Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Updated Perspectives on the Role of Biomechanics in COPD: Considerations for the Clinician
Authors Yentes JM , Liu WY , Zhang K, Markvicka E, Rennard SI
Received 10 May 2022
Accepted for publication 24 September 2022
Published 17 October 2022 Volume 2022:17 Pages 2653—2675
DOI https://doi.org/10.2147/COPD.S339195
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Min Zhang
Jennifer M Yentes,1 Wai-Yan Liu,2,3 Kuan Zhang,4 Eric Markvicka,4,5 Stephen I Rennard6
1Department of Kinesiology & Sport Management, Texas A&M University, College Station, TX, USA; 2Department of Orthopaedic Surgery & Trauma, Máxima MC, Eindhoven, the Netherlands; 3Department of Orthopaedic Surgery & Trauma, Catharina Hospital, Eindhoven, the Netherlands; 4Department of Electrical & Computer Engineering, University of Nebraska at Lincoln, Lincoln, NE, USA; 5Department of Mechanical & Materials Engineering, University of Nebraska at Lincoln, Lincoln, NE, USA; 6Department of Internal Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, University of Nebraska Medical Center, Omaha, NE, USA
Correspondence: Jennifer M Yentes, Texas A&M University, Department of Kinesiology and Sport Management, 4243 TAMU, Gilchrist 322, College Station, TX, 77843, USA, Tel +1 979-862-5396, Email [email protected]
Abstract: Patients with chronic obstructive pulmonary disease (COPD) demonstrate extra-pulmonary functional decline such as an increased prevalence of falls. Biomechanics offers insight into functional decline by examining mechanics of abnormal movement patterns. This review discusses biomechanics of functional outcomes, muscle mechanics, and breathing mechanics in patients with COPD as well as future directions and clinical perspectives. Patients with COPD demonstrate changes in their postural sway during quiet standing compared to controls, and these deficits are exacerbated when sensory information (eg, eyes closed) is manipulated. If standing balance is disrupted with a perturbation, patients with COPD are slower to return to baseline and their muscle activity is differential from controls. When walking, patients with COPD appear to adopt a gait pattern that may increase stability (eg, shorter and wider steps, decreased gait speed) in addition to altered gait variability. Biomechanical muscle mechanics (ie, tension, extensibility, elasticity, and irritability) alterations with COPD are not well documented, with relatively few articles investigating these properties. On the other hand, dyssynchronous motion of the abdomen and rib cage while breathing is well documented in patients with COPD. Newer biomechanical technologies have allowed for estimation of regional, compartmental, lung volumes during activity such as exercise, as well as respiratory muscle activation during breathing. Future directions of biomechanical analyses in COPD are trending toward wearable sensors, big data, and cloud computing. Each of these offers unique opportunities as well as challenges. Advanced analytics of sensor data can offer insight into the health of a system by quantifying complexity or fluctuations in patterns of movement, as healthy systems demonstrate flexibility and are thus adaptable to changing conditions. Biomechanics may offer clinical utility in prediction of 30-day readmissions, identifying disease severity, and patient monitoring. Biomechanics is complementary to other assessments, capturing what patients do, as well as their capability.
Keywords: kinematics, kinetics, postural control, mechanomyography, balance, wearables
A Letter to the Editor has been published for this article.
Introduction
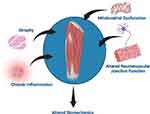
Chronic obstructive pulmonary disease (COPD) is not strictly a disease of the lungs. Extrapulmonary consequences prominently include muscle fatigue,1 muscle weakness2 and increased fall risk, which are often major patient concerns, as well as dysfunction of many other organ systems. Potential physiological explanations for alterations in skeletal muscle physiology include atrophy, fiber type shifting, mitochondrial loss and/or dysfunction, and structural changes3–8 (Figure 1). Further, patients with COPD (PwCOPD) demonstrate drastic extra-pulmonary functional decline that progresses with disease severity that may be independent of decline in lung function.9 Functional outcomes can be measured in a variety of ways. In particular, the scientific study of human movement, kinesiology, is an overarching umbrella that contains the disciplines of exercise physiology, motor control/learning, motor development, and biomechanics. In this context, functional exercise capacity, peripheral muscle strength, gait/mobility, balance, and physical activity, parameters within the scope of biomechanics, are all affected in PwCOPD.2,10–17
What is Biomechanics?
Biomechanics is defined as the scientific study of forces that act upon a body and the reactions produced. In essence, it represents “the broad interplay between mechanics and biological systems”.18 Biomechanical investigations span whole-body to tissue-level mechanics. Biomechanical areas of interest include clinical, engineering, imaging and material properties, models and robotics, sports, and animal mechanics. Clinical biomechanics, in particular, investigates injury, pathology, prosthetics, and rehabilitation. The application of biomechanics to clinical questions is extensive. Clinical research using biomechanics has included observation, diagnosis, monitoring, and rehabilitation.
Laboratory-based biomechanical analyses could be considered the microscope of movement, with accuracies of < 1 mm. Common tools or methods include the use of motion capture (kinematics), force platforms (kinetics), accelerometers, inertial measurement units, computerized dynamometers, and ultrasound (Figure 2). Motion capture is used to record where the body is in space. Using small, retroreflective markers, one can identify exactly how the body is positioned and how fast it is moving. Motion capture is used to calculate joint angles, acceleration of a limb, or even gait speed. Force platforms are used to measure the amount of force exerted by the body in three directions: vertical (synonymous with body weight when standing still), and anteroposterior and mediolateral directions (both shear forces). Force data in combination with motion capture data can provide information about torque, work, and power done at each joint. Inertial measurement units include an accelerometer, gyroscope, and magnetometer. They are found in smart phones and watches, as well as other wearables and are used for step counts and activity monitoring. In cases when motion capture is not available, they have been used to estimate body position. The most commonly known dynamometer is the hand grip dynamometer that provides a reading of grip strength. A computerized dynamometer is similar in that it will provide muscular strength or endurance information for nearly any joint. Depending on the settings, isotonic (ie, concentric or eccentric), isometric, or isokinetic strength and endurance can be tested. Rate of force production, work, and other useful measures of muscular performance can also be measured. The use of an ultrasound provides further information about the muscular tissue itself such as fascicle length and velocity as well as pennation angle (defined in Table 1).
 |
Table 1 Definitions of Biomechanical Terms |
Other techniques such as electromyography and mechanomyography have been used as well. Electromyography measures the electrical activity present during a muscle contraction. Both surface and fine-wire electromyography can be used. Surface is the most common as this uses electrodes to read the summation of electrical activity at a muscle belly. Fine-wire or intramuscular electromyography can record electrical activity of deep musculature and provide recordings of single muscle activity. Mechanomyography has been used to identify mechanical activation of muscle tissue19,20 rather than electrical activation. Typically using a microphone, the movement or displacement of a muscle can be identified.
All of the methods mentioned are typically combined with techniques from physiology such as measurement of oxygen consumption, bioelectrical impedance, and electrocardiography. In addition to traditional tools, technological improvements have allowed for the use of brain activation tools to be used during movement such as functional near-infrared spectroscopy and electroencephalography.
Starting in the last century, biomechanics focus began to be given to breathing mechanics in COPD such as force generating capacity of musculature.21 This expanded into investigations of functional outcomes in COPD such as muscle fatigue,1,22 balance,15 falling,23,24 and gait.14,25 There has even been an investigation in to the biomechanics of swallowing in PwCOPD.26 The purpose of this manuscript is to review the current knowledge regarding biomechanics in COPD and how this can be applied clinically. The status of literature regarding functional outcomes, muscle mechanics, and breathing mechanics are reviewed as well as future directions and clinical perspectives.
