Back to Journals » International Journal of General Medicine » Volume 16
Use of Chinese Herbal Medicine Was Related to Lower Risk of Osteoporotic Fracture in Sarcopenia Patients: Evidence from Population-Based Health Claims
Authors Chen WJ , Livneh H , Li HH, Wang YH, Lu MC , Tsai TY , Chien KY
Received 11 April 2023
Accepted for publication 14 June 2023
Published 7 August 2023 Volume 2023:16 Pages 3345—3354
DOI https://doi.org/10.2147/IJGM.S416705
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Wei-Jen Chen,1– 4,* Hanoch Livneh,5 Hsin-Hua Li,1 Yu-Han Wang,4,* Ming-Chi Lu,6,7 Tzung-Yi Tsai,8– 10 Kuei-Yu Chien2
1Department of Chinese Medicine, Dalin Tzu chi Hospital, The Buddhist Tzu chi Medical Foundation, Chiayi, 62247, Taiwan; 2Graduate Institute of Sports Science, National Taiwan Sport University, Taoyuan, 33301, Taiwan; 3School of Post-Baccalaureate Chinese Medicine, Tzu Chi University, Hualien, 97004, Taiwan; 4Center of Sports Medicine, Dalin Tzu chi Hospital, The Buddhist Tzu chi Medical Foundation, Chiayi, 62247, Taiwan; 5Rehabilitation Counseling Program, Portland State University, Portland, OR, 97207-0751, USA; 6Division of Allergy, Immunology and Rheumatology, Dalin Tzu chi Hospital, The Buddhist Tzu chi Medical Foundation, Chiayi, 62247, Taiwan; 7School of Medicine, Tzu Chi University, Hualien, 97004, Taiwan; 8Department of Medical Research, Dalin Tzu chi Hospital, The Buddhist Tzu chi Medical Foundation, Chiayi, 62247, Taiwan; 9Department of Nursing, Tzu Chi University of Science and Technology, Hualien, 97004, Taiwan; 10Department of Environmental and Occupational Health, College of Medicine, National Cheng Kung University, Tainan, 70428, Taiwan
*These authors contributed equally to this work
Correspondence: Kuei-Yu Chien; Tzung-Yi Tsai, Tel +886-3-3283201-2421 ; +886-5-2648000-3209, Fax +886-3-3280592 ; +886-5-2648006, Email [email protected]; [email protected]
Introduction: With population aging, sarcopenia and its accompanying risk of osteoporotic fracture has drawn increased attention. Nowadays, while Chinese herbal medicine (CHM) is often used as complementary therapy for many medical conditions, its effect against likelihood of osteoporotic fracture among sarcopenia subjects was not fully elucidated yet. We therefore conducted a population-level study to compare osteoporotic fracture risk for sarcopenia persons with or without CHM use.
Methods: Using the patient record from a nationwide insurance database, we recruited persons with newly diagnosed sarcopenia and simultaneously free of osteoporotic fracture between 2000 and 2010. Propensity score matching was then applied to randomly select sets of CHM users and non-CHM users. All of them were tracked until end of 2013 to measure the incidence and adjusted hazard ratios (HRs) for new new-onset fracture in multivariable Cox proportional hazards model.
Results: Compared to non-CHM users, the CHM users indeed had a lower incidence of osteoporotic fracture (121.22 vs 156.61 per 1000 person-years). Use of CHM correlated significantly with a lower fracture likelihood after adjusting for potential covariates, and those receiving CHM treatment for more than two years experienced a remarkably lower risk by 73%. Uses of several herbal formulae were correlated to reduced risk of osteoporotic fracture, such as Caulis Spatholobi, Xuduan, Duzhong, Danshen, Shu-Jing-Huo-Xue-Tang, Du-Huo-Ji-Sheng-Tang, Shao-Yao-Gan-Cao-Tang, and Shen-Tong-Zhu-Yu -Tang.
Conclusion: Our study depicted that cumulative CHM exposure was inversely associated with osteoporotic fracture risk in a duration-dependent manner, implying that CHM treatment may be embraced as routine care in preventing incident osteoporotic fracture.
