Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Use of Selective Alternative Therapies for Treatment of OCD
Authors Khan I , Jaura TA, Tukruna A, Arif A , Tebha SS , Nasir S, Mukherjee D, Masroor N, Yosufi A
Received 8 January 2023
Accepted for publication 30 March 2023
Published 5 April 2023 Volume 2023:19 Pages 721—732
DOI https://doi.org/10.2147/NDT.S403997
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Roger Pinder
Iman Khan,1 Taimoor Asif Jaura,2 Alaa Tukruna,3 Aabiya Arif,1 Sameer Saleem Tebha,4 Sameen Nasir,5 Dattatreya Mukherjee,6 Nadia Masroor,7 Abubakr Yosufi8
1Department of Medicine, Ziauddin Medical University, Karachi, Sindh, Pakistan; 2General Adult Psychiatry, Southwest London and Saint George’s Mental Health Nhs Trust, London, UK; 3Department of Medicine, Batterjee medical college, Jeddah, Kingdom of Saudi Arabia; 4Department of Neurosurgery and Neurology, Jinnah Medical and Dental College, Karachi, Pakistan; 5Medical College, Aga Khan University Hospital, Karachi, Pakistan; 6Department of Medicine, International School, Jinan University, Jinan, People’s Republic of China; 7Department of Medicine, Liaquat University of medical and health Sciences, Jamshoro, Sindh, Pakistan; 8Medical School, Kabul University of Medical Sciences, Kabul, Afghanistan
Correspondence: Abubakr Yosufi, Kabul University of Medical Sciences, Kabul, Afghanistan, Tel +93 747236767, Email [email protected]
Abstract: About 40% of the people with the obsessive-compulsive-disorder do not experience the desired outcome after the existing treatment, and its several side effects were reported. This systematic review was conducted to evaluate the efficacy and tolerability of alternative drugs and assess the possibility of their use as treatment options for obsessive-compulsive-disorder. The Scientific databases PubMed, Science Direct, Google Scholar, Cochrane, Directory of Open Access Journals, MedRxiv and BioRxiv, were searched from inception to March 2022, using appropriate search strategies for each drug and following the Prisma guidelines 2020. Studies were selected according to the already set criteria and assessed for bias. Data were extracted, and descriptive and continuous data were analyzed and presented as frequency/percentage and mean. A total of 16 observational and interventional studies were included for data extraction. The studies focused on four drugs, Psilocybin (n=4), Cannabis (n=7), Nicotine (n=3), and Morphine (n=2), that were used to test out their effect on OCD symptoms. Overall, the majority of the studies showed promising results by documenting a reduction in Y-BOCS scores. However, few subjects, specifically those using nicotine or Cannabis, did not affect their condition or self-reported worsening symptoms. Few side effects were also noticed. This systematic review found that the drugs mostly showed a positive response. All Psilocybin and morphine users, 88.2% and 74.1% of the nicotine and Cannabis users, respectively, reported experiencing the positive effect of these drugs, indicating that these drugs have the potential to be used in the management of OCD. However, further research is required in this arena to thoroughly understand the mechanism of action by which these drugs produce their therapeutic effect. Policies to destigmatize and encourage clinical trials with these drugs are crucial for exploring the use of these drugs as a treatment option for OCD.
Keywords: psychedelic, psychoactive, psychoneurotic disorders, obsessions, compulsions
Introduction
Obsessive-Compulsive Disorder (OCD) is a chronic psychiatric disorder characterized by obsessions, compulsions, or both. Obsessions are recurrent, unwanted thoughts, impulses, images, or urges that cause extreme anxiety, like contamination, religion, symmetry, sexual acts, and aggression. At the same time, compulsions are repetitive and wearisome behavior to counteract anxiety. Common examples include washing, ordering, checking, counting, and repeating.1 According to The Diagnostic and Statistical Manual of Mental Disorders (DSM)-5, for a condition to be described as OCD, the obsessions should be significantly distressful and time-consuming, it should not be a physiological response to any other medical condition or substance abuse, and other mental disorders should not better explain it.2 The lifetime prevalence of OCD is estimated to be 1.6% to 2.3% in the community, contributing to disability and mortality risk.3,4 There is no definitive patho-etiology of the disease, but evidence suggests genetics play a role in its prevalence, as the heritability is known to be 27% to 45% in adults. Mutations like in N-methyl-D-aspartate (NMDA) ‘s subunit “NR2” or in genes related to dopamine and serotonergic systems contribute to the disease’s symptomology.3–5 The traits of OCD are also commonly observed in approximately 5% of the first-degree relatives of the diagnosed individuals.5 Several other variables like poor motor skills and neurodevelopment risk factors, personality dimensions; positive and negative emotionality and constraint, several behavior characteristics, including social isolation, and childhood stressors like physical or sexual abuse, are found to be significantly associated with the onset of OCD in adulthood.6 There is likely to be serotonin depletion in frontrolimbic systems. Hence, selective serotonin reuptake inhibitors (SSRIs) and some antipsychotics are used for treatment but there might be some side effects. A study