum TSPO levels (all P<0.05). Subsequently, the seven variables were incorporated into the multivariate linear regression model, and it was revealed that the factors that were independently correlated with the MoCA scores were serum TSPO levels (β, −0.934; 95% CI, −1.412--0.455; VIF, 1.473; P<0.001), hematoma volume (β, −0.171; 95% CI, −0.330--0.013; VIF, 1.369; P=0.034), and NIHSS score (β, −0.594; 95% CI, −0.873--0.315; VIF, 3.705; P<0.001).
 |
Table 3 Factors in Relation to the Montreal Cognitive Assessment Scale Scores at Three Months After Acute Intracerebral Hemorrhage |
Admission Serum TSPO Levels and 3-Month Cognitive Impairment After ICH
As shown in Figure 3, serum TSPO levels were markedly higher in patients with 3-month cognitive impairment than in those without cognitive impairment (P<0.001). As shown in Table 4, compared with patients without cognitive impairment, those with cognitive impairment were significantly older and had a higher ratio of superficial to deep bleeding, NIHSS scores, hematoma volume, blood glucose levels, serum C-reactive protein levels, and serum TSPO levels (all P<0.05). As shown in Figure 4, serum TSPO levels were linearly related to the risk of 3-month cognitive impairment under a restricted cubic spline (P=0.325). All the above-mentioned seven significant variables were forced into the binary multivariate logistic regression model, and it was found that NIHSS scores (odds ratio, 1.238; 95% CI, 1.016–1.509; P=0.009), hematoma volume (odds ratio, 1.139; 95% CI, 1.068–1.215; P=0.011), and serum TSPO levels at admission (odds ratio, 1.789; 95% CI, 1.139–2.416; P=0.016) were retained as the three independent predictors of three-month cognitive impairment (using Hosmer-Lemeshow good-of-fit test, P=0.528). Subgroup analysis showed that the relationship between serum TSPO levels and cognitive impairment was not significantly affected by other parameters, such as age, sex, drinking, smoking, hypertension, diabetes mellitus, body mass index, and dyslipidemia (all P for interaction > 0.05; Figure 5).
 |
Table 4 Factors in Relation to Cognitive Impairment at 3 Months After Acute Intracerebral Hemorrhage |
Construction and Validation of the Cognitive Impairment Prediction Model
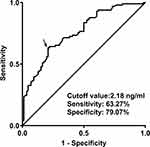
As shown in Figure 6, serum TSPO levels significantly discriminated the risk of 3-month cognitive impairment (AUC, 0.758; 95% CI, 0.690–0.818; P<0.001). Using the Youden method, serum TSPO levels more than 2.18 ng/mL predicted cognitive impairment, with a maximum Youden index (0.423). Interestingly, in contrast to NIHSS scores (AUC, 0.788; 95% CI, 0.722–0.845) and hematoma volumes (AUC, 0.782; 95% CI, 0.715–0.840), serum TSPO levels had similar predictive values (P=0.423 and 0.555, respectively). In addition, serum TSPO levels significantly improved the AUC of NIHSS scores to 0.824 (95% CI, 0.761–0.876; P=0.012) and that of hematoma volume to 0.821 (95% CI, 0.758–0.874; P=0.047). Next, a nomogram composed of NIHSS scores, hematoma volume, and serum TSPO levels at admission was constructed to assess the risk of 3-month cognitive impairment after ICH (Figure 7). The prediction model showed comparative stability using calibration curve analysis (Figure 8). This prediction model had a significantly higher predictive ability in terms of AUC than the combination of NIHSS score and hematoma volume (P=0.014; Figure 9). Furthermore, the prediction model was clinically effective based on the decision curve analysis (Figure 10). As shown in Table 5, serum TSPO levels had a similar predictive ability and additive effects on NIHSS scores, hematoma volume, and both the study and validation groups (all P>0.05).