Keywords: sarcopenia, osteoporotic fracture, risk, Chinese herbal medicines, cohort study
Introduction
Sarcopenia, an age-related musculoskeletal disease, is characterized by progressive loss of skeletal mass that leads to severe disability and premature mortality.1 The global prevalence of sarcopenia is nearly 10% of the population.2 With the aging of the population worldwide, health-care costs associated with this illness are expected to increase heavily. A recent study reports that the total annual cost of hospitalization for patients with sarcopenia in the United States was as high as USD $40.4 billion.3
During the aging process, the impact of endocrine alternations on muscle protein synthesis has attracted substantial clinical attention, as it may insidiously lead to loss in muscle mass together with muscle strength.4 Recently, the sarcopenia has been recognized as a trigger for a wide array of comorbid conditions, particularly osteoporotic fracture.5,6 One review article based on findings from 36 studies figured that individuals with sarcopenia were nearly twice as likely to experience osteoporotic fracture as those in the general population.7 At present, accumulated evidence suggests that hormonal disturbances and inflammatory molecules may be involved in the pathogenesis of sarcopenia and bone fragility.8–10 An animal study reported that the administration of interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) resulted in the degradation of skeletal muscle.11 The activation of inflammatory mediators may gradually induce bone loss via the up-regulation of pro-osteoclastogenic agents, including receptor activation of nuclear factor-kappaB (NF-kB) ligand (RANKL), thereby inciting osteoporosis fracture.12,13 Furthermore, sarcopenia increases the probability of mortality nearly threefold over that of individuals who were not diagnosed with sarcopenia.14 Given the alarming clinical findings, the availability of effective treatments to manage sarcopenia, particularly for preventing osteoporotic fracture, is of utmost importance.
Traditional Chinese herbal medicine (CHM) is often used to treat a broad range of health conditions. Several studies have uncovered the active herbal ingredients that act to lessen bone disease progression and aid in the prevention of joint deformities. For example, by abating NF-κB signaling, some Chinese herbs are believed to regulate pro-inflammatory cytokines.15 The inflammatory mediators are well known to impact bone marrow production during skeletal development and increase bone brittleness, thereby inciting the susceptibility to fracture.11,16,17 Thus, investigating whether the addition of CHM to conventional sarcopenia treatment may be helpful in preventing or delaying osteoporotic fracture.
After a thorough literature review, we found that no study has been published regarding the long-term effect of CHM in mitigating fracture risk among sarcopenia patients. To investigate this issue, we exploited a cohort study via a nationwide claims database. Results of this study could provide valuable information on the compatibility and clinical application of CHM, enabling healthcare providers to timely integrate CHM into conventional therapy for sarcopenia persons.
Materials and Methods
Data Source and Identification of Study Participants
At present, nearly 99% of Taiwan’s population is enrolled in the National Health Insurance (NHI) program of the National Health Insurance Administration Ministry in Taiwan. In this exploration, all analytical data were obtained from the Longitudinal Health Insurance Database (LHID), a data subset of the NHI program that includes the original claims data of 1 million insurants randomly extracted from all beneficiaries under the NHI program.18 This database holds the information on demographics, diagnoses, prescriptions, referrals, and hospitalisation for these subjects covered by the NHI program.
Firstly, from the database, we collected the patient records submitted for those aged 20 years or more, and they had at least one hospital admission with a diagnostic code of sarcopenia or three or more outpatient visits due to sarcopenia within 365 calendar days (International Classification of Diseases-Ninth Revision-Clinical Modification [ICD-9-CM] code of 728.2 and 728.9).19 This investigation was carried out based on the Helsinki Declaration and was approved by the local institutional review board of Buddhist Dalin Tzu Chi Hospital (No. B10803015-1). The institutional review board also waived the need for informed consent since the raw data used were on the basis of a retrospective claims data with encrypted attribution.