reporting the prevalence rate of adverse effects caused by three commonly used SSRIs; sertraline, escitalopram, and fluoxetine, found headache, decreased appetite, pruritus, memory impairment, decreased concentration and dizziness to be statistically significant. Others include tremor, weight gain, myoclonus/twitching and anxiety. (Citation: https://www.psychiatrist.com/pcc/depression/side-effect-profiles-selective-serotonin-reuptake-inhibitors-cross-sectional-study-naturalistic-setting/#:~:text=On%20the%20basis%20of%20data,8%25%20of%20patients%20receiving%20placebo.) Clomipramine, a tricyclic antidepressant (TCA), is prescribed because of its partiality for serotonin, but SSRIs are preferred after taking the side effects into account.4 Cognitive behavioral therapy (CBT) with exposure and response prevention (ERP) may be helpful, but in some cases, it may worsen the condition, aiding in a reassurance-seeking ritual.7
Around 40% of individuals with the disease are treatment-resistant. A surgical approach is considered in these cases, but it is subjected to complications as it is a complex procedure. Ablative lesion neurosurgery may cause dizziness, confusion, transient unsteady gait, isolated seizure, and urinary retention. Deep brain stimulation is reported to have a higher incidence of complications, like hardware failures, hemorrhage, and infection.8 To avoid the existing treatment’s adverse effects and take measures against treatment-resistant OCD, a safer and more effective approach is needed.
The use of alternative drugs has recently been reported to minimize the symptoms of the disease, but the literature on the influence of some of them, namely Psilocybin, Cannabis, Nicotine, and Morphine, is limited and scattered. Since the condition is considered the fourth most frequently occurring psychiatric disorder, after phobias, substance abuse, and major depression, we believe it is crucial to study these rare treatment approaches thoroughly.5 Hence, we aim to systemize their efficacy and analyze any side effects reported to aid in understanding the potential of these drugs in managing OCD.
Materials and Methods
This systematic review was consistent with the relevant criteria of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.9 The protocol was registered on PROSPERO (CRD42022326445).
Search Methods
A Systematic search, limited to human studies, was conducted from inception to March 2022 on databases: PubMed, Google Scholar, Science Direct, Directory of Open Access Journals, and Cochrane Library, implementing the Boolean (and/or) logic and using an appropriate set of Mesh terms, including but not limited to “Obsessive-Compulsive Disorders”, “OCD”, “Psilocybin”, “Cannabis”, “Marijuana”, “Nicotine”, “Tobacco”, “Morphine”. In addition, pre-printed studies on MedRxiv and BioRxiv and key reference lists were also searched for relevant articles. The search strategy used for each drug in each database is provided in the Supplementary Files as Table S1. PRISMA flowchart (Figure 1) summarizes the process through different phases of our study.
 |
Figure 1 Prisma Flowchart. |
Eligibility Criteria
Before the screening, inclusion and exclusion criteria were set to standardize the target population and avoid selection bias. Studies were included if its participants were: 1) diagnosed with OCD, using either the International Classification of Diseases or Diagnostic (ICD) and Statistical Manual of Mental Disorders criteria (DSM-III, DSM III-R, DSM-IV, DSM-IV-TR, or DSM-5). Individuals diagnosed clinically before the study took place, irrespective of the criteria mentioned, were also included 2) used Psilocybin, Cannabis, Nicotine, or Morphine as treatment 3) were of age 18 years or above 4) the study mentioned the outcome; the drug’s effect on OCD symptoms. There was no gender or ethnic restriction. Studies were excluded if participants: 1) had any systemic diseases/autoimmune diseases, 2) had any disorder that better explained the situation, 3) diagnoses were induced by substance use, and 4) a study that did not report the effect of treatment on precisely OCD symptoms.
Study Selection
After initially identifying 155,275 studies, Endnote was used to remove 5033 duplicates. Two authors then independently carried out two screening stages; a third author resolved any disagreements among them. After going through the titles and abstracts in the primary screening, 62 relevant reports were filtered out. First, any human observational and experimental study was selected. Next, any study that did not report original data, like review papers, responses, and editorials, was excluded. Finally, full-text links were thoroughly reviewed in secondary screening, and 16 studies that fulfilled the eligibility criteria were further used for data extraction and synthesis.
Data Extraction and Synthesis
Two investigators separately scanned the 16 potential studies. According to a structured data form, the following data was extracted: author names, publication date, sample size, study design, number of patients, and their age, gender, diagnostic criteria used, number of sessions of the clinical trial, onset and dosage and frequency of the drug, route of administration, adverse effects noted, use of other psychoactive drugs or therapy before or during the intervention, and the outcome along with Y-BOCS scores, if available. A decrease in Y-BOCS scores or OCD symptoms post ingestion of the drugs was considered a positive response.