Functional Outcomes
Balance
Fall rates vary among older adults according to age. However, it is estimated that 30% of persons over the age of 65 years fall each year, and this percentage increases up to 50% among people over 80 years.27,28 Early studies identified an increase in fall risk and deficiencies in balance control in PwCOPD.23,24,29–32 PwCOPD are 55% more likely to have a record of a fall compared to those without COPD,33 with an estimated prevalence of COPD fallers at 30%.34 Early evidence suggests that those with a bronchitic COPD phenotype are more likely to have poorer balance (shorter single limb stance time) and higher fall risk (longer time to complete the Timed Up and Go test) compared to those with an emphysematous phenotype.35 PwCOPD experiencing an acute disease exacerbation are likely to fall more and demonstrate balance impairments beyond those with stable disease.36 Previous literature reviews reported that balance deficiencies in COPD were related to increases in age, muscle weakness, physical inactivity, and use of supplementary oxygen and related to a decrease in exercise capacity.37,38 Number of medications, restriction of recreational activities, anxiety, and depression are also related to worse balance scores in PwCOPD.39,40 A deficiency in the production of rapid force (ie, vertical jump) has been related to the number of self-reported falls in PwCOPD.41
Postural control deficits in PwCOPD compared to those without COPD include changes in sway while quietly standing16,42–46 (Table 2). Sway is typically quantified through static posturography (ie, standing on force platform while measuring a person’s sway). Every person has a natural sway when standing, and sway naturally increases when a person closes their eyes when standing. PwCOPD have greater total sway displacement16 —greater amount of sway—compared to controls. Specifically, sway in the mediolateral direction has been found to discriminate between PwCOPD and those without COPD.45,47 Both slower sway velocity11,43 and faster sway velocity in the anteroposterior direction44 have been reported in PwCOPD. Currently, in PwCOPD, it is unclear as to how velocity or speed of sway is related to postural control and/or the risk of future falls.
 |
Table 2 Studies of Balance in PwCOPD That Used Biomechanical Measures of Postural Sway |
These differences in sway are exacerbated when sensory information is disrupted such as closing their eyes, standing on foam, or vibrations applied at the ankle joint.13,15,36,43,45,46 The greatest differences in postural control between PwCOPD and controls were found when both vision and proprioception were manipulated.30 On the other hand, additional sensory information may be helpful in rehabilitation and improvement of postural control. PwCOPD that underwent whole-body vibration training for three weeks, demonstrated changes in postural sway compared to those that underwent conventional balance training.48 As opposed to sensory manipulations, no influence of an additional cognitive load while standing was found on standing postural control.49
Caution is needed when interpreting findings of sway. Many studies conclude that decreased sway with less variability is reflective of a better postural control. However, a restrictive sway, very small sway displacement and little variability, can also indicate an unhealthy state as seen in stroke survivors and persons with Parkinson’s disease.50,51 This restrictive sway is typically employed to stabilize the head and vision to compensate for other symptoms. Therefore, research studies asking subjects to stand as still as possible could provide misleading results. Changes in sway of either direction (more or less) from controls could be considered poor postural control. The authors define healthy sway as a quasi-stable state with variability in all directions, within limits.
Dynamic activities may affect balance in patients with COPD, along with a higher incidence of falls, compared to controls.52 This is likely as control of the trunk is important for dynamic postural stability. Using balance perturbations (ie, quick shoulder flexion and extension movements), PwCOPD took longer to return to pre-perturbation velocity of sway in the anteroposterior direction, and those with more severe disease had differential abdominal muscle activity (increased external oblique and rectus abdominis both at baseline and after the perturbation) compared to controls and those with less severe disease.53 This indicates a challenge of the trunk musculature to balance both respiration and control of the trunk in PwCOPD. Similar findings have been reported in which higher muscle activation of the scalenes along with greater sway velocity was recorded during one-legged standing in PwCOPD compared to controls.54 The increased respiratory demand in PwCOPD may alter the typical biomechanical role of these muscles in postural control, possibly underpinning the balance alterations in these patients. This study also reported no difference in sway performance between PwCOPD and controls when standing with eyes open, eyes closed, or on an unstable surface. However, the gluteus medius did demonstrate a higher activation in PwCOPD during standing with eyes closed, potentially indicating a higher reliance on a hip strategy during eyes closed. Trunk symmetry in the mediolateral direction was more asymmetrical in PwCOPD and was correlated with the amount of time patients could stand on one leg.55 PwCOPD have been found to require more time during a stand-to-sit task compared to a sit-to-stand task.56 The stand-to-sit requires greater postural control of the trunk to successfully complete the task, demonstrating another way in which control of balance has been shown to be challenged in PwCOPD (Table 3).
Gait
Walking is an important characteristic of many instrumental activities of daily living. An important functional measure of gait is its speed.57 In older adults, a higher risk of mortality, mobility limitation, mobility disability (eg falling), and cardiovascular disease was associated with taking longer to complete 400 m of walking.58,59 Gait speed has been associated with survival in older adults,60 hospitalization,61 and disability.62 In PwCOPD, gait speed slows with disease severity,63 and is moderately associated with lung function, dyspnea, quality of life, muscle strength, and physical activity.64,65 Gait speed at hospital discharge is a way to identify PwCOPD at risk for readmission.66 Moreover, it is possible that pulmonary rehabilitation will improve gait in PwCOPD.67,68
Despite the importance of walking, there is a dearth of studies using biomechanical tools to investigate gait patterns in PwCOPD. It is currently unclear as to the magnitude of gait deficiencies associated with the pathophysiology of COPD and whether or not these gait deficiencies are related to fall risk.69 Further, it is unknown if gait deficiencies associated with COPD are more or less severe than those associated with other diseases. One such study suggests that gait deficiencies in COPD are not as severe as those associated with peripheral artery disease.70
Due to the higher risk of falls in PwCOPD, there has been interest in investigating dynamic stability during walking. Potentially to increase stability during walking, PwCOPD walk with increased step time, shorter steps, and wider steps65,71,72 (Table 4). Ankle kinetics (ie, generating torque and absorbing power) is reportedly affected in PwCOPD compared to controls.72 PwCOPD walk with larger margins of stability compared to healthy controls, meaning that in the mediolateral direction, they have a wider lateral foot placement (from the extrapolated center of mass) compared to aged-matched controls.73 Specific, acute, walking-based, stability training appears to modify step behavior, and possibly gait stability outcomes.74
 |
Table 3 Studies of Balance in PwCOPD Using Non-Biomechanical Tests |
The six-minute walk test (6MWT) has been considered an important functional outcome in PwCOPD.10 It has been used as a surrogate for peak oxygen uptake, aerobic endurance, and exercise capacity. During a 6MWT, PwCOPD walked with slower cadence (ie, strides/min) and increased trunk acceleration variability (ie, increased between-stride time variability) compared to controls75 as well as altered electromyography of the locomotor muscles.76 However, it should be considered that the 6MWT differs depending on the length of the walking track,77 if the subject can rest as long and as much as they want during the test,78 and if the test involves turns—a biomechanically different task from walking and a task that can take significant time to complete.79 To mitigate these issues, research has been conducted on completing the 6MWT on a treadmill combined with virtual reality with similar gait deficits reported in other studies.12,67 However, there are limitations to this approach as walking on a treadmill may alter muscle activation and gait as compared to walking overground. Though virtual reality might address this issue by providing optic flow and a more realistic environment to the patient.
The variability of movement is an important outcome measure regarding health and disease status.80 As Frey80 has identified, there is a functional variability between movements with health representing a mid-zone between those that are too rigid or those that have lost control. When walking, PwCOPD demonstrated less control, or less consistent, hip and knee joint movement patterns, especially at faster speeds.81 Step time and step width variability was altered in PwCOPD,71 as well as step length.13 Stride frequency variability, as measured by the coefficient of variation, was increased in PwCOPD as compared to controls at speeds faster and slower than their self-selected walking speed; however, these group differences were not found when speed was controlled.82 When performing a cognitive task while walking, stride time variability was significantly increased in PwCOPD compared to controls.83
Muscle Mechanics
The biomechanical analysis of muscle tissue is typically focused on the contractile and elastic components of the sarcomere. The four biomechanical properties of muscle tissue include tension, extensibility, elasticity, and irritability (for definitions, see Table 1). Much of the work published on muscle dysfunction in COPD has focused on physiology of muscle tissue. There is a paucity of literature focused on biomechanical properties of muscle tissue in COPD (Table 5).
 |
Table 4 Studies of Gait in PwCOPD |
To produce a similar relative force as healthy controls, PwCOPD required a higher stimulation frequency of the vastus lateralis, yet the contractile properties, when corrected for muscle cross sectional areas, were preserved in those with COPD.84 This is in agreement with other studies.85 Recent research has reported decreased muscle stiffness and viscosity during passive flexion and extension of the knee.86 Muscle stiffness of the quadriceps, measured by shear wave elastography (for definition, see Table 1), is decreased in PwCOPD and is inversely associated with lung function, exercise tolerance, muscle strength, and dyspnea.87 Unlike the quadriceps, muscle stiffness as measured by shear wave velocity has been found to be increased in the diaphragm, indicating a higher muscle stiffness.88 The stretch-shortening cycle of the vastus lateralis showed higher potentiation in PwCOPD compared to those without.89 This was also inversely associated with muscle thickness, pennation angle, and habitual gait speed. Smaller pennation angles and reduced muscle thickness have been reported in PwCOPD compared to healthy men.90
The altered biomechanical properties of skeletal muscle could lead to increased energy expenditure, decreased force production, and/or abnormal joint motion. They are possible mechanisms for decreased gait speed, increased fatigue, altered gait kinematics, and increased fall risk in PwCOPD. These findings support many of the physiological studies demonstrating abnormalities in the skeletal muscle tissue in PwCOPD. The availability of biomechanical measures could facilitate the development of therapeutic interventions designed to address these issues.
