Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
Use of a 4-week up-titration regimen of roflumilast in patients with severe COPD
Authors Watz H, Bagul N, Rabe KF , Rennard S, Alagappan VKT, Román J, Facius A , Calverley PMA
Received 13 October 2017
Accepted for publication 28 December 2017
Published 6 March 2018 Volume 2018:13 Pages 813—822
DOI https://doi.org/10.2147/COPD.S154012
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Henrik Watz,1 Nitin Bagul,2 Klaus F Rabe,3,4 Stephen Rennard,5,6 Vijay KT Alagappan,7 Jonas Román,8 Axel Facius,9 Peter MA Calverley10
1Pulmonary Research Institute at LungenClinic Grosshansdorf, Airway Research Center North, German Center for Lung Research, Grosshansdorf, Germany; 2DNA Medical Ltd, Langley, UK; 3Department of Pulmonary Medicine, LungenClinic Grosshansdorf, Airway Research Center North, German Center for Lung Research, Grosshansdorf, 4Department of Medicine, Christian Albrecht University Kiel, Kiel, Germany; 5Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA; 6AstraZeneca, Cambridge, UK; 7AstraZeneca, Gaithersburg, MD, USA; 8AstraZeneca R&D, Gothenburg, Sweden; 9thinkQ2 AG, Baar, Switzerland; 10Department of Clinical Sciences, Institute of Ageing and Chronic Disease, University of Liverpool, Liverpool, UK
Background: The oral selective phosphodiesterase-4 inhibitor roflumilast (ROF) reduces exacerbations in patients with severe COPD. Adverse events (AEs) can cause early ROF discontinuation. Alternative dosing strategies may help patients continue their therapy.
Methods: In this multicenter, double-blind trial, 1,321 patients with severe COPD were randomized 1:1:1 to 4 weeks’ treatment with ROF 250 µg once daily (OD), 500 µg every other day (EOD), or 500 µg OD, each followed by ROF 500 µg OD for 8 weeks, plus standard therapy. The primary end point was the percentage of patients prematurely discontinuing study treatment.
Results: Patients in the 250 µg OD/500 µg OD group had significantly fewer treatment discontinuations (odds ratio [OR] 0.66 [95% CI 0.47–0.93], p=0.017) and lower rates of AEs of interest such as diarrhea, nausea, headache, decreased appetite, insomnia and abdominal pain (OR 0.63 [95% CI 0.47–0.83], p=0.001) compared with those in the 500 µg OD group. Although rates of discontinuation and AEs of interest were numerically lower with ROF 500 µg EOD/500 µg OD, the difference was not significant (OR 0.76, p=0.114, and OR 0.78, p=0.091, respectively) compared with ROF 500 µg OD.
Conclusion: A dose of ROF 250 µg OD for 4 weeks before escalation to the approved maintenance dose of 500 µg OD resulted in reduced treatment discontinuation and improved tolerability.
Keywords: roflumilast, COPD, discontinuation, adverse event
Introduction
Severe exacerbations of COPD are associated with a poor prognosis.1–3 Roflumilast (ROF) is a selective, oral phosphodiesterase-4 (PDE4) inhibitor used for the treatment of patients with severe COPD associated with chronic bronchitis and a history of exacerbations.4 Previous studies have shown that ROF as an add-on to inhaled COPD therapy reduces exacerbations in this patient population.5,6 More recently, this has been shown in the ROF and Exacerbations in patients receiving Appropriate Combination Therapy (REACT) study – in patients using ROF therapy in addition to an inhaled corticosteroid (ICS)/long-acting beta agonist (LABA) ± long-acting muscarinic antagonist (LAMA) combination7 – a finding most evident in those with a history of hospitalization.8
Patients initiating treatment with the approved 500 μg dose of ROF may report side effects in the first few weeks, including diarrhea, nausea, headache, insomnia, abdominal pain, loss of appetite and a reduction in body weight.4,6,9 These are predominantly mild to moderate in severity and, with the exception of body weight reduction, typically transient – often resolving within the first few weeks of treatment.10 However, they are a common cause of early treatment discontinuation. Overall rates of discontinuations for patients taking 500 μg of ROF in recent 52-week clinical trials have been in the region of 30%,7,11 although the rates of discontinuation are thought to be higher in clinical practice.12 Therefore, alternative dosing strategies to improve tolerability over the first few weeks of treatment may help patients continue their therapy.