A shared excel spreadsheet was used to organize the extracted data. If possible, descriptive and continuous data were analyzed and presented as frequency/percentage and mean with standard deviation.
Risk of Bias Assessment
Appropriate quality assessment tools for case study/series, observational studies, and control trials developed by the National Heart, Lung, and Brain Institute (NHLBI) and Research Triangle Institute International were used by two authors independently.10 The internal validity was assessed, as shown in Supplementary File as Tables S2-S5 and categorized as “good quality”, “fair quality”, and “poor quality”. Scores were assigned accordingly, and “poor quality” studies were removed.
Results
Of the 16 chosen studies, 4 (25.0%) were on Psilocybin, 7 (43.7%) of them used Cannabis as an intervention, 3 (18.8%) studies focused on nicotine, and 2 (12.5%) were on Morphine that reported data of total 779 participants.11–26 The selected studies’ study designs have been displayed in Figure 2. Data extracted from each study have been summarized in Table 1.
 |
Table 1 Summary of Studies Included |
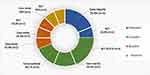 |
Figure 2 Study designs. |
Psilocybin
There were 3 (75.0%) case studies and 1 (25.0%) random control trial focused on Psilocybin’s effect on OCD symptoms. The sample size of the selected 4 studies was 12, ranging from 1 (Case study) to 9 (Random control trial), with a total mean age of 35.72 years. Participants in the studies combined were primarily males, 10 (83.3%), while there were only 2 females (16.7%).
All participants experienced a decrease in symptoms after ingesting Psilocybin. Three (25.0%) took 2 grams of the ‘magic “mushroom”, but at a different frequency; twice a week, once every two, and once every three weeks. The remaining 9 (75.0%) people, in a control trial, were weekly given 4 different doses; 25 (very low dose [VLD]), 100 (low dose [LD]), 200 (medium dose [MD]), and 300 (high dose [HD]) µg/kg of body weight. LD, MD, and HD were assigned in that order, whereas VLD was given in a random double-blind manner at any time after the first dose (LD). 2 (16.7%) subjects had a previous failed drug history against OCD. One had taken diazepam, fluoxetine, and buspirone clomipramine and underwent psychoanalysis, supportive psychotherapy, and several months of cognitive-behavioral therapy. The other one showed no improvement with sertraline, escitalopram, mirtazapine, fluoxetine, venlafaxine, paroxetine, duloxetine, milnacipran, and intranasal ketamine. Celecoxib with escitalopram was partially useful. One of the patients (8.3%) previously took Peyote cactus a few times but experienced long-lasting side effects, whereas 9 (75.0%) were only included in the clinical trial if they had not taken serotonin reuptake inhibitors (SRIs) for the past 12 weeks. 2 studies used the Yale-Brown Obsessive-Compulsive Scale (YBOCS) to measure outcome showed a significant reduction in the scores. 4 (33.3%) people reported side effects, which include transient hypertension (n=1, 8.3%), anxiety-provoking feeling (n=1, 8.3%), dizziness (n=1, 8.3%), nausea (n=1, 8.3%), vomiting (n=1, 8.3%) and feeling of dissociation (n=3, 25%).
Cannabis
7 of the selected studies that highlighted the use of Cannabis in treating OCD had a combined sample population of 726, with 348 females (47.9%) and 347 males (47.8%). Thirty (4.1%) participants identified as transgender/other, and 1(0.1%) did not disclose this information. The mean age of the total participants was 29.03 years.