Identification of Osteoporotic Fracture Outcome
The primary outcome in this study was first-time diagnosis of osteoporotic fracture, which was defined as the first appearance in the inpatient or outpatient claim records of ICD-9-CM codes 733.1 and 805–829 or fracture-related surgery indicated by ICD-9-CM code 78.1 (application of external fixation device), 78.4 (other repair or plastic operation on bone), 78.5 (internal fixation of bone without fracture reduction), 78.9 (insertion of bone growth stimulator), 79 (reduction of fracture and dislocation), or 81 (repair and plastic operations on joint structures).20 To ascertain the causal estimates, we removed the enrollees if they had the diagnosis of fracture before sarcopenia (n = 8317), as well as those who had missing values or were followed up for less than 365 days after cohort entry (n = 896). The final cohort comprised 13,985 enrollees with sarcopenia (Figure 1).
 |
Figure 1 Flowchart showing the method of selecting and following study subjects. |
Definition of CHM Use
To define patients’ CHM use, we reviewed individual CHM treatment records from cohort entry date to index date. In this study, CHM users were defined as those who ever received the relevant CHM treatment for a period of 31 days or longer, due to a diagnosis of sarcopenia. To reduce selection bias arising from participation/non-participation in CHM treatment, we randomly selected a comparison cohort via the propensity score matching on a 1:1 ratio. The predicted probability of participating in treatment by CHM was calculated by a logistic regression model based on the enrollee’s baseline characteristics (Table 1). Afterwards, we categorized the cumulative days of CHM treatment as follows: non-CHM use (<31 days), CHM use for 31–365 days, CHM use for 366–730 days, and CHM use for more than 730 days. This approach would allow us to carefully determine the duration-dependent effect of CHM in preventing fracture chance. Moreover, we calculated person-years (PY) of CHM use starting from initiation of CHM treatment to correct for immortal time for subjects who received CHM.21 The index date of the follow-up period for non-CHM users was assigned as the date of the first sarcopenia diagnosis, whereas that for CHM users was the time of the first CHM prescription. All participants were followed up until the end of 2013 to measure the incidence of osteoporotic fracture.
 |
Table 1 Patient Demographic Data and Comorbidities |
Information Regarding Covariates
Covariates in the statistical analysis included age, sex, monthly income, prior comorbidities, and urbanization of individual residential area. With regard to the monthly income, it was transformed to ordinal variables based on individual income-related insured amount as follows, namely New Taiwan Dollars [NTD] ≦17,880, 17,881–40,000 and ≥400,001. Furthermore, the urbanization degree was classified into three types of settlements based on former rule, which comprised cities, towns and semi-dense areas, and rural areas.22 Baseline comorbidities were defined as occurring within one year preceding sarcopenia onset, and all of them were calculated by the established Charlson-Deyo comorbidity index (CCI).23 The Deyo-adapted CCI incorporated 17 diseases and each disease was rated on the scale of 1–6, with higher total scores indicating more severe comorbid disease burden. As for medication usage, we stratified all enrollees into two groups based on if they ever received, or not received, any one of the anti-osteoporotic medications, which included calcium supplements, vitamin D, calcitonin, bisphosphates, selective estrogen receptor modulators (SERMs), sex hormones, strontium and RANKL inhibitors for more than six months.
Statistics
In all comparisons, P value < 0.05 was considered significant. Firstly, we reported continuous variables using mean and standard deviation (SD) and categorical variables using frequencies and percentages. Distributions of sociodemographic data and number of comorbidities between two groups were compared with the standardized differences. It is more appropriate for comparing the balance of covariate distribution between treated and untreated groups as it is not subject to the impact of sample size.24 Incidence rate of osteoporotic fracture was presented as the number of cases per 1000 PY. The Kaplan–Meier method was employed to compare the cumulative incidence of osteoporotic fracture between two groups, and a Log rank test was applied to examine the significance level of differences between the two groups. We used Cox proportion hazard regression analyses to estimate hazard ratio (HR) and 95% confidence interval (CI) of osteoporotic fracture for patients with sarcopenia, using the non-CHM users as the reference group. The proportional hazards assumption was evaluated by testing the Schoenfeld residuals and constructing a log–log plot, and based on these, it was concluded that there was no evidence of violation. All of the analyses were carried out using SAS version 9.3 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Baseline and Demographic Characteristics of the Study Population
The inclusive studied cohort was comprised of 2822 CHM users and 2822 non-CHM users. The mean age of patients was 47.6 ± 14.9 years, with female predominance (53.2%). Most of the enrollees had monthly incomes of NTD less than 17,880 (49.6%) and lived in urbanized areas (57.4%) (Table 1). After the matching procedure, there were no significant differences between the treated CHM and untreated CHM cohorts in age, sex, comorbidities, geographic region, urbanization level and medication usage (Table 1).