The present study, referred to as OPTIMIZE (ClinicalTrials.gov: NCT02165826), investigated whether treatment discontinuation rates could be reduced and tolerability could be improved by using a reduced dose of ROF for a short initial treatment period. Phase I dose-ranging and modeling studies13 have previously suggested that a daily dose of 250 μg is associated with an improved side effect profile compared with the approved 500 μg dose. However, the 250 μg dose is less efficacious, associated with less forced expiratory volume in 1 second (FEV1) improvement, than the 500 μg dose14,15 and is not appropriate for long-term maintenance therapy.
The 12-week OPTIMIZE study evaluated the tolerability and discontinuation rate associated with a daily dose of 250 μg ROF for the first 4 weeks, before escalation to 500 μg for 8 weeks. In a parallel treatment arm, 500 μg was given on alternate days for the first 4 weeks of treatment, before increasing to 500 μg daily. The results of both up-titration strategies were compared with 500 μg dose daily, taken continuously for 12 weeks. Pharmacokinetic analyses were undertaken to evaluate drug exposure in patients receiving the three treatment strategies to assess any correlation between drug exposure and ability to tolerate ROF.
Methods
Patients
Patients aged ≥40 years with a history of COPD associated with chronic productive cough, ≥1 moderate or severe exacerbation in the previous 12 months, and who were former/current smokers (history of ≥10 pack-years) were eligible for enrollment. A post-bronchodilator FEV1 ≤50% of predicted and an FEV1/forced vital capacity (FVC) ratio <70% were required. Patients had to be receiving standard of care COPD treatment (LABA or LAMA or a combination of the two for at least 12 weeks). Patients were excluded if they had a COPD exacerbation ongoing at screening, a lower respiratory tract infection unresolved within 4 weeks prior to screening, asthma/other relevant lung disease, or known α1-antitrypsin deficiency (refer Supplementary materials for the full list of inclusion/exclusion criteria). ICS and theophylline were permitted if taken at a constant daily dose for 12 weeks prior. All patients involved in the OPTMIZE trial provided their written informed consent.
Study design and interventions
OPTIMIZE was a multicenter, double-blind, Phase III randomized trial conducted over 12 weeks, which included an initial 4-week up-titration period. All patients discontinuing the trial were offered the opportunity to enter an 8-week open-label down-titration phase, during which they received ROF 250 μg OD (Figure S1).
Patients were randomized (by a computerized interactive voice response system/interactive web response system) to one of three treatment regimens (1:1:1) (Figure S1): ROF 250 μg once daily (OD) for 4 weeks and then 500 μg OD thereafter (250 μg OD/500 μg OD), ROF 500 μg every other day (EOD) for 4 weeks and then 500 μg OD thereafter (500 μg EOD/500 μg OD) or ROF 500 μg OD for 12 weeks (500 μg OD). Patients continued receiving their usual maintenance therapy.
The first 4 weeks (up-titration) of the trial were double blinded, and the remaining 8 weeks (maintenance period) were single blinded, with only the sponsor and investigators aware that the patient was receiving ROF 500 μg OD. The original randomized treatment regimen remained blinded to all parties involved in the study for the duration of the study. ROF 250 and 500 μg and placebo were supplied as identical white, round, biplane tablets in wallet cards containing 20 tablets, with identical labeling and packaging.
Patients attended clinics at screening, randomization and Weeks 2, 4, 8 and 12. Those discontinuing and entering the down-titration phase also attended clinic on Weeks 2, 4 and 8 of the down-titration. Study medication was accounted for at each visit to assess compliance.
The study protocol was approved by each respective institutional review board and followed established good clinical practice guidelines. A list of all approving institutional review boards is available in Table S1. All patients gave written informed consent for this study.