Breathing Mechanics
Changes in relative movement of the abdomen and rib cage are critically important to breathing mechanics. Muscles of the rib cage and abdomen work in coordination to assist the diaphragm in healthy adults, even when the work load is minimal.91,92 This is not the case in PwCOPD. Early results demonstrated that dyssynchronous thoracoabdominal motion, “suggesting ineffective diaphragmatic function”, is associated with disease severity in COPD.93 Exercise appears to change thoracoabdominal motion in persons with airway obstruction94,95 (Table 6). Arm exercise, whether unsupported arm lifts or arm cycle ergometry, led to dyssynchronous breathing.96,97 In PwCOPD, unsupported arm exercise yet not leg cycling, induced dyssynchronous breathing, which was associated with dyspnea.98 However, during leg cycling exercise in PwCOPD, an increased percent contribution of abdominal motion to tidal volume was greater than the increased percent contribution of ribcage motion. Abdominal motion continued to contribute a greater percentage to tidal volume as exercise workload increased.99 Moreover, dyssynchronous thoracoabdominal motion (ie, less rib cage excursion and increased abdominal excursion in PwCOPD compared to controls) impacts clinical outcomes such as the 6MWT in PwCOPD.100 Rib cage excursion independently predicted the distance walked during the 6MWT and further, rib cage excursion was inversely related to lung hyperinflation.100 Hyperinflation, in turn, may be either static, resulting from reduced lung elastic recoil and alterations in the chest wall, or dynamic, resulting from the inability of the lungs to deflate fully due to reduced expiratory airflows.101 The degree to which dyssynchronous breathing impacts hyperinflation is not established. Finally, hyperinflation can be addressed by both pharmacologic and surgical approaches.102 Understanding the contribution of biomechanical dysfunction to these processes will be important to optimize these treatments.
 |
Table 5 Studies of Muscle Mechanics in PwCOPD |
 |
Table 6 Studies of Breathing Mechanics in PwCOPD |
Optoelectronic plethysmography (OEP) using motion capture to record breathing mechanics based upon movement of the thorax and abdomen was first introduced in 1999.103 Chest wall kinematics are directly measured from this technique104 and regional compartmental volumes can be accurately estimated.105,106 End-expiratory lung volume107 and inspiratory capacity108 measured from OEP are valid against gold standards. In PwCOPD, OEP has been used to investigate dynamic hyperinflation, dyspnea, chest wall volumes, and the effect of bronchodilators during exercise.105,109–111 For a more exhaustive review regarding the use of OEP in clinic and research see Massaroni et al.112
Mechanomyography is another tool that has been used to assess respiratory muscle activation during breathing. Using a microphone or accelerometer, the oscillations of the muscle surface due to change in muscle shape from contraction can be recorded. This technique has not been widely used in PwCOPD. The few studies that have been done suggest its use for respiratory effort, muscular function such as efficiency, and asynchrony.113–116 In PwCOPD, muscle activation using mechanomyography has a strong to moderate association with pulmonary function.117 In PwCOPD and heart failure, mechanomyography has shown potential at validly identifying respiratory muscle activation.118 Most recently, mechanomyography has been combined with bioimpedance to monitor respiratory activity.119 Additionally, these sensors may hold vast clinical utility of monitoring pulmonary disease through monitoring of lung sounds and breathing patterns.120
Future Directions
With the advancements in technology, it is logical to question whether or not laboratory-based biomechanical evaluations will be needed. Motion analysis in the laboratory is still the gold standard. However, laboratory- or even clinic-based movement does not always reflect everyday movement. Take for example gait speed. Gait speed differs based on the method in which it is recorded.121 If taken in clinic, it may not accurately reflect gait speed in community settings.122
Wearable sensors offer an avenue to capture everyday movement passively. In particular, wearables data are being added to large scale projects such as the COPD Biomarker Qualification Consortium and Mobilise-D. Compared to adults aged 18–49 years, those aged 50 years and older use wearables, smartphones, and smart home technology at the same pace.123 These technologies contain a wide variety of sensors from inertial measurement units that contain an accelerometer, gyroscope, and magnetometer, bioelectrical impedance, to optical and temperature sensors. Major advancements in materials and mechanics have resulted in the development of soft, “skin-like” devices that closely match the mechanical properties of biological tissue.124–126 The ability for these electronic devices to bend, twist, and stretch is accomplished by utilizing elastically deformable electrical conductors that provides mechanically robust electrical connectivity between solid-state elements. Current approaches include deterministically patterned metal wiring,127–130 percolating networks of conductive nanoparticles,131–133 and conductive fluids such as carbon filled grease,134 liquid metals,135–138 and ionic fluids.139–142 For widespread use and entry into clinical research, these soft wearable sensors must be validated against existing clinical gold-standards and seek US Food and Drug Administration approval.
Monitoring movement and health status using wearables will present clinicians and researchers with a new challenge: big data. It will also create opportunities to provide for “real-time” monitoring of “real-world” functioning. Deciphering what features of collected data is relevant for clinical care. Continuously sending raw data from users to a cloud center raises challenges for communications networks. It inevitably creates computing bottlenecks of health information management at the cloud center. More importantly, sharing user’s health information to untrusted cloud server is not acceptable due to privacy concerns. And the sensed health data has numerous features/attributes, which should be carefully extracted for modeling. The computational cost of extracting massive features increases exponentially, so that the problem cannot be easily solved in a reasonable time. To this end, distributed learning can offload computations for relevant data models to the local devices, such as smartphones. Only the relevant information is sent to the cloud center to be aggregated and used to update a universal decision-making model, which in turn saves communication cost and protects the raw data confidentiality. On the other hand, cloud-based systems may increase opportunity for open datasets being prioritized in grant applications. Wearables offer an immense opportunity for open science and data sharing.
COPD is a markedly heterogeneous condition. However, subtyping PwCOPD into meaningful subgroups has been problematic. Studies of biomechanics in PwCOPD, like many other studies, have not focused on the identification of subtypes. This may be a fruitful area for future studies.
Advanced Analytics
In addition to identifying relevant features in sensor data, these individual data streams can provide a wealth of information. Healthy rhythmic biologic processes are marked by variability, complexity, and homeokinesis, which is the ability to maintain an ordered system that fluctuates within an acceptable range.143–145 The ability to make flexible adaptations to everyday stresses allows a wide range of potential behaviors, leading to systems that are adaptable and flexible in an unpredictable and ever-changing environment.80 These fluctuations can be quantified by assessing variability, which measures a characteristic of the distribution, and by assessing their complexity, which quantifies their irregularity and thus their independence from other rhythmic processes. Biological rhythms not only fluctuate, they interact with each other. As consequence, they can be coupled. PwCOPD demonstrate abnormal coupling of gait and respiratory rhythms, demonstrating a very rigid coupling across different walking speeds.146,147
Clinical Perspectives
It is clear that PwCOPD have functional limitations that can be assessed by biomechanical measures. Development of biomechanical outcomes could provide important novel measures to guide clinical care and facilitate the development of novel treatments. It will be important for clinical care to include assessments of balance and gait during patient intake as these are clinically important outcomes in their own right. Potentially adding a balance confidence or previous falls question will identify individuals that are at high risk for future falls. Further, review of balance and balance recovery training in pulmonary rehabilitation programs may provide an opportunity to improve programming and enhance these skills. Gait speed may be a potentially simple assessment to add to office visit intake. A log of gait speed over multiple office visits will allow healthcare providers to identify a minimal clinically important difference in the decline in speed over time. Recording gait speed over 4 meters has been shown to be responsive to longitudinal change in COPD.148 In addition, standardizing 6MWT assessments across healthcare providers will ensure that changes in distance are due to the patient and not the methods used for testing.10
In the official ATS/ETS statement on pulmonary rehabilitation,149 balance assessment is encouraged yet no information is provided on what kind of assessment or how often this should be done. A recent review of balance assessments in COPD38 offers guidance with regard to falls and fall history screening; however, this is without a clear delineation of which tool would be best as there are multiple factors that go into proper selection of an assessment tool. Balance training during pulmonary rehabilitation has been shown to be effective in improving balance, yet unclear regarding the long-term effect on falls.150 As balance assessment and training in PwCOPD are still relatively new concepts, further development of interventions is required. As of 2021, balance assessment and training were not listed as essential components of pulmonary rehabilitation by the ATS,151 indicating work still needs to be done in establishing them as critical components. Biomechanical assessments would strongly assist in guiding best practices of which assessment tools and/or interventions are most effective.