CHM Use and Subsequent Risk of Osteoporotic Fracture Among Enrollees
Review of the total cohort identified 3273 cases of osteoporotic fracture, 1655 in non-CHM users and 1618 in CHM users during follow-up periods of 10567.96 PY and 13347.88 PY, respectively. Incidence of fracture during the study period was 156.61 per 1000 PY among non-CHM users as compared to 121.22 per 1000 PY among CHM users. Those receiving CHM services had a reduced risk of osteoporotic fracture after controlling for baseline characteristics (adjusted HR, 0.77; 95% CI, 0.73–0.85) (Table 2). Subgroup analysis also revealed a duration-dependent inverse relation between the CHM use period and fracture risk (Table 2). Those receiving CHM treatment in addition to the conventional treatment for more than two years had a marked 73% reduction in fracture risk. Kaplan–Meier survival curve analysis and Log rank tests revealed a significant difference in osteoporotic-fracture-free survival across the four groups (p < 0.001) (Figure 2).
 |
Table 2 Risk of Osteoporotic Fracture Among Sarcopenia Patients with and without CHM Use |
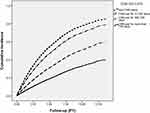 |
Figure 2 Cumulative incidence of osteoporotic fracture across four groups. |
Association of CHM Use with Subsequent Risk of Osteoporotic Fracture by Sex and Age
According to the multivariable analysis stratified by age and sex, we noted that benefit of CHM therapy in reducing osteoporotic fracture was more predominant in females, with an adjusted HR of 0.72 (95% CI, 0.67–0.78) (Table 3). Among the commonly used herbal products, some prescriptions were related to decreased risk of osteoporotic fracture, containing Caulis Spatholobi, Xuduan, Duzhong, Danshen, Shu-Jing-Huo-Xue-Tang (SJHXT), Du-Huo-Ji-Sheng-Tang (DHJST), Shao-Yao-Gan-Cao-Tang (SYGCT), and Shen-Tong-Zhu-Yu -Tang (STZYT) (Figure 3).
 |
Table 3 Incidence and Osteoporotic Fracture Risk for Sarcopenia Patients with and without CHM Use Stratified by Sex and Age |
Discussion
Faced with no specific treatments for the prevention of fracture incident after onset of sarcopenia, seeking promptly complementary therapies in managing the bone-muscle crosstalk appears to be the privileged direction in clinical practice. Few studies, if any, have been conducted to explore the long-term effect of CHM on the reduction of osteoporosis fracture incident among sarcopenia subjects. We observed that if individuals with sarcopenia received CHM treatment in addition to the conventional treatment, they would experience a lower risk of osteoporotic fracture than did those who did not receive CHM treatment. On top of that, the longer the duration of CHM use, the greater more notable impact of CHM in reducing incident osteoporotic fracture. Those who received CHM treatment for more than two years had a substantially lower risk of osteoporotic fracture by 73%, as compared to those who did not receive CHM. Despite the lack of comparable studies, the beneficial effect of CHM in preventing osteoporotic fracture observed herein contributes to a growing body of evidence indicating the clinical efficacy of CHM for individuals with chronic diseases.16,25
Findings from the present study indicate that female patients benefited more from CHM use than did males. As others have shown, females often possess better knowledge, attitudes, and self-care practices than males;26 accordingly, they may tend to adhere to the prescribed medical regimen to minimize the likelihood of osteoporotic fracture. Another possible reason for this may be tied to the production of sex hormones, especially estrogen. Decline of estrogen would contribute to the releases of pro-inflammatory mediators, such as IL-6 and TNF-α,27 both of which are viewed as underlying mechanism for osteoporotic fracture.11,12
A major contribution of this study is the identification of specific herbal products that may exert therapeutic effects in reducing susceptibility to osteoporotic fracture. Of the single-herb products commonly used to treat sarcopenia, Caulis Spatholobi may decrease the vulnerability to bone fracture. This reaction may have several scientific explanations behind it. First, a recent in vivo study found that Caulis Spatholobi stems inhibit lipopolysaccharide-induced production of pro-inflammatory cytokines in murine macrophage cells.28 Second, another pharmacological study reported that formononetin, a major component of Caulis Spatholobi, may dose-dependently suppress the proliferation and differentiation of primary mononuclear macrophages and osteoclast activation.29 These explanations may have been put forward to explain the protective effect of Caulis Spatholobi.