The majority of the selected population experienced relief in OCD symptoms after cannabis intake. One hundred eighty-five (25.5%), however, felt worsening of obsessions (n=101, 13.9%) and compulsions (n=81,11.2%), or both (n=3,0.4%). From the available data, 604 (83.2%) of the participants inhaled the drug, that included through vaporizing (n=183, 25.2%), Joints/Blunts (n=105, 14.5%), Pipes (n=113, 15.6%) or Bongs (n=104, 14.3%), while 67 (9.2%) of them took it orally. Eleven (1.5%) of them, in a clinical trial, took 1 mg of a synthetic form of Δ-9-tetrahydrocannabinol; nabilone, twice a day for 28 days, of which 5(0.7%) also went through exposure and ritual prevention psychotherapy (EX/RP). 3(0.4%) cases reported usage of 10mg (n=2, 0.3%), twice and thrice a day, and 20mg (n=1,0.1%), daily, of Dronabinol. Twelve (1.7%) subjects in a control trial were asked to randomly smoke three different varieties of approximately 800mg of cannabis; THC (7.0% THC/0.18% CBD), CBD (0.4% THC/10.4% CBD), and placebo (0% THC/0% CBD), over the period of three sessions, while 12(1.7%) randomly received different sessions of low THC/High CBD Marijuana (THC <1% and CBD (>10%), High THC/Low CBD Marijuana (THC 5–10%, CBD (<1%), and placebo comparator with no THC or CBD content. In a cross-sectional survey of 601(82.9%) participants, 543(74.8%) of them reported to have inhaled high THC (>10%), 40 (6.7%) took Low THC (<10%), 186 (25.6%) had high CBD (>10%) and 294 (48.92%) responded to Low CBD (<10%). Finally, 87(12.0%) out of 726 people with OCD measured their cannabis intake through the mean number of puffs (S.D) they used to relieve the symptoms and the mean of its THC and CBD concentration. For intrusions they took 6.50 (4.35) puffs; THC= 16.46 (5.11), CBD= 0.96 (3.37), for compulsions 10.27 (6.21) puffs; THC: 13.47 (6.87), CBD: 3.55 (5.77), and for anxiety by 8.50 (6.09) number of puffs; THC: 13.62 (7.23), CBD: 3.41 (5.75). 465(64.0%) participants had history of previous treatment that showed no or little improvement, that included SRIs (SSRIs or antidepressants)(n = 251, 34.6%), other medications, like antipsychotics etc, (n=334, 46.0%), Transcranial Magnetic Stimulation (n=10, 1.4%) and alternative (mindfulness meditation, yoga, herbal supplements) (n=360, 49.6%), while 377 went through psychotherapy Treatment (n= 337, 76.4%), including Exposure and response prevention (n= 99, 13.6%), Cognitive therapy (without exposure)(n= 259,35.7%), Acceptance and commitment therapy (n=67,9.2%), Psychodynamic psychotherapy (n=35,4.8%) and electro convulsant therapy (n=1, 0.1%).
YBOCS scores were calculated in 639 (88%) people, and all reported a general decrease from the baseline score. A slight increase in YBOCS score after some time from its previous score was noted in 17(2.3%) participants; 5 (0.7%) cases showed a 1.6 increase, after 2 weeks, from the scores previously rated after 2 weeks of use. The rest of the 12(1.7%) people, instead of showing a progressive decrease in YBOCS scores after every 20 minutes, showed a slight increase after 180 minutes from the last rated scores at 90 minutes. Increase in score was of 1.18 (high THC(5–10%) /low CBD(<1%); and 2 (low THC(<1%) /high CBD). Of the 38(5.2%) cases with this information, 25(3.4%) reported few side effects, out of which 13 (1.8%) experienced more than one symptom. They were dry mouth (n=13, 52%), nervousness (n=9, 36%), “felt high” feeling (n=1, 4%), drowsiness (n = 7, 28%), difficulty concentrating (n = 4, 16%), disorientation (n = 4, 16%), and forgetfulness (n = 4, 16%), constipation (n= 2.8%) and hypotension (n=1, 4%). Adverse effects reported after 4 weeks were dry mouth (n=5), forgetfulness (n=5), disorientation (n=4) and difficulty concentrating (n=3). 13(1.8%) of the remaining 38 participants said no mild or severe side effects. Only 12 (1.7%) of the total population reported mortality status; no deaths were reported.
Nicotine
A total of 3 studies (1 RCT, 1 case series, and 1 case report) were retrieved from multiple databases that reported the effect of nicotine on OCD symptoms. Seventeen individuals, in total, were administered nicotine. Of these 17 participants, 9 (52.9%) were males, and the mean age of the patients was 29.7 years. All participants were diagnosed with OCD according to either the DSM-IV or DSM-IV-TR criteria.
Of the included studies, nicotine was administered only through transdermal patches in 11 (64.7%) participants. 4 (23.5%) participants were given transdermal patches for three weeks, followed by nicotine chewing gums for five additional weeks. The rest consumed nicotine only by chewing gum 2 (11.8%). The dosage for the dermal patches was 17.5 mg per patch for 11 (64.7%) participants, which was given for five days (one patch a day). For 4 (23.5%) participants, the transdermal patch dosage was 5 mg/16 h during the first week and 10 mg/16 h during the second and third weeks. The chewing gums consumed ranged from 1.1/day to 10/day. 15 (88.2%) patients showed improvement in OCD symptoms, with a decrease in YBOCS score after the intervention. Out of the 15 participants, 10 (66.7%) participants had decreased compulsion scores, but no reduction in obsession scores was seen. Only 5(33.3%) participants had a reduction in both the compulsion and obsession scores. 2 (11.8%) participants reported no change in their YBOCS scores; in addition, they reported experiencing side effects like nausea, tremors, and headaches due to nicotine.
Previous use of CBT, SSRI, monoamine oxidase inhibitor, typical antipsychotics, atypical antipsychotics, anticonvulsants, and tricyclic antidepressants with limited or no success was reported in 3(17.6%), 5(29.4%), 1(5.9%), 1 (5.9%), 2 (11.8%), 1(5.9%) and 3 (17.6%) participants, respectively.