There is further evidence that non-specific pulmonary rehabilitation has been effective in improving gait speed152 and biomechanical gait outcomes67 in a subset of PwCOPD. Updated pulmonary rehabilitation programs are encouraged to include gait assessment and training153 suggesting that gait training could have a clinical impact on 6MWD, balance, and fall risk. Biomechanical assessment of gait may be useful to identify PwCOPD that have an altered gait pattern providing those that need it most with additional/specific training sessions during pulmonary rehabilitation. Further, there is a lack of knowledge regarding whether or not gait improvements made during pulmonary rehabilitation have translated to daily life.
Breathing exercises have played an important role in treatment of PwCOPD. Monitoring thoracoabdominal motion during breathing therapy is important as abdominodiaphragmatic breathing (“inspiration with the diaphragm ‘alone’ and expiration with a contraction of the abdominal muscles”) may induce the most dyssynchronous thoracoabdominal motion;154 whereas, diaphragmatic breathing (“belly breathing”) is associated with more effective breathing outcomes.155–157 During the 6MWT, rib cage excursion was an independent predictor of total 6MWT distance.100 Therefore, understanding the extent of dyssynchronous thoracoabdominal motion in a patient will have an impact on treatment. Important too will be biomechanical analyses to evaluate the effectiveness of newer interventions to improve breathing mechanics, and potentially balance and gait, such as singing for lung health,158–160 dancing,161,162 yoga,163,164 liuzijue qigong,165,166 and tai chi.167–169
Muscle mechanics may have an impact on clinical care; however, this is yet to be fully determined. Additional research is needed to understand the effects of COPD on muscle tissue tension, extensibility, elasticity, and irritability. Based on current pulmonary rehabilitation recommendations, a comprehensive assessment should include inspiratory and peripheral muscle strength and endurance assessments.151 Further, pulmonary rehabilitation programs should include resistance and strength training as the benefits have been well documented.149
Advancements in wearable technology will likely have a significant impact on clinical care. For example, sensors attached to the arm used to record upper extremity function (ie, rapid elbow flexion) in PwCOPD hospitalized for an exacerbation may be useful for prediction of 30-day readmission.170 Another potential target for wearables is in identifying disease severity during everyday activities such as walking.171 In addition to prognostic value, the ability to capture real-time data in real-world settings opens up entirely novel opportunities for patient monitoring. For example, objective patient monitoring could provide more accurate information regarding falls and physical activity. Recall of falls is subject to memory and recall of physical activity is subject to bias, stigma, and/or embarrassment. This can provide novel outcomes to facilitate the development of new treatments and to create measures to gauge the impact of treatments in the “at home” setting.
Conclusion
In conclusion, biomechanical assessments are complementary to physiological assessments. Biomechanical measures offer an additional dimension of assessment compared to physiological laboratory measures, as biomechanics can capture what patients are actually doing as well as their capability. Consideration of function and movement in clinical care will lead to more comprehensive treatments or referrals to therapists (eg, physical therapy). Ensuring that PwCOPD are capable of more than just movements for instrumental activities of daily living, will make a direct impact on their quality of life. Having the confidence to move about in their communities or play with their grandchildren is important in maintaining social connections and overall happiness.
Acknowledgments
This work was supported by the National Institutes of Health (L30 HL129255).
Disclosure
Dr Stephen I Rennard reports personal fees from BoehringerIngelheim, GSK, Sanofi, and Verona. Dr Stephen I Rennard is the founder and president of Great Plains Biometrix and Drs. Jennifer M Yentes and Eric Markvicka sit on the Board of Directors, outside the submitted work. In addition, Drs. Jennifer M Yentes and Stephen I Rennard have a patent for gait respiratory coupling issued and licensed by UNeMed, and a patent wearable multifunction sensor pending with Dr. Eric Markvicka. The authors report no other conflicts of interest in this work.
References
1. Saey D, Debigare R, LeBlanc P., et al. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(4):425–430. doi:10.1164/rccm.200208-856OC
2. Seymour JM, Spruit MA, Hopkinson NS, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36(1):81–88. doi:10.1183/09031936.00104909
3. Gea J, Agusti A, Roca J. Pathophysiology of muscle dysfunction in COPD. J Appl Physiol. 2013;114(9):1222–1234. doi:10.1152/japplphysiol.00981.2012
4. Barreiro E, Gea J. Molecular and biological pathways of skeletal muscle dysfunction in chronic obstructive pulmonary disease. Chron Respir Dis. 2016;13(3):297–311. doi:10.1177/1479972316642366
5. Barreiro E, Jaitovich A. Muscle atrophy in chronic obstructive pulmonary disease: molecular basis and potential therapeutic targets. J Thorac Dis. 2018;10(Suppl 12):S1415–S1424. doi:10.21037/jtd.2018.04.168
6. Gosker HR, Wouters EF, van der Vusse GJ, Schols AM. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectives. Am J Clin Nutr. 2000;71(5):1033–1047. doi:10.1093/ajcn/71.5.1033
7. Jaitovich A, Barreiro E. Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. What We Know and Can Do for Our Patients. Am J Respir Crit Care Med. 2018;198(2):175–186. doi:10.1164/rccm.201710-2140CI
8. Mathur S, Brooks D, Carvalho C. Structural alterations of skeletal muscle in COPD. Front Physiol. 2014;5::104 doi:10.3389/fphys.2014.00104.
9. Tudorache E, Oancea C, Avram C, Fira-Mladinescu O, Petrescu L, Timar B. Balance impairment and systemic inflammation in chronic obstructive pulmonary disease. COPD. 2015;1847. doi:10.2147/COPD.S89814
10. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi:10.1183/09031936.00150314
11. Jirange P, Vaishali K, Sinha MK, Bairapareddy KC, Alaparthi GK, Melani AS. A Cross-Sectional Study on Balance Deficits and Gait Deviations in COPD Patients. Canadian Respir J. 2021;2021:1–5. doi:10.1155/2021/6675088
12. Liu WY, Spruit MA, Delbressine JM, et al. Spatiotemporal gait characteristics in patients with COPD during the Gait Real-time Analysis Interactive Lab-based 6-minute walk test. PLoS One. 2017;12(12):e0190099. doi:10.1371/journal.pone.0190099
13. Morlino P, Balbi B, Guglielmetti S, et al. Gait abnormalities of COPD are not directly related to respiratory function. Gait Posture. 2017;58:352–357. doi:10.1016/j.gaitpost.2017.08.020
14. Yentes JM, Sayles H, Meza J, Mannino DM, Rennard SI, Stergiou N. Walking abnormalities are associated with COPD: an investigation of the NHANES III dataset. Respir Med. 2011;105(1):80–87. doi:10.1016/j.rmed.2010.06.007
15. Butcher SJ, Meshke JM, Sheppard MS. Reductions in functional balance, coordination, and mobility measures among patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2004;24(4):274–280 doi:10.1097/00008483-200407000-00013.
16. de Castro LA, Ribeiro LR, Mesquita R, et al. Static and Functional Balance in Individuals With COPD: comparison With Healthy Controls and Differences According to Sex and Disease Severity. Respir Care. 2016;61(11):1488–1496. doi:10.4187/respcare.04749
17. Troosters T, Demeyer H. Physical Inactivity as a Missing Link in Understanding the Progression of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2015;192(3):267–269 doi:10.1164/rccm.201506-1123ED.