Outcomes and end points
The primary end point was the percentage of patients prematurely discontinuing study treatment for any reason during the 12-week study period. Secondary end points included the percentage of patients with adverse events (AEs) of interest (diarrhea, nausea, headache, decreased appetite, insomnia and abdominal pain) during the trial, percentage of patients prematurely discontinuing study treatment for any reason during the down-titration phase, and change in pre-bronchodilator FEV1 during both the trial and the down-titration phase. The six types of AEs of interest used to evaluate tolerability were selected as they are the most common AEs associated with ROF treatment, the main reason for treatment discontinuation, and assumed to be related to PDE4 inhibition.
Safety assessments included monitoring AEs and assessment of Columbia-Suicide Severity Rating Scale (C-SSRS), body weight, and body mass index (BMI). AEs of interest (occurrence and intensity) were assessed on a daily basis using diary cards. Intensity (mild/moderate/severe) was evaluated using a 7-point Likert scale.
Pharmacokinetic evaluations
Pharmacokinetics (PK) of ROF and ROF N-oxide were measured using 6 mL blood samples by Pharmaceutical Product Development (Middleton, WI, USA) using a high-performance liquid chromatography tandem mass spectrometer, as described previously.16
An integrated population PK (popPK) model was developed to predict individual ROF/ROF N-oxide exposure levels and total PDE4 inhibitory activity (tPDE4i) levels and assess whether patients unable to tolerate ROF 500 μg OD have drug exposure with 250 μg OD, similar to patients on a 500 μg OD dose. The integrated popPK model was developed from an earlier base model in which the structural parameters were fixed to estimates from a dataset of 21 Phase I and two Phase II/III studies.16 This base model used combined REACT/OPTIMIZE datasets.7,17
Statistical analyses
A hierarchical testing approach was followed; if significance (5%) was not reached, subsequent tests were exploratory. The hierarchy of testing for the null hypothesis was first: if the percentage of patient discontinuations on ROF 250 μg OD/500 μg were not lower than or equal to that on ROF 500 μg OD by Week 12, then the percentage of patient discontinuations on ROF 500 μg EOD/500 μg OD were not lower than or equal to that on ROF 500 μg OD by Week 12 (refer Supplementary materials for full list).
The primary end point and secondary safety end points were based on the safety analysis set (SAS) and performed using a logistic regression model. For the primary end point and AEs of interest, the treatment odds ratio (OR) and relative risk (RR) between groups18 were calculated. The secondary efficacy end point, change in pre-bronchodilator FEV1, was based on the full analysis set (FAS) and analyzed using an analysis of covariance model.
Additional post hoc exploratory analyses were undertaken to further assess whether weight loss on ROF was related to, 1) baseline BMI, and 2) gastrointestinal AEs and/or decreased appetite (no formal statistical testing performed).
For calculation of sample size, rates of discontinuation for any reason were estimated based on pooled data from the ROF COPD pivotal studies,6,9 which included a similar patient population to the OPTIMIZE study. Assuming a discontinuation rate of 20% with ROF 500 μg OD and 13% with either up-titration regimen, a total of 441 patients per treatment arm, 1,323 overall, would provide 80% power to declare superiority of each of ROF 250 μg OD/500 μg OD and ROF 500 μg EOD/500 μg OD versus ROF 500 μg OD for 12 weeks.
Results
Patients
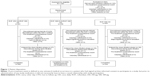
The study was conducted at 161 sites across 15 countries (refer Supplementary materials for full list) between April 2014 and October 2015. In total, 1,323 patients were randomized, of whom 1,321 received treatment. A total of 104 patients entered the down-titration phase (Figure 1). Data presented hereafter are for the 12-week trial population; data on the down-titration phase are included in the Supplementary materials.
Baseline characteristics were generally balanced across the treatment arms (Table 1). Patients had a mean age of 64.6 years and were predominantly male (74.4%). Compliance with study medication ranged from 101% to 104% across treatment arms. Demographic and baseline characteristics of patients who entered the down-titration phase are presented in Table S2.