 |
Table 5 Additive Effects of Serum Translocator Protein Levels Between Study Group and Validation Group with Respect to Predicting Cognitive Impairment at 3 Months After Acute Intracerebral Hemorrhage |
Discussion
TSPO is primarily derived from glial cells, and TSPO has been regarded as a biomarker of brain injury and neuroinflammatory responses in the central nervous system.8–10 Gradually, circulating TSPO has been accepted as a biochemical marker that reflects the severity and clinical outcomes of ischemic stroke, traumatic brain injury, and ICH.17–19 We found some noteworthy results: (1) serum TSPO levels, which were independently correlated with 3-month MoCA scores, were independently associated with 3-month cognitive impairment after ICH; (2) serum TSPO levels displayed substantial predictive ability for cognitive impairment at 3 months after ICH, which was verified using a series of statistical methods in the study group; and (3) additive effects of serum TSPO levels on NIHSS scores and hematoma volume were demonstrated in both the study and validation groups. Thus, serum TSPO level may be a reliable predictor of cognitive impairment after ICH.
Cognitive impairment is a common neurodegenerative disease characterized by a decline in memory and/or other cognitive processes, and is often associated with poor patients.21 Previously, cognitive impairment was considered a chronic condition, which is apt to occur in the elderly;22 gradually, a new concept was formed that acute brain injury, whether hemorrhagic or ischemic stroke, can also lead to the occurrence of cognitive impairment.23 The pathophysiological mechanisms implicated in cognitive impairment are complex and may be related to ischemia, hypoxia, neuroinflammation, oxidation, apoptosis and necrosis.24 The MoCA is a conventional scale used to assess cognitive impairment.20 However, early prediction remains a major clinical challenge. For acute brain injury diseases, clinical and radiological severity scales such as NIHSS and hematoma volume may be potential predictive factors for cognitive impairment after ICH.4 Notably, some biomarkers have drawn extensive interest for decades with respect to their predictive significance in cognitive impairment.5–7
TSPO is mainly expressed in the glial cells of brain tissues.8 This expression pattern coincides with microglial activation.25 Subsequently, brain and even circulating TSPO were reported to be related to the extent of both brain injury and neuroinflammatory responses in various brain injury illnesses, including brain trauma, hemorrhage and ischemia.11–13,17–19 Thus, TSPO is identified as a biomarker of brain injury and neuroinflammation.11–13,17–19 Accumulating evidence has shown that TSPO participates in a diverse spectrum of microglial inflammatory responses, including cytokine secretion, proliferation, motility, and phagocytosis.26,27 Recently, TSPO ligands have been found to have protective potential against ischemic stroke, ICH, and traumatic brain injury.14–16 Thus, TSPO may act as a protective receptor against acute brain injury.
However, results regarding the effects of TSPO on cognitive function are inconsistent. TSPO ligands can reverse Alzheimer’s related pathologies in mice.28 Another study supported the protective role of TSPO ligands in a tauopathy mouse model.29 Similarly, short-term treatment with the TSPO ligand PK11195 effectively improves behavioral and pathological conditions in aged female mice.30 In addition, an experimental study demonstrated that TSPO may play pivotal roles in microglial respiratory-glycolytic metabolism and phagocytosis, and its deletion may reduce the removal of toxic protein aggregates in Alzheimer’s disease, indicating its protective function.31 In contrast, TSPO ligands may alter synaptic plasticity, thereby causing cognitive impairment and indicating their detrimental effects.32 Overall, TSPO may play a dual role in the occurrence and development of cognitive impairment. Thus, treatments of cognitive impairment, which targets TSPO, warrant to be further studied.