The present study also identified Xuduan and Danshen as agents that may offer protection against osteoporotic fracture. These two remedies have long been used in CHM for the treatment of bone diseases.30,31 Salvianolic acid B and asperosaponin VI, two major compounds purified from these herbal products, have been found to substantially induce osteoblast maturation and increase bone formation, mainly through the activation of PI3K/AKT osteogenic pathway.31,32 This pathway plays an indispensable role in the survival, proliferation, migration, and differentiation of bone mesenchymal stem cells. Furthermore, both in vivo and in vitro studies implicated the PI3K/AKT signaling pathway in the inhibition of articular cartilage destruction.33
Another herbal product proven effective in lessening osteoporotic fracture risk in this study is Duzhong. In a study involving rats fed 100 mg/kg Duzhong twice per day, for four days, this herb was found to significantly promote osteocalcin and increase the releases of alkaline phosphatase along with collagen I in osteoblasts via regulation of RANKL-induced NF-κB signaling pathway.34 There are also data that suggested this pathway plays the role in aggressiveness of immune cells and osteoclast formation.17
Findings of this study indicate that individuals who used SJHXT and SYGCT had a lower incidence of fracture. Clinically, these two herbs are often prescribed to arthritis patients for the treatment of muscle pain. An earlier study in a rodent model revealed that SJHXT could exert anti-inflammatory and analgesic effects by modulating α-2 adrenoceptor activity.35 A review article reported that dysregulation of the α2- adrenoceptor pathway would contribute to aberrant cytokine gene expression.36 Additionally, SYGCT is often used to relieve muscle cramps in arthritis patients. One recent report by Chang et al showed that this compound markedly ameliorated the inflammatory state in rats with polycystic ovary syndrome by blocking activation of the TLR4/NF-κB signaling pathway.37 Under pathological conditions such as inflammation, activated B cells and T cells are known to secrete large concentrations of RANKL, which in turn increases osteoclastogenesis and bone loss.17,33
We also identified positive therapeutic effects of DHJST together with STZYT on the subsequent predisposition to fracture. Both animal and human studies have provided evidence that herbs such as gentianine from DHJST have anti-inflammatory properties.38 The mechanism by which this ingredient exerts potent anti-inflammatory effect may include the inhibition of Rho/NF- κB signaling pathway activation.39 We observed that STZYT was associated with a decreased risk of osteoporotic fracture as well. In one recent animal study, this decoction resulted in marked inhibition of the inflammatory response and alleviated the symptoms of arthritis through the MAPK p38/PPARγ/CTGF signaling pathway.40 This pathway was involved in a diverse array of cellular processes, including inflammation, angiogenesis, and cell proliferation,41 and is also implicated in the release of myokines,8 thus playing a decisive role in the generation of bone loss.