18. American Society of Biomechanics: about; 2021. Available from: https://asbweb.org/about/.
19. Stokes MJ, Dalton PA. Acoustic myography for investigating human skeletal muscle fatigue. J Appl Physiol. 1991;71(4):1422–1426. doi:10.1152/jappl.1991.71.4.1422
20. Oster G, Jaffe JS. Low frequency sounds from sustained contraction of human skeletal muscle. Biophys J. 1980;30(1):119–127. doi:10.1016/S0006-3495(80)85080-6
21. Yan S, Sinderby C, Bielen P, Beck J, Comtois N, Sliwinski P. Expiratory muscle pressure and breathing mechanics in chronic obstructive pulmonary disease. Eur Respir J. 2000;16(4):684–690. doi:10.1034/j.1399-3003.2000.16d20.x
22. Mador MJ, Deniz O, Aggarwal A, Kufel TJ. Quadriceps fatigability after single muscle exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(1):102–108. doi:10.1164/rccm.200202-080OC
23. Roig M, Eng JJ, Road JD, Reid WD. Falls in patients with chronic obstructive pulmonary disease: a call for further research. Respir Med. 2009;103(9):1257–1269. doi:10.1016/j.rmed.2009.03.022
24. Beauchamp MK, Hill K, Goldstein RS, Janaudis-Ferreira T, Brooks D. Impairments in balance discriminate fallers from non-fallers in COPD. Respir Med. 2009;103(12):1885–1891. doi:10.1016/j.rmed.2009.06.008
25. Nantsupawat N, Lane P, Siangpraipunt O, Gadwala S, Nugent K. Gait Characteristics in Patients With Chronic Obstructive Pulmonary Disease. J Prim Care Community Health. 2015;6(4):222–226. doi:10.1177/2150131915577207
26. Steidl EMDS. Outcomes of manual therapy on the biomechanics of swallowing in individuals with COPD. Codas. 2021;33(5):e20200203. doi:10.1590/2317-1782/20192020203
27. Bergen G, Stevens MR, Burns ER. Falls and Fall Injuries Among Adults Aged >/=65 Years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(37):993–998. doi:10.15585/mmwr.mm6537a2
28. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of Interventions for Preventing Falls in Older Adults: a Systematic Review and Meta-analysis. JAMA. 2017;318(17):1687–1699. doi:10.1001/jama.2017.15006
29. Roig M, Eng JJ, MacIntyre DL, et al. Falls in people with chronic obstructive pulmonary disease: an observational cohort study. Respir Med. 2011;105(3):461–469. doi:10.1016/j.rmed.2010.08.015
30. Roig M, Eng JJ, Macintyre DL, Road JD, Reid WD. Postural Control Is Impaired in People with COPD: an Observational Study. Physiother Can. 2011;63(4):423–431. doi:10.3138/ptc.2010-32
31. Beauchamp MK, Sibley KM, Lakhani B, et al. Impairments in systems underlying control of balance in COPD. Chest. 2012;141(6):1496–1503. doi:10.1378/chest.11-1708
32. Ozalevli S, Ilgin D, Narin S, Akkoclu A. Association between disease-related factors and balance and falls among the elderly with COPD: a cross-sectional study. Aging Clin Exp Res. 2011;23(5–6):372–377 doi:10.1007/BF03325235.
33. Hakamy A, Bolton CE, Gibson JE, McKeever TM. Risk of fall in patients with COPD. Thorax. 2018;73(11):1079–1080. doi:10.1136/thoraxjnl-2017-211008
34. Oliveira CC, Annoni R, Lee AL, McGinley J, Irving LB, Denehy L. Falls prevalence and risk factors in people with chronic obstructive pulmonary disease: a systematic review. Respir Med. 2021;176:106284. doi:10.1016/j.rmed.2020.106284
35. Voica A, Oancea C, Tudorache E, et al. Chronic obstructive pulmonary disease phenotypes and balance impairment. COPD. 2016:919. doi:10.2147/COPD.S101128
36. Oliveira CC, Lee AL, McGinley J, et al. Balance and Falls in Acute Exacerbation of Chronic Obstructive Pulmonary Disease: a Prospective Study. COPD. 2017;14(5):518–525. doi:10.1080/15412555.2017.1342232
37. Porto EF, Castro AA, Schmidt VG, et al. Postural control in chronic obstructive pulmonary disease: a systematic review. Int J Chron Obstruct Pulmon Dis. 2015;10:1233–1239. doi:10.2147/COPD.S63955
38. Loughran KJ, Atkinson G, Beauchamp MK, et al. Balance impairment in individuals with COPD: a systematic review with meta-analysis. Thorax. 2020;75(7):539–546. doi:10.1136/thoraxjnl-2019-213608
39. Crisan AF, Oancea C, Timar B, Fira-Mladinescu O, Tudorache V. Falls, an underestimated risk in COPD. Eur Respir J. 2015;46(suppl):59. doi:10.1183/13993003.congress-2015.PA3070
40. Jácome C, Cruz J, Gabriel R, Figueiredo D, Marques A. Functional balance in older adults with chronic obstructive pulmonary disease. J Aging Phys Act. 2014;22(3):357–363. doi:10.1123/japa.2012-0319
41. Schons P, da Silva ES, Coertjens M, et al. The relationship between height of vertical jumps, functionality and fall episodes in patients with chronic obstructive pulmonary disease: a case-control study. Exp Gerontol. 2021;152:111457. doi:10.1016/j.exger.2021.111457
42. Eymir M, Yakut H, Özalevli S, Alpaydın AÖ. Static and dynamic balance impairment and relationship with disease-related factors in patients with chronic obstructive pulmonary disease: a cross-sectional study. Wien Klin Wochenschr. 2021;133(21–22):1186–1194. doi:10.1007/s00508-021-01918-8
43. Almeida CNS. Static balance in older adults with chronic obstructive pulmonary disease undergoing pulmonary rehabilitation. Geriatrics Gerontol Aging. 2020;14(2):98–107. doi:10.5327/Z2447-212320201900091
44. Park JK, Deutz NEP, Cruthirds CL, et al. Risk Factors for Postural and Functional Balance Impairment in Patients with Chronic Obstructive Pulmonary Disease. JCM. 2020;9(2):609. doi:10.3390/jcm9020609
45. Smith MD, Chang AT, Seale HE, Walsh JR, Hodges PW. Balance is impaired in people with chronic obstructive pulmonary disease. Gait Posture. 2010;31(4):456–460. doi:10.1016/j.gaitpost.2010.01.022
46. Janssens L, Brumagne S, McConnell AK, et al. Proprioceptive changes impair balance control in individuals with chronic obstructive pulmonary disease. PLoS One. 2013;8(3):e57949. doi:10.1371/journal.pone.0057949
47. Molouki A, Roostayi MM, Abedi M, Fakharian A, Akbarzadeh Baghban A. Postural Balance Evaluation in Patients with Chronic Obstructive Pulmonary Disease. Tanaffos. 2020;19(4):392–400.
48. Gloeckl R, Schneeberger T, Leitl D, et al. Whole-body vibration training versus conventional balance training in patients with severe COPD—a randomized, controlled trial. Respir Res. 2021;22(1):138. doi:10.1186/s12931-021-01688-x
49. Van Hove O, Cebolla AM, Andrianopoulos V, et al. The influence of cognitive load on static balance in chronic obstructive pulmonary disease patients. Clin Respir J. 2021;15(3):351–357. doi:10.1111/crj.13307
50. Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992;111(1):46–58. doi:10.1016/0022-510x(92)90111-w
51. Yamamoto T, Suzuki Y, Nomura K, et al. A Classification of Postural Sway Patterns During Upright Stance in Healthy Adults and Patients with Parkinson’s Disease. J Adv Comput Intelligence Intelligent Informatics. 2011;15(8):997–1010. doi:10.20965/jaciii.2011.p0997
52. Porto EF, Pradella CO, Rocco CM, et al. Comparative Postural Control in COPD Patients and Healthy Individuals During Dynamic and Static Activities. J Cardiopulm Rehabil Prev. 2017;37(2):139–145. doi:10.1097/HCR.0000000000000246
53. Smith MD, Chang AT, Hodges PW. Balance recovery is compromised and trunk muscle activity is increased in chronic obstructive pulmonary disease. Gait Posture. 2016;43:101–107. doi:10.1016/j.gaitpost.2015.09.004
54. Araújo de Castro L, Morita AA, Sepúlveda-Loyola W, et al. Are there differences in muscular activation to maintain balance between individuals with chronic obstructive pulmonary disease and controls? Respir Med. 2020;173:106016. doi:10.1016/j.rmed.2020.106016
55. Terui Y, Iwakura M, Suto E, et al. New evaluation of trunk movement and balance during walking in COPD patients by a triaxial accelerometer. COPD. 2018;13:3957–3962. doi:10.2147/COPD.S184212
56. Janssens L, Brumagne S, McConnell AK, et al. Impaired Postural Control Reduces Sit-to-Stand-to-Sit Performance in Individuals with Chronic Obstructive Pulmonary Disease. PLoS One. 2014;9(2):e88247. doi:10.1371/journal.pone.0088247
57. Karpman C, Benzo R. Gait speed as a measure of functional status in COPD patients. COPD. 2014;9(1):1315–1320. doi:10.2147/COPD.S54481
58. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi:10.1001/jama.295.17.2018
59. Vestergaard S, Patel KV, Walkup MP, et al. Stopping to rest during a 400-meter walk and incident mobility disability in older persons with functional limitations. J Am Geriatr Soc. 2009;57(2):260–265. doi:10.1111/j.1532-5415.2008.02097.x
60. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi:10.1001/jama.2010.1923
61. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51(3):314–322 doi:10.1046/j.1532-5415.2003.51104.x.
62. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol a Biol Sci Med Sci. 2000;55(4):M221–31 doi:10.1093/gerona/55.4.m221.