Study discontinuations
The greatest between-group difference in discontinuations occurred in the first few weeks of treatment (Figure 2A). Significantly fewer patients discontinued treatment (for any reason) in the ROF 250 μg OD/500 μg OD group compared with the ROF 500 μg OD group (18.4% versus 24.6%; RR 0.72 [95% CI 0.54–0.95], OR 0.66 [95% CI 0.47–0.93], p=0.017; Figure 2B). There were fewer treatment discontinuations in the ROF 500 μg EOD/500 μg OD group compared with the ROF 500 μg OD group, but this difference did not reach statistical significance (20.1% versus 24.6%; RR 0.81 [95% CI 0.62–1.05], OR 0.76 [95% CI 0.55–1.07], p=0.114; Figure 2). Based on the hierarchical testing approach, all interpretations of p-values after the second hierarchical test are exploratory.
Tolerability
Significantly fewer patients experienced AEs of interest in the ROF 250 μg OD/500 μg OD arm compared with the ROF 500 μg OD arm (45.4% versus 54.2%; RR 0.79 [95% CI 0.66–0.91], OR 0.63 [95% CI 0.47–0.83], p=0.001; Figure 3). The frequency of all AEs of interest was lower, and the median time to onset was longer in the ROF 250 μg OD/500 μg OD arm compared with the ROF 500 μg OD arm (Table S3). The proportion of patients reporting AEs of interest was numerically lower in the ROF 500 μg EOD/500 μg OD arm compared with the 500 μg OD arm, but the difference was not significant (48.3% versus 54.2%; OR 0.78 [95% CI 0.59–1.04, p=0.091]); (Figure 3 and Table S3). The AEs of interest were predominantly mild or moderate (Table S4) and of short duration (2–4 days; data include discontinued patients; Table S3). For each treatment arm, among patients who discontinued, the most commonly reported AE of interest was diarrhea (19.8%, 31.8%, and 33%, in the ROF 250 μg OD/500 μg OD, 500 μg EOD/500 μg OD and the 500 μg OD arms respectively; Table S5).
The percentage of patients who withdrew from treatment due to AEs was 13.2%, 15.3%, and 17.4% with ROF 250 μg OD/500 μg OD, 500 μg EOD/500 μg OD, and 500 μg OD, respectively. AEs that most commonly led to discontinuation in the ROF 250 μg OD/500 μg OD arm, 500 μg EOD/500 μg OD arm, and 500 μg OD arm, were diarrhea (3.6%, 6.4%, and 8.1% of patients, respectively), decreased appetite (3.6%, 4.6%, and 7.4% of patients, respectively), and nausea (3.9%, 3.7%, 6.1% of patients, respectively).
Overall, 63.7% of patients experienced any AE: 61.2%, 64.3%, and 65.7% with ROF 250 μg OD/500 μg OD, 500 μg EOD/500 μg OD, and 500 μg OD, respectively. The most frequently reported AEs were those assessed as AEs of interest. During the trial, 4.6% of patients experienced a serious AE and six deaths were reported (three in the 250 μg OD/500 μg OD arm [COPD, cardiac failure, and cardiopulmonary failure], one in the 500 μg EOD/500 μg OD arm [pneumothorax spontaneous], and two in the 500 μg OD arm [lung adenocarcinoma and myocardial infarction]). Suicidal ideation was reported in one patient in the ROF 500 μg OD group during the trial, and this patient discontinued treatment as a result.
Weight decrease was self-reported by 2.3%, 2.1%, and 3.8% of patients who received 250 μg OD/500 μg OD, 500 μg EOD/500 μg OD, and 500 μg OD, respectively. Reduction in body weight was evident from Week 2 and was of a similar magnitude across treatment groups (mean decrease 1.02, 0.82, and 0.98 kg, respectively; Figure 4). In post hoc analyses, weight loss tended to be greater in patients with a BMI >25 kg/m2 compared with those with a BMI ≤25 kg/m2 in all treatment groups (Table S6).
Lung function
Improvements from baseline in pre-bronchodilator FEV1 over the trial were minimally different between ROF 250 μg OD/500 μg OD, 500 μg EOD/500 μg OD, and 500 μg OD treatment groups (least squares [LS] mean change 90, 130, and 110 mL, respectively).