Under normal physiological conditions, TSPO is scarcely expressed in brain tissues. Acute brain injuries, such as ICH, traumatic brain injury, and cerebral ischemia, can increase the expression of TSPO in brain tissues.14–16 In patients with Alzheimer’s disease, radioligand binding to the brain TSPO is strongly correlated with disease severity.33 Also, a meta-analysis, which contained twenty-eight studies enrolling 318 healthy individuals, 168 subjects with mild cognitive impairment and 269 patients with Alzheimer’s disease, as well as assessing 37 brain regions, showed that TSPO could be markedly upregulated throughout the whole brain of patients with mild cognitive impairment or Alzheimer’s disease.34 The above evidence strongly supports the assumption that TSPO may be a potential biomarker of cognitive impairment.
Clearly, 90-day or 3-month neurological outcome is conventionally used as a prognostic parameter of ICH, because patients will be at stable state of neurological function after post-ICH 90 days or 3 months.35–37 In other words, 90-day or 3-month cognitive impairment is believably accepted as a parameter for assessing status of cognitive function among ICH patients.4,38 In our study, the MoCA, which was assessed at 3 months after ICH, was regarded as a categorical or continuous variable. Multivariate logistic regression analysis showed that apart from NIHSS scores and hematoma volume, serum TSPO emerged as an independent predictor of cognitive impairment following ICH. Regarding the additive effects of serum TSPO on NIHSS scores and hematoma volume alone or in combination, serum TSPO showed significant efficiency in terms of AUC for predicting cognitive impairment after ICH. To effectively predict cognitive impairment at three months after ICH, a nomogram was configured that incorporated NIHSS scores, hematoma volume, and serum TSPO. The predictive model performed well in the validation group. Also, on subgroup analysis with cognitive impairment as a dependent variable, there were no substantial interactions of serum TSPO levels with other parameters, such as age, sex, drinking, smoking, hypertension, diabetes mellitus, body mass index, and dyslipidemia. Hypothetically, serum TSPO level may be a promising predictor of cognitive impairment after ICH.
Conclusions
Admission serum TSPO levels, which were significantly inversely correlated with MoCA scores, were markedly enhanced in patients with a 3-month cognitive impairment after ICH. Serum TSPO, NIHSS scores, and hematoma volume appeared to be three independent predictors of cognitive impairment at three months following ICH. Moreover, the prediction model, which was composed of NIHSS scores, hematoma volume, and serum TSPO, effectively predicts post-ICH 3-month cognitive impairment three months post-ICH in the study and validation groups. Presumably, serum TSPO may serve as a valuable predictive biomarker of cognitive impairment in patients with ICH.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available because they are personal data, but are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was performed in accordance with the tenets of the Declaration of Helsinki, and the protocol was approved by the ethics committee of Shengzhou People’s Hospital (No. 2020-K-Y-007-01). Signed informed consent was obtained from patients’ proxies.
Acknowledgments
We gratefully thank all study participants, their relatives, and the staff at the recruitment centers for their invaluable contributions.
Funding
This study was financially supported by the Zhejiang Provincial Medical and Health Technology Plan Project (No. 2021KY1170).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450–1460. doi:10.1056/NEJM200105103441907