63. Lahousse L, Verlinden VJ, van der Geest JN, et al. Gait patterns in COPD: the Rotterdam Study. Eur Respir J. 2015;46(1):88–95. doi:10.1183/09031936.00213214
64. Ilgin D, Ozalevli S, Kilinc O, Sevinc C, Cimrin AH, Ucan ES. Gait speed as a functional capacity indicator in patients with chronic obstructive pulmonary disease. Ann Thorac Med. 2011;6(3):141–146. doi:10.4103/1817-1737.82448
65. Iwakura M, Okura K, Shibata K, et al. Gait characteristics and their associations with clinical outcomes in patients with chronic obstructive pulmonary disease. Gait Posture. 2019;74:60–65. doi:10.1016/j.gaitpost.2019.08.012
66. Walsh JA, Barker RE, Kon SSC, et al. Gait speed and adverse outcomes following hospitalised exacerbation of COPD. Eur Respir J. 2021;58(5):2004047. doi:10.1183/13993003.04047-2020
67. Liu WY, Meijer K, Delbressine J, Willems P, Wouters E, Spruit M. Effects of Pulmonary Rehabilitation on Gait Characteristics in Patients with COPD. JCM. 2019;8(4):459. doi:10.3390/jcm8040459
68. Yentes JM, Blanke D, Rennard SI, Stergiou N. The Effect of a Short Duration, High Intensity Exercise Intervention on Gait Biomechanics in Patients With COPD: findings From a Pilot Study. Chronic Obstr Pulm Dis. 2014;1(1):133–147. doi:10.15326/jcopdf.1.1.2013.0002
69. Zago M, Sforza C, Bonardi DR, Guffanti EE, Galli M. Gait analysis in patients with chronic obstructive pulmonary disease: a systematic review. Gait Posture. 2018;61:408–415. doi:10.1016/j.gaitpost.2018.02.007
70. McCamley JD, Pisciotta EJ, Yentes JM, et al. Gait deficiencies associated with peripheral artery disease are different than chronic obstructive pulmonary disease. Gait Posture. 2017;57:258–264. doi:10.1016/j.gaitpost.2017.06.018
71. Yentes JM, Rennard SI, Schmid KK, Blanke D, Stergiou N. Patients with Chronic Obstructive Pulmonary Disease Walk with Altered Step Time and Step Width Variability as Compared with Healthy Control Subjects. Ann Am Thorac Soc. 2017;14(6):858–866. doi:10.1513/AnnalsATS.201607-547OC
72. Yentes JM, Schmid KK, Blanke D, Romberger DJ, Rennard SI, Stergiou N. Gait mechanics in patients with chronic obstructive pulmonary disease. Respir Res. 2015;16:31. doi:10.1186/s12931-015-0187-5
73. Fallahtafti F, Curtze C, Samson K, Yentes JM. Chronic obstructive pulmonary disease patients increase medio-lateral stability and limit changes in antero-posterior stability to curb energy expenditure. Gait Posture. 2020;75:142–148. doi:10.1016/j.gaitpost.2019.10.025
74. McCrum C, Vaes AW, Delbressine JM, et al. A pilot study on the feasibility and effectiveness of treadmill-based perturbations for assessing and improving walking stability in chronic obstructive pulmonary disease. Clin Biomechanics. 2022:91. doi:10.1016/j.clinbiomech.2021.105538
75. Annegarn J, Spruit MA, Savelberg HH, et al. Differences in walking pattern during 6-min walk test between patients with COPD and healthy subjects. PLoS One. 2012;7(5):e37329. doi:10.1371/journal.pone.0037329
76. Marquis N, Debigare R, Bouyer L, et al. Physiology of walking in patients with moderate to severe chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2009;41(8):1540–1548. doi:10.1249/MSS.0b013e31819c717f
77. Scivoletto G, Tamburella F, Laurenza L, Foti C, Ditunno JF, Molinari M. Validity and reliability of the 10-m walk test and the 6-min walk test in spinal cord injury patients. Spinal Cord. 2011;49(6):736–740. doi:10.1038/sc.2010.180
78. ATS. ATS Statement. Am J Respir Crit Care Med. 2002;166(1):111–117. doi:10.1164/ajrccm.166.1.at1102
79. Saraiva NAO, Ferreira AS, Papathanasiou JV, Guimarães FS, Lopes AJ. Kinematic evaluation of patients with chronic obstructive pulmonary disease during the 6-min walk test. J Bodyw Mov Ther. 2021;27:134–140. doi:10.1016/j.jbmt.2021.01.005
80. Frey U, Maksym G, Suki B. Temporal complexity in clinical manifestations of lung disease. J Appl Physiol. 2011;110(6):1723–1731. doi:10.1152/japplphysiol.01297.2010
81. Liu WY, Schmid KK, Meijer K, Spruit MA, Yentes JM. Subjects With COPD Walk With Less Consistent Organization of Movement Patterns of the Lower Extremity. Respir Care. 2020;65(2):158–168. doi:10.4187/respcare.06743
82. Sanseverino MA, Pecchiari M, Bona RL, et al. Limiting Factors in Walking Performance of Subjects With COPD. Respir Care. 2018;63(3):301–310. doi:10.4187/respcare.05768
83. Heraud N, Alexandre F, Gueugnon M, et al. Impact of Chronic Obstructive Pulmonary Disease on Cognitive and Motor Performances in Dual-Task Walking. COPD. 2018;15(3):277–282. doi:10.1080/15412555.2018.1469607
84. Debigaré R, Côte CH, Hould FS, LeBlanc P, Maltais F. In vitro and in vivo contractile properties of the vastus lateralis muscle in males with COPD. Eur Respir J. 2003;21(2):273–278. doi:10.1183/09031936.03.00036503
85. Degens H, Sanchez Horneros JM, Heijdra YF, Dekhuijzen PNR, Hopman MTE. Skeletal muscle contractility is preserved in COPD patients with normal fat-free mass. Acta Physiol Scand. 2005;184(3):235–242. doi:10.1111/j.1365-201X.2005.01447.x
86. Valle MS, Casabona A, Di Fazio E, et al. Impact of chronic obstructive pulmonary disease on passive viscoelastic components of the musculoarticular system. Sci Rep. 2021;11(1):18077. doi:10.1038/s41598-021-97621-9
87. Deng M, Zhou X, Li Y, et al. Ultrasonic Elastography of the Rectus Femoris, a Potential Tool to Predict Sarcopenia in Patients With Chronic Obstructive Pulmonary Disease. Front Physiol. 2021;12:783421. doi:10.3389/fphys.2021.783421
88. Xu JH, Wu ZZ, Tao FY, et al. Ultrasound Shear Wave Elastography for Evaluation of Diaphragm Stiffness in Patients with Stable COPD: a Pilot Trial. J Ultrasound Med. 2021;40(12):2655–2663. doi:10.1002/jum.15655
89. Navarro-Cruz R, Alcazar J, Rodriguez-Lopez C, et al. The Effect of the Stretch-Shortening Cycle in the Force–Velocity Relationship and Its Association With Physical Function in Older Adults With COPD. Front Physiol. 2019;1:10 doi:10.3389/fphys.2019.00316.