Morphine
Currently, there is only one randomized control trial and one case study assessing the effect of Morphine on OCD. Twenty-four patients with OCD were given morphine, of which 17 (70.8%) were male, and 7 (29.2%) were females, with a mean age of 40.6 years.
All participants’ symptoms did not improve substantially from taking typical treatments. All participants tried and failed at least 2 SSRI trials. 1 (4.2%) participant tried electroconvulsive therapy, CBT psychosurgical procedure, and Naltrexone and experienced either limited or no improvement. 5 (20.8%) participants were on Gabapentin, 4 (16.7%) on Clomipramine, 10 (41.7%) on benzodiazepine, 7 (29.2%) on atypical anti-psychotics, 1 (4.2%) on Topiramate, 1 (4.2%) on Trazodone, and 15 (62.5%) were still taking SSRIs.
23 (95.8%) participants received a starting dose of 30mg orally once a week for two weeks. Dosage for these participants was adjusted in the second week according to their tolerability, ranging from 15-to 45 mg. 1 (4.2%) participant was administered morphine 10mg subcutaneously for 24 hours and then converted to 30mg orally later on. The Median YBOCS score at the highest morphine dose for 23 of the 24 participants had a median drop of 13%, where the score dropped from a median value of 29 (28–30) to a median value of 25 (12–34). The rest also reported a drastic improvement in OCD symptoms due to morphine. 7 out of 24 (29.2%) reported a reduction of ≥ 25 in their YBCOS score. However, some patients reported experiencing side effects like dizziness 5 (21.0%), fatigue 3 (12.5), nausea 6 (25%), and sedation 13 (52.3%).
Discussion
To our best understanding and knowledge, this systematic review is the first to study the four investigational and experimental drugs, Psilocybin, Cannabis, nicotine, and Morphine, as the possible treatment for OCD. Despite the literature being scattered, this study scrutinizes the efficacy of the drugs by using the measure of outcome and analyzing them. Adverse effects were summarized to assess the safety and tolerability of the drugs.
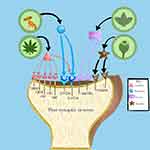
The interventions of our review can be broadly divided according to their interactions. Psilocybin and Cannabis have similar interactions with serotonin receptors. This can possibly contribute to the reason it improves OCD symptoms. However, unlike Psilocybin, cannabis treatment is divided into different doses and concentrations to reduce its adverse effects.27 Nicotine and Morphine primarily act on different receptors, but participants using them experienced fewer side effects than the drugs acting on serotonin receptors. These drug interactions with the different receptors are shown in Figure 3.
The amino acid L-tryptophan is converted into psilocin, the active compound in magic mushrooms. It is phosphorylated by alkaline phosphate into its prodrug, Psilocybin. Psilocin is an antidepressant and anti-anxiolytic as its serotonin (5-hydroxytryptamine) receptor agonist, with high affinity at 5-HT2A and lesser affinity at 5-HT1A.28 Because of this, it is also considered for treating other psychiatric disorders, for example, anxiety, depression, and substance abuse disorders, like alcohol and tobacco addiction, and has reported similar positive outcomes to OCD, improving the condition.28–30 Psilocybin is also reported to modulate functional connectivity, like between the amygdala and ventral striatum, which typically converts emotional information into motor response, and between the amygdala and frontal lobe, which normally justifies the information recognized by the amygdala, leading to the disintegration of associative networks.31–33 Likewise, the most common adverse effect found in our studies was the feeling of dissociation after a few hours of ingesting the drug. Other studies have also highlighted a significant association of dissociation with disruption in areas responsible for memory and emotions (like the amygdala and hippocampus), cognitive control, and arousal modulation.34 Other side effects were increased blood pressure, heart rate, nausea, vomiting, and transient anxiety. An animal model of OCD, after taking lower doses of psilocybin argentipes, reduced its marble-burying behavior without affecting locomotor activity, further confirming the tolerability of the drug.35
As of March 2021, recreational Cannabis has been legalized in a few countries, specifically Georgia, Uruguay, South Africa, Canada, and parts of the United States. It is still illegal in places throughout Central and South America and Europe.36 Cannabis, a genus of flowering plants in the family Cannabaceae and also known as marijuana, has components that interact with the endocannabinoid system (ECS) or other pathways. There are two cannabinoid receptors, CB1, expressed in the central nervous system, and CB2 in the periphery, responsible for immunomodulating and anti-inflammatory effects after some conditions.37 Our studies mainly reported decreasing Y-BOCS scores after inhaling or ingesting the drug. Other studies also support this anxiolytic effect. The exact mechanism is unknown, but CBD interacts with receptors responsible for anxiety and fear, like the serotonin 5-HT1A receptor, cannabinoid type 1 receptor (CB1R), and the transient receptor potential (TRP) vanilloid type 1 (TRPV1) receptor. A few articles in our review that, after repeated administration, reported a slight increase in Y-BOCS scores near the end of the trial, which means the improvement in symptoms was not maintained. This might hint at the negative effect of chronic OCD symptoms.15,17,38 In our selected articles, a combination of different components of Cannabis in different concentrations is used, including THC and CBD. Their co-administration reduces the neuropsychiatric and cognitive adverse drug effects, as the effect of THC is reduced by CBD.37 One of our studies supports this where there was mild; slight hypertension, heart rate, and most commonly dry mouth., side effects for THC (7.0% THC/0.18% CBD), whereas CBD (0.4% THC/10.4% CBD) and placebo (0% THC/0% CBD) had no adverse effect.15