Despite being a pioneer study in exploring the effect of CHM on risk of developing osteoporotic fracture for sarcopenia patients, this study has several noteworthy limitations. First, data used in this work are from a claims-based database; accordingly, no detailed information regarding biochemical data, family history, lifestyle behaviors, or body weight was recorded in the database. Thus, it is inevitable that residual confounding by a few of these factors may partially bias the association herein. Therefore, a large cohort size of sarcopenia patients and adopting prospective randomized trials are warranted to explore the potential mechanisms underpinning the clinical benefits of CHM. Second, in this study, all enrollees were assigned a diagnosis based on the ICD-9-CM code only, thus risking an inaccurate diagnosis of disease. To ameliorate this issue, we capitalized on procedural claims data to confirm ambulatory diagnostic codes along with inpatient claims data to minimize the possibility of misclassification. On this note, it should also be highlighted that probability of exposure being misclassified is independent of disease status and the probability of disease status being misclassified is independent of exposure status, which in turn leads to an underestimate (dilution) of the true strength of an association between exposure and disease. Third, a surveillance bias might arise since the CHM users would be more likely to seek additional healthcare services as compared to those who did not receive CHM treatment. To confront this concern, the frequency of medical visits for each participant was estimated and inserted into the multivariate regression model. The reanalysis supported that the positive effect of CHM remained invariable after adjusting the surveillance bias, with an adjusted HR of 0.78 (95% CI, 0.73–0.84). These limitations notwithstanding, this study has several strengths that bolster its merits. The first strength stems from the use of a large population database. Over 90% of the Taiwanese population and healthcare providers are covered by the NHI program, which included a representative Taiwanese sample, leaving little room for non-response or loss to follow-up. The second strength is the employment of a long observation time. Given that fracture is one of the major contributors to disability and the need for medical care, a 10-year observation period and the large sample size used in this work ensure a better empirical assessment of the treatment employed. The third strength relates to the application of propensity score matching to select the subjects with and without CHM exposure, thus reducing the possibility of confounding by indication.
Conclusion
In sum, this population-based cohort study depicted that, during conventional treatment for sarcopenia, the integration of CHM would nearly reduce the chance of osteoporotic fracture by 23%. Long-term use of CHM may potently bring the inverse association with fracture risk among persons with sarcopenia. In addition to the positive effect of CHM reported herein, the findings of this study further indicated those commonly prescribed herbal products that are likely to be associated with lower osteoporotic fracture risk, thus paving the way for further pharmacological investigations.
Abbreviations
CHM, Chinese herbal medicine; HRs, hazard ratios; IL, interleukin; TNF-α, tumor necrosis factor-α; NF-kB, nuclear factor-kappaB; RANKL, receptor activation of nuclear factor-kappaB ligand; NHI, National Health Insurance; LHID, Longitudinal Health Insurance Database; ICD-9-CM, International Classification of Diseases-Ninth Revision-Clinical Modification; PY, person-years; NTD, New Taiwan Dollars; CCI, Charlson–Deyo comorbidity index; SD, standard deviation; CI, confidence interval; SJHXT, Shu-Jing-Huo-Xue-Tang; DHJST, Du-Huo-Ji-Sheng-Tang; SYGCT, Shao-Yao-Gan-Cao-Tang; STZYT, Shen-Tong-Zhu-Yu –Tang.
Acknowledgments
This study uses data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes, Taiwan. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. WJC, HL, HHL and YHW contributed equally to this work.
Funding
This project was supported by the Tzu Chi Medical Foundation (TCMF-CM2-111-07).
Disclosure
The authors declare that they have no conflicting interests.