2. Rost NS, Brodtmann A, Pase MP, et al. Post-Stroke Cognitive Impairment and Dementia. Circ Res. 2022;130(8):1252–1271. doi:10.1161/CIRCRESAHA.122.319951
3. Kazim SF, Ogulnick JV, Robinson MB, et al. Cognitive impairment after intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg. 2021;148:141–162. doi:10.1016/j.wneu.2021.01.026
4. Griauzde J, Lisabeth LD, Li C, et al. A population-based study of the outcomes of intracerebral hemorrhage survivors. J Stroke Cerebrovasc Dis. 2019;28(1):49–55. doi:10.1016/j.jstrokecerebrovasdis.2018.09.005
5. Gao Y, Wang B, Miao Y, Han Y. Serum neuroglobin as a potential prognostic biomarker for cognitive impairment after intracerebral hemorrhage. Front Neurol. 2022;13:885323. doi:10.3389/fneur.2022.885323
6. Xia M, Su Y, Fu J, et al. Use of serum matrix metalloproteinases in cerebral amyloid angiopathy-related intracerebral hemorrhage and cognitive impairment. J Alzheimers Dis. 2021;82(3):1159–1170. doi:10.3233/JAD-210288
7. Lan TY, Cui DY, Liu TT, Pan JY, Wang X. Correlation of serum ferritin levels with neurological function-related indices and cognitive dysfunction in patients with cerebral hemorrhage. Clin Neuropathol. 2021;40(6):333–340. doi:10.5414/NP301368
8. Papadopoulos V, Baraldi M, Guilarte TR, et al. Translocator protein (18kDa): a new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27(8):402–409. doi:10.1016/j.tips.2006.06.005
9. Singh P, Adhikari A, Singh D, Gond C, Tiwari AK. The 18-kDa translocator protein PET tracers as a diagnostic marker for neuroinflammation: development and current standing. ACS Omega. 2022;7(17):14412–14429. doi:10.1021/acsomega.2c00588
10. Liu GJ, Middleton RJ, Hatty CR, et al. The 18 kDa translocator protein, microglia and neuroinflammation. Brain Pathol. 2014;24(6):631–653. doi:10.1111/bpa.12196
11. Messmer K, Reynolds GP. Increased peripheral benzodiazepine-binding sites in the brains of patients with Huntington disease. Neurosci Lett. 1998;241(1):53–56. doi:10.1016/s0304-3940(97)00967-1
12. Zhou R, Ji B, Kong Y, et al. PET imaging of neuroinflammation in Alzheimer’s disease. Front Immunol. 2021;12:739130. doi:10.3389/fimmu.2021.739130
13. Dupont AC, Largeau B, Santiago Ribeiro MJ, Guilloteau D, Tronel C, Arlicot N. Translocator Protein-18 kDa (TSPO) Positron Emission Tomography (PET) imaging and its clinical impact in neurodegenerative diseases. Int J Mol Sci. 2017;18(4):785. doi:10.3390/ijms18040785
14. Li HD, Li M, Shi E, et al. A translocator protein 18 kDa agonist protects against cerebral ischemia/reperfusion injury. J Neuroinflammation. 2017;14(1):151. doi:10.1186/s12974-017-0921-7
15. Li M, Ren H, Sheth KN, Shi FD, Liu Q. TSPO ligands attenuate brain injury after intracerebral hemorrhage. FASEB J. 2017;31(8):3278–3287. doi:10.1096/fj.201601377RR
16. Papadopoulos V, Lecanu L. Translocator protein (18 kDa) TSPO: an emerging therapeutic target in neurotrauma. Exp Neurol. 2009;219(1):53–57. doi:10.1016/j.expneurol.2009.04.016
17. Luo LF, Weng JF, Cen M, et al. Prognostic significance of serum translocator protein in patients with traumatic brain injury. Clin Chim Acta. 2019;488:25–30. doi:10.1016/j.cca.2018.10.035
18. Chen WH, Yeh HL, Tsao CW, et al. Plasma translocator protein levels and outcomes of acute ischemic stroke: a pilot study. Dis Markers. 2018;2018:9831079. doi:10.1155/2018/9831079
19. Jiang W, Jin P, Bao Q, Wei W, Jiang W. Prognostic significance of serum translocator protein in patients with spontaneous intracerebral hematoma: preliminary findings. Neurol Res. 2021;43(5):412–417. doi:10.1080/01616412.2020.1866372