90. Coratella G, Rinaldo N, Schena F. Quadriceps concentric-eccentric force and muscle architecture in COPD patients vs healthy men. Hum Mov Sci. 2018;59:88–95. doi:10.1016/j.humov.2018.03.015
91. Aliverti A, Cala SJ, Duranti R, et al. Human respiratory muscle actions and control during exercise. J Appl Physiol. 1997;83(4):1256–1269. doi:10.1152/jappl.1997.83.4.1256
92. Sanna A, Bertoli F, Misuri G, et al. Chest wall kinematics and respiratory muscle action in walking healthy humans. J Appl Physiol. 1999;87(3):938–946. doi:10.1152/jappl.1999.87.3.938
93. Sharp JT, Goldberg NB, Druz WS, Fishman HC, Danon J. Thoracoabdominal motion in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1977;115(1):47–56. doi:10.1164/arrd.1977.115.1.47
94. Dodd DS, Brancatisano T, Engel LA. Chest wall mechanics during exercise in patients with severe chronic air-flow obstruction. Am Rev Respir Dis. 1984;129(1):33–38. doi:10.1164/arrd.1984.129.1.33
95. Delgado HR, Braun SR, Skatrud JB, Reddan WG, Pegelow DF. Chest wall and abdominal motion during exercise in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1982;126(2):200–205. doi:10.1164/arrd.1982.126.2.200
96. Breslin EH, Garoutte BC. Respiratory responses to unsupported arm lifts paced during expiration. West J Nurs Res. 1995;17(1):91–100. doi:10.1177/019394599501700108
97. Castro AAM, Porto EF, Feltrim MIZ, Jardim JR. Asynchrony and hyperinflation in patients with chronic obstructive pulmonary disease during two types of upper limbs exercise. Arch Bronconeumol. 2013;49(6):241–248. doi:10.1016/j.arbres.2012.12.009
98. Celli BR, Rassulo J, Make BJ. Dyssynchronous breathing during arm but not leg exercise in patients with chronic airflow obstruction. N Engl J Med. 1986;314(23):1485–1490. doi:10.1056/NEJM198606053142305
99. Alves GS, Britto RR, Campos FC, Vilaça ABO, Moraes KS, Parreira VF. Breathing pattern and thoracoabdominal motion during exercise in chronic obstructive pulmonary disease. Braz J Med Biol Res. 2008;41(11):945–950. doi:10.1590/s0100-879x2008001100001
100. Chien J-Y, Ruan S-Y, Huang Y-CT, Yu C-J, Yang P-C. Asynchronous thoraco-abdominal motion contributes to decreased 6-minute walk test in patients with COPD. Respir Care. 2013;58(2):320–326. doi:10.4187/respcare.01522
101. O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180–184. doi:10.1513/pats.200508-093DO
102. Shah PL, Herth FJ, van Geffen WH, Deslee G, Slebos D-J. Lung volume reduction for emphysema. Lancet Respir Med. 2017;5(2):147–156. doi:10.1016/S2213-2600(16)30221-1
103. Iandelli I, Rosi E, Scano G. The ELITE system. Monaldi Archives for Chest Disease = Archivio Monaldi per le Malattie Del Torace. 1999;54(6):498–501.
104. Pereira MC, Porras DC, Lunardi AC, et al. Thoracoabdominal asynchrony: two methods in healthy, COPD, and interstitial lung disease patients. PLoS One. 2017;12(8):e0182417. doi:10.1371/journal.pone.0182417
105. Aliverti A. Regional chest wall volumes during exercise in chronic obstructive pulmonary disease. Thorax. 2004;59(3):210–216. doi:10.1136/thorax.2003.011494
106. Cala SJ, Kenyon CM, Ferrigno G, et al. Chest wall and lung volume estimation by optical reflectance motion analysis. J Appl Physiol. 1996;81(6):2680–2689. doi:10.1152/jappl.1996.81.6.2680
107. Dellaca RL, Aliverti A, Pelosi P, et al. Estimation of end-expiratory lung volume variations by optoelectronic plethysmography. Crit Care Med. 2001;29(9):1807–1811. doi:10.1097/00003246-200109000-00026
108. Duranti R, Filippelli M, Bianchi R, et al. Inspiratory capacity and decrease in lung hyperinflation with albuterol in COPD. Chest. 2002;122(6):2009–2014. doi:10.1378/chest.122.6.2009
109. Georgiadou O, Vogiatzis I, Stratakos G, et al. Effects of rehabilitation on chest wall volume regulation during exercise in COPD patients. Eur Respir J. 2006;29(2):284–291. doi:10.1183/09031936.00121006
110. Bianchi R, Gigliotti F, Romagnoli I, et al. Patterns of chest wall kinematics during volitional pursed-lip breathing in COPD at rest. Respir Med. 2007;101(7):1412–1418. doi:10.1016/j.rmed.2007.01.021
111. Coutinho Myrrha MA, Vieira DSR, Moraes KS, Lage SM, Parreira VF, Britto RR. Chest wall volumes during inspiratory loaded breathing in COPD patients. Respir Physiol Neurobiol. 2013;188(1):15–20. doi:10.1016/j.resp.2013.04.017
112. Massaroni C, Carraro E, Vianello A, et al. Optoelectronic Plethysmography in Clinical Practice and Research: a Review. Respiration. 2017;93(5):339–354. doi:10.1159/000462916
113. Torres A, Sarlabous L, Fiz JA, et al. Noninvasive measurement of inspiratory muscle performance by means of diaphragm muscle mechanomyographic signals in COPD patients during an incremental load respiratory test. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2493–2496. doi:10.1109/IEMBS.2010.5626618
114. Sarlabous L, Torres A, Fiz JA, Gea J, Martinez-Llorens JM, Jane R. Evaluation of the respiratory muscular function by means of diaphragmatic mechanomyographic signals in COPD patients. Annu Int Conf IEEE Eng Med Biol Soc. 2009;2009:3925–3928. doi:10.1109/IEMBS.2009.5333536
115. Estrada L, Torres A, Sarlabous L, et al. Estimation of bilateral asynchrony between diaphragm mechanomyographic signals in patients with chronic obstructive pulmonary disease. Annu Int Conf IEEE Eng Med Biol Soc. 2014;2014:3813–3816. doi:10.1109/EMBC.2014.6944454
116. Sarlabous L, Torres A, Fiz JA, et al. Evaluation of the respiratory muscles efficiency during an incremental flow respiratory test. Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:3820–3823. doi:10.1109/IEMBS.2011.6090775
117. Sarlabous L, Torres A, Fiz JA, Martínez-Llorens JM, Gea J, Jané R. Inspiratory muscle activation increases with COPD severity as confirmed by non-invasive mechanomyographic analysis. PLoS One. 2017;12(5):e0177730. doi:10.1371/journal.pone.0177730
118. Petrocelli LCE, Lozano-García M, Moore A, et al. Assessment of inspiratory muscle activation using surface mechanomyography in COPD patients with comorbid heart failure. Eur Respir J. 2019;54(suppl):63. doi:10.1183/13993003.congress-2019.PA3932
119. Blanco-Almazán D, Groenendaal W, Lozano-García M, et al. Combining Bioimpedance and Myographic Signals for the Assessment of COPD During Loaded Breathing. IEEE Trans Biomed Eng. 2021;68(1):298–307. doi:10.1109/TBME.2020.2998009
120. Gupta P, Wen H, Di Francesco L, Ayazi F. Detection of pathological mechano-acoustic signatures using precision accelerometer contact microphones in patients with pulmonary disorders. Sci Rep. 2021;11(1):13427. doi:10.1038/s41598-021-92666-2
121. Stuck AK, Bachmann M, Füllemann P, Josephson KR, Stuck AE. Effect of testing procedures on gait speed measurement: a systematic review. PLoS One. 2020;15(6):e0234200. doi:10.1371/journal.pone.0234200
122. Taylor D, Stretton CM, Mudge S, Garrett N. Does clinic-measured gait speed differ from gait speed measured in the community in people with stroke? Clin Rehabil. 2006;20(5):438–444. doi:10.1191/0269215506cr945oa
123. Nelson Kakulla B. 2020 Tech Trends of the 50+. AARP Res. 2020. doi:10.26419/res.00329.001
124. Rogers JA, Someya T, Huang Y. Materials and mechanics for stretchable electronics. Science. 2010;327(5973):1603–1607. doi:10.1126/science.1182383
125. Park YG, Lee GY, Jang J, Yun SM, Kim E, Park JU. Liquid Metal-Based Soft Electronics for Wearable Healthcare. Adv Healthcare Mater. 2021;10(17):2002280. doi:10.1002/adhm.202002280
126. Hammock ML, Chortos A, Tee BCK, Tok JBH, Bao Z. 25th Anniversary Article: the Evolution of Electronic Skin (E-Skin): a Brief History, Design Considerations, and Recent Progress. Adv Mater. 2013;25(42):5997–6038. doi:10.1002/adma.201302240
127. Markvicka EJ, Wang G, Lee YC, Laput G, Majidi C, Yao L. ElectroDermis: fully Untethered, Stretchable, and Highly-Customizable Electronic Bandages.
128. Kim DH, Lu N, Ma R, et al. Epidermal Electronics. Science. 2011;333(6044):838–843. doi:10.1126/science.1206157
129. Bartlett MD, Markvicka EJ, Majidi C. Rapid fabrication of soft, multilayered electronics for wearable monitoring. Adv Funct Mater. 2016;26(46):8496–8504.
130. Fan JA, Yeo WH, Su Y, et al. Fractal design concepts for stretchable electronics. Nat Commun. 2014;5(1):3266. doi:10.1038/ncomms4266
131. Weigel M, Lu T, Bailly G, Oulasvirta A, Majidi C, Steimle J iSkin: flexible, Stretchable and Visually Customizable On-Body Touch Sensors for Mobile Computing. In:
132. Lipomi DJ, Vosgueritchian M, Tee BCK, et al. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nature Nanotech. 2011;6(12):788–792. doi:10.1038/nnano.2011.184
133. Russo A, Ahn BY, Adams JJ, Duoss EB, Bernhard JT, Lewis JA. Pen-on-Paper Flexible Electronics. Adv Mater. 2011;23(30):3426–3430. doi:10.1002/adma.201101328
134. Muth JT, Vogt DM, Truby RL, et al. Embedded 3D printing of strain sensors within highly stretchable elastomers. Adv Mater. 2014;26(36):6307–6312. doi:10.1002/adma.201400334
135. Markvicka EJ, Bartlett MD, Huang X, Majidi C. An autonomously electrically self-healing liquid metal–elastomer composite for robust soft-matter robotics and electronics. Nat Mater. 2018;17(7):618–624 doi:10.1038/s41563-018-0084-7.