Pharmacokinetics
Individual PK parameters and tPDE4i values were derived for 1,238 patients with at least one quantifiable PK sample,17 including 101 patients who discontinued the 12-week trial and entered the down-titration phase, of whom 76 entered after not tolerating at least one dose of ROF 500 μg. Patients who discontinued ROF 500 μg OD in the trial because of AEs of interest had a slightly higher median tPDE4i (>10%) than those able to tolerate this dose (median tPDE4i level: 1.28 and 1.16, respectively; Figure 5). However, as expected with linear PK, reducing the dose to 250 μg OD in these patients reduced tPDE4i to below that typically observed in patients able to tolerate the 500 μg OD (median tPDE4i level: 0.65).
  | Figure 5 Comparison of tPDE4i levels achieved with ROF 500 μg or 250 μg according to whether patients were able to tolerate the dose. |
Discussion
Although the side effects that may occur soon after initiation of ROF are often transient in nature and predominantly mild to moderate in severity, they are a common reason for discontinuing treatment. Using an alternative reduced dosing regimen for a short initial treatment period (as used successfully with other systemic therapies)19–22 is one strategy to help increase the acceptability of ROF treatment and help patients stay on therapy. The OPTIMIZE study supports this concept. While use of a reduced dose of ROF as long-term maintenance therapy may not induce sufficient PDE4 inhibition to exert the clinical efficacy of the 500 μg dose,14,15 it can be used as part of an up-titration regimen to overcome tolerability issues in the first weeks of treatment and hence reduce discontinuation rates.
Initiating ROF at a 250 μg daily dose for 4 weeks, before escalating to 500 μg daily dose for 8 weeks, was associated with a statistically significant decrease in the percentage of patients discontinuing treatment for any reason and reporting side effects of interest. The overall discontinuation rate for patients taking ROF 500 μg daily in the current study was in line with that observed in recent ROF clinical trials,7,11 and for patients starting at the reduced dosing of 250 μg daily, the odds of early discontinuation and experiencing side effects of interest were 34% and 37% lower (RR reduction 28% and 21%). Patients starting ROF at 500 μg on alternate days also reported fewer side effects and lower treatment discontinuation rates, but this was not significantly different compared with the 500 μg daily regimen.
The AEs of interest selected as a focus for OPTIMIZE study have previously been identified as limiting tolerability to ROF.4,6,9 Diarrhea and other gastrointestinal side effects particularly, have been perceived as a barrier to ROF treatment. Diarrhea accounted for approximately one-third of discontinuations with the 500 μg dose, and this was lowered to approximately 20% with reduced dosing.
The mechanisms by which ROF causes nausea and diarrhea are believed to be distinct. Nausea is mediated by PDE4-D; located outside the blood–brain barrier, this subtype is accessible to ROF.23 The mechanism behind diarrhea is less clear; activators of the cystic fibrosis transmembrane conductance regulator (CFTR) are known to be potent inducers of diarrhea, and it is thought that PDE4 inhibition could activate this mechanism.24,25
Weight loss is a known systemic side effect of ROF6,9 and has been reported with both selective and nonselective PDE inhibitors.26 However, it did not appear to be a significant reason for discontinuation in OPTIMIZE, accounting for <1% of discontinuations overall. Reductions in body weight were similar between all three dosing regimens and comparable with previous studies when treatment length is taken into account.6,9 Absolute weight loss tended to be more pronounced in patients with a higher baseline BMI and in those who also experienced gastrointestinal side effects or loss of appetite with ROF (as seen previously).6 While the mechanism by which ROF causes weight decrease remains to be fully elucidated, it is thought to be related to the effects of increased cAMP on signaling pathways regulating lipolysis.15
Frequency of individual side effects of interest was always lowest in the 250 μg/500 μg OD group followed by the 500 μg EOD/500 μg OD group and then the 500 μg OD group. One exception was abdominal pain; the reason for this was unclear. Frequencies of some side effects of interest were higher in this study than those reported in previous studies;7,11 one explanation for this finding may be that OPTIMIZE, in contrast to previous studies, used a daily patient-assessed diary method to record side effects of interest. As OPTIMIZE did not include a placebo arm, the impact of the use of daily diary cards on side effect rates cannot be assessed. Additionally, levels of theophylline were not known in the 80 patients who were taking it, but may have contributed to increased reporting of side effects in these patients.