Nicotine, derived from the tobacco plant, binds to the acetylcholine receptors (nAChRs) in the brain, releasing many neurotransmitters, including dopamine, and controlling cell signaling mechanisms and neuronal excitability.39 The central cholinergic system might also play a role in the pathophysiology of OCD, along with glutamate and serotonin. Multiple factors can be suggestive of this, like the hyperactive orbitofrontal cortex receiving cholinergic innervation, growth hormone released in response to OCD competes with the acetylcholinesterase inhibitor pyridostigmine which is then found to be increased, the amygdala is responsible for aversion and fear processes, has a glutamatergic association with the orbitofrontal cortex. Lastly, clomipramine, an SSRI, is used to treat OCD and has anticholinergic characteristics. We evaluated studies using chewing gums, cigarettes, or transdermal nicotine patches to treat OCD with severe symptoms. The outcome was favorable, and both obsessions and compulsions were improved. A case study by Stefan Lundberg et al showed worsening of symptoms after 8 weeks, reportedly due to stress regarding withdrawal.22 This can represent how likely one can develop substance abuse symptoms as nicotine is highly addictive; hence close monitoring is required.40 Our studies did not show any serious adverse effects, but nicotine abuse can lead to increased risk of gastrointestinal, respiratory, and cardiovascular disorders and decreased immunity. It can also cause DNA mutations leading to cancer, for example, gastrointestinal and lung carcinoma, or breast cancer.41
The classic opioid analgesic, Morphine, has an affinity for kappa, delta, and mu-opioid receptors. It activates descending inhibitory pathways of the central nervous system and stops the nociceptive afferent neurons of the peripheral nervous system, which then leads to a reduction of nociceptive transmission. It showed satisfactory results in this study, as the symptoms of OCD improved after its administration. Many studies focused on the anxiolytic function of the drug-like Kerstin Roeska et al concluded that Morphine and gabapentin successfully reduced anxiety-like behavior and mechanical hypersensitivity.42 Our findings were consistent with two small-blinded studies on the effect of morphine antagonists that led to the worsening of the symptoms. However, according to a study conducted on rats, chronic administration can cause depression and anxiety instead.43 Morphine may also be used in other psychiatric disorders like posttraumatic stress disorder.44 One of our studies reported participants to have experienced dizziness (22%), fatigue (13%), nausea (26%), and sedation (44%). Similarly, a prospective survey showed symptoms like dry mouth, constipation, sedation, and myoclonus associated with Morphine; hence it should be carefully administered.45
Limitations
Our study is subjected to some limitations. The use of these under-the-table drugs for OCD is rare, due to which data was scarce. Hence our sample size was small and cannot be applied to a broader population. The studies found after the vigorous screening process were low-quality primary evidence, the majority being observational case studies/series or cross-sectional. There are walls of negative stigma built around us regarding psychiatric disorders, including OCD. This has led to delays in help-seeking behavior and treatment because of which the condition can go unnoticed or undiagnosed for an extended period, and patients can present at a severe stage, hence can cause over and underestimation of side effects and positive results. Recreational drugs are still illegal in many eastern and western countries. Due to this and the social pressure, self-medicated cases might go unreported, contributing to reporting bias. Most studies in this review did not have a long-term intervention or follow-up; hence, adverse effects, including mortality, with chronic use of these drugs could not be assessed. Many studies assessed the outcome using self-rated scales, leading to self-report bias and giving their perception about improvement instead of the exact findings. Our systematic review lacked homogeneity, due to which we could not compare the inter-drug comparison findings in terms of effectiveness and toxicity.
Conclusion
We systematically reviewed the effect of Psilocybin, Cannabis, Nicotine, and Morphine on OCD. The majority of patients in this systematic review showed positive outcome. Subjects of Psilocybin (n=12) and Morphine (n=2) showed the most favorable implications, with a decrease in the symptoms of OCD noticed in all of them. On the other hand, 11.8% people after administration of nicotine (n=2) and 25.9% participants after use of Cannabis (n=185) reported worsening of symptoms. With less severe adverse effects reported, their role in decreasing compulsions and obsessions could be noteworthy. Our review can help lay down the design for future clinical trials, highlighting the need for more comprehensive and interventional studies, which are required to authenticate these findings further and understand the drug’s mechanism of action in improving the symptoms. These findings should further encourage official sectors to modify policies or establish new ones regarding their use as a treatment, especially in the psychiatric domain.