20. Li Y, Chen X, Zhou R, et al. Correlation between cognitive impairment, homocysteine, and S100B levels in patients with progressive ischemic stroke. Neuropsychiatr Dis Treated. 2023;19:209–217. doi:10.2147/NDT.S393624
21. Huang YY, Chen SD, Leng XY, et al. Post-stroke cognitive impairment: epidemiology, risk factors, and management. J Alzheimers Dis. 2022;86(3):983–999. doi:10.3233/JAD-215644
22. Sanford AM. Mild Cognitive Impairment. Clin Geriatr Med. 2017;33(3):325–337. doi:10.1016/j.cger.2017.02.005
23. Kim KY, Shin KY, Chang KA. Potential biomarkers for post-stroke cognitive impairment: a systematic review and meta-analysis. Int J Mol Sci. 2022;23(2):602. doi:10.3390/ijms23020602
24. Zhang X, Bi X. Post-stroke cognitive impairment: a review focusing on molecular biomarkers. J Mol Neurosci. 2020;70(8):1244–1254. doi:10.1007/s12031-020-01533-8
25. Choi J, Ifuku M, Noda M, et al. Translocator protein (18 kDa)/peripheral benzodiazepine receptor-specific ligands induce microglial functions consistent with an activated state. Glia. 2011;59(2):219–230. doi:10.1002/glia.21091
26. Karlstetter M, Nothdurfter C, Aslanidis A, et al. The translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. J Neuroinflammation. 2014;11:3. doi:10.1186/1742-2094-11-3
27. Yao R, Pan R, Shang C, et al. Translocator protein 18 kDa (TSPO) deficiency inhibits microglial activation and impairs mitochondrial function. Front Pharmacol. 2020;11:986. doi:10.3389/fphar.2020.00986
28. Barron AM, Garcia-Segura LM, Caruso D, et al. The ligand for the translocator protein reverses the pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2013;33(20):8891–8897. doi:10.1523/JNEUROSCI.1350-13.2013
29. Fairley LH, Sahara N, Aoki I, et al. Neuroprotective effects of the mitochondrial translocator protein ligand in a mouse model of tauopathy. J Neuroinflammation. 2021;18(1):76. doi:10.1186/s12974-021-02122-1
30. Christensen A, Pike CJ. TSPO ligand PK11195 improves Alzheimer-related outcomes in aged female 3xTg-AD mice. Neurosci Lett. 2018;683:7–12. doi:10.1016/j.neulet.2018.06.029
31. Fairley LH, Lai KO, Wong JH, et al. Mitochondrial control of microglial phagocytosis by translocator protein and hexokinase 2 in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2023;120(8):e2209177120. doi:10.1073/pnas.2209177120
32. Shi Y, Cui M, Ochs K, et al. Long-term diazepam treatment enhances microglial spine engulfment and impairs cognitive performance via the mitochondrial 18 kDa translocator protein (TSPO). Nat Neurosci. 2022;25(3):317–329. doi:10.1038/s41593-022-01013-9
33. Kreisl WC, Lyoo CH, McGwier M, et al. In vivo radioligand binding to translocator proteins correlates with AD severity. Brain. 2013;136(Pt 7):2228–2238. doi:10.1093/brain/awt145
34. Bradburn S, Murgatroyd C, Ray N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: a meta-analysis. Ageing Res Rev. 2019;50:1–8. doi:10.1016/j.arr.2019.01.002
35. Feng H, Wang X, Wang W, Zhao X. Lipid levels and 3-month prognosis after spontaneous intracerebral hemorrhage in women. Front Neurol. 2021;12:690194. doi:10.3389/fneur.2021.690194
36. James ML, Langefeld CD, Sekar P, et al. Assessment of the interaction of age and sex on 90-day outcome after intracerebral hemorrhage. Neurology. 2017;89(10):1011–1019. doi:10.1212/WNL.0000000000004255
37. Sagar R, Kumar A, Verma V, et al. Incremental accuracy of blood biomarkers for predicting clinical outcomes after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2021;30(3):105537. doi:10.1016/j.jstrokecerebrovasdis.2020.105537
38. You S, Wang X, Lindley RI, et al. Early cognitive impairment after intracerebral hemorrhage in the INTERACT1 Study. Cerebrovasc Dis. 2017;44(5–6):320–324. doi:10.1159/000481443
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.