The 250 μg daily dose was selected as the appropriate reduced dose following modeling studies, suggesting an improved side effect profile, compared with the 500 μg maintenance dose.13 Lung function measurements in OPTIMIZE revealed that the initial 4-week up-titration regimens did not appear to reduce the effect of the drug on lung function. FEV1 improvements of ~100 mL in all treatment arms were observed; this improvement is at the upper range of changes compared with previous studies.6,7,9,11
Following oral dosing, ROF is rapidly converted by cytochrome P450 enzymes to its active metabolite ROF N-oxide, which contributes to ~90% of the tPDE4i activity.27,28 ROF N-oxide is primarily cleared by the enzyme CYP3A4, and a number of covariates can affect the activity.29,30 PK analyses found that patients who discontinued ROF 500 μg daily in the main 12-week trial of OPTIMIZE because of side effects of interest had a slightly higher median tPDE4i than those able to tolerate this dose. It is plausible that higher tPDE4i levels lead to more potential off-target effects of ROF such as nausea and diarrhea. However, as expected with linear PK, reducing the dose to 250 μg daily in these patients reduced tPDE4i to well below that typically observed in patients able to tolerate 500 μg daily. However, a daily dose of 250 μg should not be considered suitable as a therapeutic maintenance dose, as efficacy in exacerbation reduction has not been adequately demonstrated in clinical studies.
A recent Korean study retrospectively analyzed data from 85 patients with severe/very severe COPD taking either 500 μg or 250 μg ROF daily, up-titrated to 500 μg daily up to 3 months after. There was a trend toward fewer AEs and discontinuations with the reduced initial dose.31 Similarly, a recent PK modeling analysis13 predicted that ROF 250 μg daily and 500 μg on alternate days were associated with lower plasma drug concentrations, lower tPDE4i, and lower incidence of diarrhea, nausea, and headache compared with ROF 500 μg daily. These data support the findings of the OPTIMIZE study.
Limitations of the current study include the lack of a placebo arm, the low sample size in the down-titration phase, and a short study duration of 12 weeks compared with a long-term maintenance treatment. Nonetheless, the study population is one of the largest to investigate side effects in a targeted COPD population, and gives us confidence in the validity of our conclusions.
Conclusion
Starting ROF treatment at 250 μg daily for 4 weeks before increasing to the therapeutic maintenance dose of 500 μg daily reduced treatment discontinuations and improved the tolerability profile compared with initiating treatment at 500 μg daily. In practice, this should help patients with COPD to stay longer on treatment with the therapeutic dose. However, use of 250 μg OD as long-term maintenance therapy may not induce sufficient PDE4 inhibition to exert clinical efficacy.
Acknowledgments
Ken Nip (Takeda) provided additional statistical assistance, and Udo-Michael Goehring provided study interpretation. Ella Palmer, PhD (Synergy Vision, London, UK, supported by AstraZeneca) provided writing and editorial assistance with the preparation of this manuscript, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). This study was funded by Takeda Pharmaceutical Company and AstraZeneca. Data from the OPTIMIZE study were presented as an abstract (PA308) at the European Respiratory Society International Congress, 3–7 September 2016, London, UK. Additionally, the pharmacokinetic and pharmacodynamics data from this study were presented as an abstract (A1337) at the American Thoracic Society 2017 International Conference, 19–14 May 2017, Washington DC, USA.
Disclosure
NB was employed by Takeda Development Centre Europe Ltd, London, UK. The authors report no other conflicts of interest in this work.