Data Sharing Statement
All data is in the main manuscript and Supplemental File.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Richter PMA, Ramos RT. Obsessive-compulsive disorder. Continuum. 2018;24(3):828–844. doi:10.1212/CON.0000000000000603
2. van Ameringen M, Patterson B, Simpson W. DSM-5 obsessive-compulsive and related disorders: clinical implications of new criteria. Depress Anxiety. 2014;31(6):487–493. doi:10.1002/da.22259
3. Obsessive-Compulsive Disorder - StatPearls - NCBI Bookshelf. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553162/.
4. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334–341. doi:10.1001/JAMAPSYCHIATRY.2014.2502
5. Aouizerate B, Guehl D, Cuny E, et al. Pathophysiology of obsessive-compulsive disorder: a necessary link between phenomenology, neuropsychology, imagery and physiology. Prog Neurobiol. 2004;72(3):195–221. doi:10.1016/J.PNEUROBIO.2004.02.004
6. Grisham JR, Fullana MA, Mataix-Cols D, Moffitt TE, Caspi A, Poulton R. Risk factors prospectively associated with adult obsessive-compulsive symptom dimensions and obsessive-compulsive disorder. Psychol Med. 2011;41(12):2495–2506. doi:10.1017/S0033291711000894
7. International OCD Foundation | ineffective and Potentially Harmful Psychological Interventions for Obsessive-Compulsive Disorder. Available from: https://iocdf.org/expert-opinions/ineffective-and-potentially-harmful-psychological-interventions-for-obsessive-compulsive-disorder/.
8. Doshi PK. Surgical treatment of obsessive compulsive disorders: current status. Indian J Psychiatry. 2009;51(3):221–226. doi:10.4103/0019-5545.55095
9. PRISMA. Available from: http://www.prisma-statement.org/.
10. Study Quality Assessment Tools | NHLBI, NIH. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
11. Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry. 2006;67(11):1735–1740. doi:10.4088/JCP.V67N1110
12. Wilcox JA. Psilocybin and Obsessive Compulsive Disorder. J Psychoactive Drugs. 2014;46(5):393–395. doi:10.1080/02791072.2014.963754
13. Moreno FA, Delgado PL. Hallucinogen-induced relief of obsessions and compulsions. Am J Psychiatry. 1997;154(7):1037–1038. doi:10.1176/AJP.154.7.1037B
14. Lugo-Radillo A, Cortes-Lopez JL. Long-term Amelioration of OCD Symptoms in a Patient with Chronic Consumption of Psilocybin-containing Mushrooms. J Psychoactive Drugs. 2021;53(2):146–148. doi:10.1080/02791072.2020.1849879
15. Kayser RR, Haney M, Raskin M, Arout C, Simpson HB. Acute effects of cannabinoids on symptoms of obsessive-compulsive disorder: a human laboratory study. Depress Anxiety. 2020;37(8):801–811. doi:10.1002/DA.23032
16. Cooper JJ, Grant J. Refractory OCD Due to Thalamic Infarct With Response to Dronabinol. J Neuropsychiatry Clin Neurosci. 2017;29(1):77–78. doi:10.1176/APPI.NEUROPSYCH.16030053
17. Kayser RR, Raskin M, Snorrason I, Hezel DM, Haney M, Simpson HB. Cannabinoid Augmentation of Exposure-Based Psychotherapy for Obsessive-Compulsive Disorder. J Clin Psychopharmacol. 2020;40(2):207–210. doi:10.1097/JCP.0000000000001179
18. Schindler F, Anghelescu I, Regen F, Jockers-Scherubl M. Improvement in refractory obsessive compulsive disorder with dronabinol. Am J Psychiatry. 2008;165(4):536–537. doi:10.1176/APPI.AJP.2007.07061016
19. Kayser RR, Senter MS, Tobet R, Raskin M, Patel S, Simpson HB. Patterns of Cannabis Use Among Individuals with Obsessive-Compulsive Disorder: results from an Internet Survey. J Obsessive Compuls Relat Disord. 2021;30. doi:10.1016/J.JOCRD.2021.100664
20. Mauzay D, LaFrance EM, Cuttler C. Acute Effects of Cannabis on Symptoms of Obsessive-Compulsive Disorder. J Affect Disord. 2021;279:158–163. doi:10.1016/J.JAD.2020.09.124
21. NCT03274440. Effects of Marijuana on Symptoms of OCD. Available from: https://clinicaltrials.gov/show/NCT03274440.
22. Lundberg S, Carlsson A, Norfeldt P, Carlsson ML. Nicotine treatment of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(7):1195–1199. doi:10.1016/J.PNPBP.2004.06.014
23. Pasquini M, Garavini A, Biondi M. Nicotine augmentation for refractory obsessive-compulsive disorder. A case report. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(1):157–159. doi:10.1016/J.PNPBP.2004.08.011
24. Changes in compulsion and anxiety symptoms with nicotine transdermal patches in non-smoking obsessive-compulsive disorder patients - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/15011734/.