References
1. Dhillon RJ, Hasni S. Pathogenesis and management of sarcopenia. Clin Geriatr Med. 2017;33(1):17–26. doi:10.1016/j.cger.2016.08.002
2. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. 2017;16:21. doi:10.1186/s40200-017-0302-x
3. Goates S, Du K, Arensberg MB, Gaillard T, Guralnik J, Pereira SL. Economic impact of hospitalizations in US adults with sarcopenia. J Frailty Aging. 2019;8(2):93–99. doi:10.14283/jfa.2019.10
4. Giudice J, Taylor JM. Muscle as a paracrine and endocrine organ. Curr Opin Pharmacol. 2017;34:49–55. doi:10.1016/j.coph.2017.05.005
5. An HJ, Tizaoui K, Terrazzino S, et al. Sarcopenia in autoimmune and rheumatic diseases: a comprehensive review. Int J Mol Sci. 2020;21(16):5678. doi:10.3390/ijms21165678
6. Schaap LA, van Schoor NM, Lips P, Visser M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: the longitudinal aging study Amsterdam. J Gerontol a Biol Sci Med Sci. 2018;73(9):1199–1204. doi:10.1093/gerona/glx245
7. Yeung SSY, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10(3):485–500. doi:10.1002/jcsm.12411
8. Nishikawa H, Fukunishi S, Asai A, Yokohama K, Nishiguchi S, Higuchi K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int J Mol Med. 2021;48(2):156. doi:10.3892/ijmm.2021.4989
9. Lin HC, Lin CL, Huang WY, et al. The use of adjunctive traditional Chinese medicine therapy and survival outcome in patients with head and neck cancer: a nationwide population-based cohort study. QJM. 2015;108(12):959–965. doi:10.1093/qjmed/hcv079
10. Zhang H, Hu Y, Chen X, et al. Expert consensus on the bone repair strategy for osteoporotic fractures in China. Front Endocrinol. 2022;13:989648. doi:10.3389/fendo.2022.989648
11. Tazawa R, Uchida K, Fujimaki H, et al. Elevated leptin levels induce inflammation through IL-6 in skeletal muscle of aged female rats. BMC Musculoskelet Disord. 2019;20(1):199. doi:10.1186/s12891-019-2581-5
12. Abu-Amer Y. NF-κB signaling and bone resorption. Osteoporos Int. 2013;24(9):2377–2386. doi:10.1007/s00198-013-2313-x
13. Guo J, Wang F, Hu Y, et al. Exosome-based bone-targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. Cell Rep Med. 2023;4(1):100881. doi:10.1016/j.xcrm.2022.100881
14. Beaudart C, Zaaria M, Pasleau F, Reginster J-Y, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017;12(1):e0169548. doi:10.1371/journal.pone.0169548
15. Li W, Huang H, Zhang Y, et al. Anti-inflammatory effect of tetrahydrocoptisine from Corydalis impatiens is a function of possible inhibition of TNF-α, IL-6 and NO production in lipopolysaccharide-stimulated peritoneal macrophages through inhibiting NF-κB activation and MAPK pathway. Eur J Pharmacol. 2013;715(1):62–71. doi:10.1016/j.ejphar.2013.06.017
16. Wang K, Zhang D, Liu Y, et al. Traditional Chinese medicine formula Bi-Qi capsule alleviates rheumatoid arthritis-induced inflammation, synovial hyperplasia, and cartilage destruction in rats. Arthritis Res Ther. 2018;20(1):43. doi:10.1186/s13075-018-1547-6
17. Weitzmann MN. Bone and the immune system. Toxicol Pathol. 2017;45(7):911–924. doi:10.1177/0192623317735316
18. Center for Biomedical Resources of NHRI. National health insurance research database. Taiwan: LHID; 2012. Available from: http://nhird.nhri.org.tw/en/index.htm.
19. Lin MH, Chiu SY, Chang PH, Lai YL, Chen PC, Ho WC. Hyperlipidemia and statins use for the risk of new diagnosed sarcopenia in patients with chronic kidney: a population-based study. Int J Environ Res Public Health. 2020;17(5):1494. doi:10.3390/ijerph17051494
20. Lin TK, Liou YS, Lin CH, Chou P, Jong GP. High-potency statins but not all statins decrease the risk of new-onset osteoporotic fractures: a nationwide population-based longitudinal cohort study. Clin Epidemiol. 2018;10:159–165. doi:10.2147/CLEP.S145311
21. Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol. 2008;19(5):841–843. doi:10.1681/ASN.2007121354