References
Mullerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. | ||
Soler-Cataluna J, Martinez-Garcia M, Roman Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. | ||
Hartl S, Lopez-Campos JL, Pozo-Rodriguez F, et al. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD Audit. Eur Respir J. 2016;47(1):113–121. | ||
Wedzicha JA, Calverley PM, Rabe KF. Roflumilast: a review of its use in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:81–90. | ||
Bateman ED, Rabe KF, Calverley PM, et al. Roflumilast with long-acting {beta}2 agonists for COPD: influence of exacerbation history. Eur Respir J. 2011;38(3):553–560. | ||
Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 Study Groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685–694. | ||
Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385(9971):857–866. | ||
Rabe KF, Calverley PMA, Martinez FJ, Fabbri LM. Effect of roflumilast in patients with severe COPD and a history of hospitalisation. Eur Respir J. 2017;50(1):1700158. | ||
Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374(9691):695–703. | ||
Calverley PM, Martinez FJ, Fabbri LM, Goehring UM, Rabe KF. Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol. Int J Chron Obstruct Pulmon Dis. 2012;7:375–382. | ||
Martinez FJ, Rabe KF, Sethi S, et al. Effect of roflumilast and inhaled corticosteroid/long-acting beta2-agonist on chronic obstructive pulmonary disease exacerbations (RE(2)SPOND). A randomized clinical trial. Am J Respir Crit Care Med. 2016;194(5):559–567. | ||
Munoz-Esquerre M, Diez-Ferrer M, Monton C, et al. Roflumilast added to triple therapy in patients with severe COPD: a real life study. Pulm Pharmacol Ther. 2015;30:16–21. | ||
Lahu G, Facius A. Application of population pharmacokinetic modeling to explore the impact of alternative roflumilast dosing regimens on tolerability. Int J Clin Pharmacol Ther. 2013;51(11):832–836. | ||
Rabe KF. Roflumilast for the treatment of chronic obstructive pulmonary disease. Expert Rev Respir Med. 2010;4(5):543–555. | ||
Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163(1):53–67. | ||
Lahu G, Hunnemeyer A, Diletti E, et al. Population pharmacokinetic modelling of roflumilast and roflumilast N-oxide by total phosphodiesterase-4 inhibitory activity and development of a population pharmacodynamic-adverse event model. Clin Pharmacokinet. 2010;49(9):589–606. | ||
Facius A, Bagul N, Gardiner P, Watz H. Pharmacokinetics of a 4-week up-titration regimen of roflumilast in the OPTIMIZE study. Am J Respir Crit Care Med. 2017;195:A1337. | ||
Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. | ||
Leung LK, Patafio FM, Rosser WW. Gastrointestinal adverse effects of varenicline at maintenance dose: a meta-analysis. BMC Clin Pharmacol. 2011;11:15. | ||
Drugs.com [webpage on the Internet]. Varenicline Tablets (Dosage) – Summary of Product Characteristics (SPC) – (FDA). 2016. [updated March 2, 2017]. Available from: https://www.drugs.com/dosage/varenicline.html. Accessed March 29, 2017. | ||
Medicines.org.uk [webpage on the Internet]. Vargatef 100 mg and 150 mg Soft Capsules – Summary of Product Characteristics (SPC) – (eMC). 2016. [updated March 9, 2017]. Available from: https://www.medicines.org.uk/emc/medicine/29790. Accessed March 29, 2017. | ||
Medicines.org.uk [webpage on the Internet]. Esbriet 267 mg Hard Capsules – Summary of Product Characteristics (SPC) – (eMC). 2015. [updated August 5, 2016]. Available from: https://www.medicines.org.uk/emc/medicine/29932. Accessed March 29, 2017. | ||
Spina D. PDE4 inhibitors: current status. Br J Pharmacol. 2008;155(3):308–315. | ||
Akabas MH. Cystic fibrosis transmembrane conductance regulator. Structure and function of an epithelial chloride channel. J Biol Chem. 2000;275(6):3729–3732. | ||
Blanchard E, Zlock L, Lao A, et al. Anchored PDE4 regulates chloride conductance in wild-type and DeltaF508-CFTR human airway epithelia. FASEB J. 2014;28(2):791–801. | ||
Boswell-Smith V, Cazzola M, Page CP. Are phosphodiesterase 4 inhibitors just more theophylline? J Allergy Clin Immunol. 2006;117(6):1237–1243. | ||
Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297(1):267–279. | ||
Hauns B, Hermann R, Hunnemeyer A, et al. Investigation of a potential food effect on the pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46(10):1146–1153. | ||
Bebia Z, Buch SC, Wilson JW, et al. Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin Pharmacol Ther. 2004;76(6):618–627. | ||
Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. | ||
Hwang H, Shin JY, Park KR, et al. Effect of a dose-escalation regimen for improving adherence to roflumilast in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis (Seoul). 2015;78(4):321–325. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.