25. Koran LM, Aboujaoude E, Bullock KD, Franz B, Gamel N, Elliott M. Double-blind treatment with oral morphine in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2005;66(3):353–359. doi:10.4088/JCP.V66N0312
26. Warneke L. A possible new treatment approach to obsessive-compulsive disorder. Can J Psychiatry. 1997;42(6):667–668. doi:10.1177/070674379704200624
27. Psilocybin vs. THC: differences, Similarities, Uses and Cautions. Available from: https://leafwell.com/blog/shrooms-vs-weed/.
28. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165–1180. doi:10.1177/0269881116675512
29. Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa P, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29(3):289–299. doi:10.1177/0269881114565144
30. Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983–992. doi:10.1177/0269881114548296
31. Grimm O, Kraehenmann R, Preller KH, Seifritz E, Vollenweider FX. Psilocybin modulates functional connectivity of the amygdala during emotional face discrimination. Eur Neuropsychopharmacol. 2018;28(6):691–700. doi:10.1016/J.EURONEURO.2018.03.016
32. Cho YT, Ernst M, Fudge JL. Cortico–Amygdala–Striatal Circuits Are Organized as Hierarchical Subsystems through the Primate Amygdala. J Neurosci. 2013;33(35):14017. doi:10.1523/JNEUROSCI.0170-13.2013
33. The Amygdala-Prefrontal Cortex Connection is Crucial | the Raising of America. Available from: https://www.raisingofamerica.org/amygdala-prefrontal-cortex-connection-crucial.
34. Krause-Utz A, Frost R, Winter D, Elzinga BM. Dissociation and Alterations in Brain Function and Structure: implications for Borderline Personality Disorder. Curr Psychiatry Rep. 2017;19:1. doi:10.1007/S11920-017-0757-Y
35. Matsushima Y, Shirota O, Kikura-Hanajiri R, Goda Y, Eguchi F. Effects of Psilocybe argentipes on marble-burying behavior in mice. Biosci Biotechnol Biochem. 2009;73(8):1866–1868. doi:10.1271/BBB.90095
36. Areesantichai C, Perngparn U, Pilley C. Current cannabis-related situation in the Asia-Pacific region. Curr Opin Psychiatry. 2020;33(4):352–359. doi:10.1097/YCO.0000000000000616
37. Brown JD, Rivera Rivera KJ, Hernandez LYC, et al. Natural and Synthetic Cannabinoids: pharmacology, Uses, Adverse Drug Events, and Drug Interactions. J Clin Pharmacol. 2021;61(S2):S37–S52. doi:10.1002/JCPH.1871
38. Spradlin A, Mauzay D, Cuttler C. Symptoms of obsessive-compulsive disorder predict cannabis misuse. Addict Behav. 2017;72:159–164. doi:10.1016/J.ADDBEH.2017.03.023
39. Wonnacott S, Barik J. Molecular and cellular mechanisms of action of nicotine in the CNS. Handb Exp Pharmacol. 2009;192(192):173–207. doi:10.1007/978-3-540-69248-5_7
40. Benowitz NL. Nicotine addiction. Prim Care. 1999;26(3):611–631. doi:10.1016/S0095-4543(05)
41. Mishra A, Chaturvedi P, Datta S, Sinukumar S, Joshi P, Garg A. Harmful effects of nicotine. Indian J Med Paediatr Oncol. 2015;36(1):24–31. doi:10.4103/0971-5851.151771
42. Roeska K, Doods H, Arndt K, Treede RD, Ceci A. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain. 2008;139(2):349–357. doi:10.1016/J.PAIN.2008.05.003
43. Motaghinejad M, Fatima S, Banifazl S, Bangash M, Karimian M. Study of the effects of controlled morphine administration for treatment of anxiety, depression and cognition impairment in morphine-addicted rats. Adv Biomed Res. 2016;5(1):178. doi:10.4103/2277-9175.188491
44. Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biol Psychiatry. 2009;65(5):438–440. doi:10.1016/J.BIOPSYCH.2008.10.032
45. Glare P, Walsh D, Sheehan D. The adverse effects of morphine: a prospective survey of common symptoms during repeated dosing for chronic cancer pain. Am J Hosp Palliat Care. 2006;23(3):229–235. doi:10.1177/1049909106289068
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.