22. Liu CY, Hung YT, Chuang YL, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag. 2006;4(1):1–22.
23. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi:10.1016/0895-4356(92)90133-8
24. Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27(12):2037–2049. doi:10.1002/sim.3150
25. Matos LC, Machado JP, Monteiro FJ, Greten HJ. Understanding traditional Chinese medicine therapeutics: an overview of the basics and clinical applications. Healthcare. 2021;9(3):257. doi:10.3390/healthcare9030257
26. Shih CC, Liao CC, Su YC, Tsai CC, Lin JG. Gender differences in traditional Chinese medicine use among adults in Taiwan. PLoS One. 2012;7(4):e32540. doi:10.1371/journal.pone.0032540
27. Shivers KY, Amador N, Abrams L, Hunter D, Jenab S, Quiñones-Jenab V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine. 2015;72(2):121–129. doi:10.1016/j.cyto.2015.01.007
28. Tu NT, Huyen CT, Duc NV, et al. Anti-inflammatory secondary metabolites from the stems of millettia dielsiana harms ex diels. Carbohydr Res. 2019;484:107778. doi:10.1016/j.carres.2019.107778
29. Hong YB, Jiang H, Wang JW, Yu PF, You WL. Experimental study on the inhibition of Formononetin on the differentiation of osteoclasts induced by RANKL. Zhongguo Gu Shang. 2020;33(1):64–70. doi:10.3969/j.issn.1003-0034.2020.01.012
30. Tao Y, Chen L, Yan J. Traditional uses, processing methods, phytochemistry, pharmacology and quality control of dipsacus asper Wall. ex C.B. Clarke: a review. J Ethnopharmacol. 2020;258:112912. doi:10.1016/j.jep.2020.112912
31. Tan Q, Liu Y, Lei T, et al. Study on the mechanism of salvia miltiorrhiza in the treatment of traumatic bone defects. J Chemistry. 2021;2021:8646394. doi:10.1155/2021/8646394
32. Ke K, Li Q, Yang X, et al. Asperosaponin VI promotes bone marrow stromal cell osteogenic differentiation through the PI3K/AKT signaling pathway in an osteoporosis model. Sci Rep. 2016;6:35233. doi:10.1038/srep35233
33. Li X, Wang L, Huang B, et al. Targeting actin-bundling protein L-plastin as an anabolic therapy for bone loss. Sci Adv. 2020;6(47):eabb7135. doi:10.1126/sciadv.abb7135
34. Liang H, Yu F, Liu X, Yuan B, Zhao Z, Wu S. Effect of eucommia ulmoides extract on osteoblast proliferation. Trop J Pharm Res. 2017;16(11):2675–2679. doi:10.4314/tjpr.v16i11.15
35. Shu H, Arita H, Hayashida M, et al. Anti-hypersensitivity effects of Shu-jing-huo-xue-tang, a Chinese herbal medicine, in CCI-neuropathic rats. J Ethnopharmacol. 2010;131(2):464–470. doi:10.1016/j.jep.2010.07.004
36. Scanzano A, Cosentino M. Adrenergic regulation of innate immunity: a review. Front Pharmacol. 2015;6:171. doi:10.3389/fphar.2015.00171
37. Chang ZP, Deng GF, Shao YY, et al. Shaoyao-gancao decoction ameliorates the inflammation state in polycystic ovary syndrome rats via remodeling gut microbiota and suppressing the TLR4/NF-κB pathway. Front Pharmacol. 2021;12:670054. doi:10.3389/fphar.2021.670054
38. Huang CY, Cheng CJ, Chiou WF, Chang WC, Kang YN, Lee MH. Efficacy and safety of Duhuo Jisheng Decoction add-on bisphosphonate medications in patients with osteoporosis: a meta-analysis of randomized controlled trials. J Ethnopharmacology. 2022;283:114732. doi:10.1016/j.jep.2021.114732
39. Wenjin C, Jianwei W. Protective effect of gentianine, a compound from Du Huo Ji Sheng Tang, against freund’s complete adjuvant-induced arthritis in rats. Inflammation. 2017;40(4):1401–1408. doi:10.1007/s10753-017-0583-8
40. Han Y, Wang J, Jin M, Jia L, Yan C, Wang Y. Shentong zhuyu Decoction inhibits inflammatory response, migration, and invasion and promotes apoptosis of rheumatoid arthritis fibroblast-like synoviocytes via the MAPK p38/PPARγ/CTGF pathway. Biomed Res Int. 2021;2021:6187695. doi:10.1155/2021/6187695
41. Baeza-Raja B, Muñoz-Cánoves P. p38 MAPK-induced nuclear factor-kappaB activity is required for skeletal muscle differentiation: role of interleukin-6. Mol Biol Cell. 2004;15(4):2013–2026. doi:10.1091/mbc.e03-08-0585
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