136. Tutika R, Haque ABM, Bartlett MD. Self-healing liquid metal composite for reconfigurable and recyclable soft electronics. Commun Materials. 2021;2(1):1–8 doi:10.1038/s43246-021-00169-4.
137. Liu S, Shah DS, Kramer-Bottiglio R. Highly stretchable multilayer electronic circuits using biphasic gallium-indium. Nat Mater. 2021;20(6):851–858. doi:10.1038/s41563-021-00921-8
138. Lu T, Markvicka EJ, Jin Y, Majidi C. Soft-Matter Printed Circuit Board with UV Laser Micropatterning. ACS Appl Mater Interfaces. 2017;9(26):22055–22062. doi:10.1021/acsami.7b05522
139. Ma Y, Pharr M, Wang L, et al. Soft Elastomers with Ionic Liquid-Filled Cavities as Strain Isolating Substrates for Wearable Electronics. Small. 2017;13(9):1602954. doi:10.1002/smll.201602954
140. Chen Z, Gao N, Chu Y, He Y, Wang Y. Ionic Network Based on Dynamic Ionic Liquids for Electronic Tattoo Application. ACS Appl Mater Interfaces. 2021;13(28):33557–33565. doi:10.1021/acsami.1c09278
141. Chossat JB, Tao Y, Duchaine V, Park YL Wearable soft artificial skin for hand motion detection with embedded microfluidic strain sensing.
142. Sun JY, Keplinger C, Whitesides GM, Suo Z. Ionic skin. Adv Mater. 2014;26(45):7608–7614. doi:10.1002/adma.201403441
143. Macklem PT. Complexity and respiration: a matter of life and death. Physiol Basis Respir Dis. 2005;1:605–609.
144. Macklem PT. Emergent phenomena and the secrets of life. J Appl Physiol. 2008;104(6):1844–1846. doi:10.1152/japplphysiol.00942.2007
145. Lipsitz LA, Goldberger AL. Loss of Complexity and Aging - Potential Applications of Fractals and Chaos Theory to Senescence. JAMA. 1992;267(13):1806–1809. doi:10.1001/jama.267.13.1806
146. Yentes JM, Denton W, Samson K, Schmid KK, Wiens C, Rennard S. Energy efficient physiologic coupling of gait and respiration is altered in chronic obstructive pulmonary disease. Acta Physiologica. 2018:2:548 doi:10.1111/apha.13217.
147. McCamley J, Denton W, Lyden E, Yentes JM. Measuring Coupling of Rhythmical Time Series Using Cross Sample Entropy and Cross Recurrence Quantification Analysis. Comput Math Methods Med. 2017;2017:7960467. doi:10.1155/2017/7960467
148. Kon SSC, Canavan JL, Nolan CM, et al. The 4-metre gait speed in COPD: responsiveness and minimal clinically important difference. Eur Respir J. 2014;43(5):1298–1305. doi:10.1183/09031936.00088113
149. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64. doi:10.1164/rccm.201309-1634ST
150. Delbressine JM, Vaes AW, Goërtz YM, et al. Effects of Exercise-Based Interventions on Fall Risk and Balance in Patients With Chronic Obstructive Pulmonary Disease: a SYSTEMATIC REVIEW. J Cardiopulm Rehabil Prev. 2020;40(3):152–163. doi:10.1097/HCR.0000000000000513
151. Holland AE, Cox NS, Houchen-Wolloff L, et al. Defining Modern Pulmonary Rehabilitation. An Official American Thoracic Society Workshop Report. Annals ATS. 2021;18(5):e12–e29. doi:10.1513/AnnalsATS.202102-146ST
152. McClellan R, Amiri HM, Limsuwat C, Nugent KM. Pulmonary Rehabilitation Increases Gait Speed in Patients With Chronic Lung Diseases. Health Serv Res Manag Epidemiol. 2014;1:2333392814533659. doi:10.1177/2333392814533659
153. Wouters EF, Posthuma R, Koopman M, et al. An update on pulmonary rehabilitation techniques for patients with chronic obstructive pulmonary disease. Expert Rev Respir Med. 2020;14(2):149–161. doi:10.1080/17476348.2020.1700796
154. Willeput R, Vachaudez JP, Lenders D, Nys A, Knoops T, Sergysels R. Thoracoabdominal motion during chest physiotherapy in patients affected by chronic obstructive lung disease. Respiration. 1983;44(3):204–214. doi:10.1159/000194550
155. Fernandes M, Cukier A, Feltrim MI. Efficacy of diaphragmatic breathing in patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2011;8(4):237–244. doi:10.1177/1479972311424296
156. Yamaguti WP, Claudino RC, Neto AP, et al. Diaphragmatic breathing training program improves abdominal motion during natural breathing in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Arch Phys Med Rehabil. 2012;93(4):571–577. doi:10.1016/j.apmr.2011.11.026
157. Cancelliero-Gaiad KM, Ike D, Pantoni CBF, Borghi-Silva A, Costa D. Respiratory pattern of diaphragmatic breathing and pilates breathing in COPD subjects. Braz J Phys Ther. 2014;18(4):291–299. doi:10.1590/bjpt-rbf.2014.0042
158. McNamara RJ, Epsley C, Coren E, McKeough ZJ. Singing for adults with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;2017(12):CD012296. doi:10.1002/14651858.CD012296.pub2
159. Kaasgaard M, Rasmussen DB, Andreasson KH, et al. Use of Singing for Lung Health as an alternative training modality within pulmonary rehabilitation for COPD: a randomised controlled trial. Eur Respir J. 2022;59(5):51. doi:10.1183/13993003.01142-2021
160. Lewis A, Philip KEJ, Lound A, Cave P, Russell J, Hopkinson NS. The physiology of singing and implications for ‘Singing for Lung Health’ as a therapy for individuals with chronic obstructive pulmonary disease. BMJ Open Respir Res. 2021;8(1):e000996. doi:10.1136/bmjresp-2021-000996
161. MacBean V, Reilly CC, Rafferty GF, Kolyra E. Dance as a rehabilitative strategy for patients with COPD. Eur Respir J. 2017;50(suppl 61):2154. doi:10.1183/1393003.congress-2017.PA3715
162. Harrison S, Bierski K, Burn N, et al. Dance for people with chronic breathlessness: a transdisciplinary approach to intervention development. BMJ Open Respir Res. 2020;7(1):e000696. doi:10.1136/bmjresp-2020-000696
163. Fulambarker A, Farooki B, Kheir F, Copur AS, Srinivasan L, Schultz S. Effect of yoga in chronic obstructive pulmonary disease. Am J Ther. 2012;19(2):96–100. doi:10.1097/MJT.0b013e3181f2ab86
164. Kaminsky DA, Guntupalli KK, Lippmann J, et al. Effect of Yoga Breathing (Pranayama) on Exercise Tolerance in Patients with Chronic Obstructive Pulmonary Disease: a Randomized, Controlled Trial. J Altern Complement Med. 2017;23(9):696–704. doi:10.1089/acm.2017.0102
165. Gao P, Tang F, Liu W, He K, Mo Y. Effect of liuzijue qigong on patients with stable chronic obstructive pulmonary disease. Medicine. 2021;100(41):e27344. doi:10.1097/MD.0000000000027344
166. Xu S, Zhang D, He Q, et al. Efficacy of Liuzijue Qigong in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Complement Ther Med. 2022;65:102809. doi:10.1016/j.ctim.2022.102809
167. Ngai SPC, Jones AYM, Tam WWS. Tai Chi for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2016;4(6):CD009953. doi:10.1002/14651858.CD009953.pub2
168. Gilliam EA, Cheung T, Kraemer K, et al. The impact of Tai Chi and mind-body breathing in COPD: insights from a qualitative sub-study of a randomized controlled trial. PLoS One. 2021;16(4):e0249263. doi:10.1371/journal.pone.0249263
169. Kantatong T, Panpanich R, Deesomchok A, Sungkarat S, Siviroj P. Effects of the tai chi qigong programme on functional capacity, and lung function in chronic obstructive pulmonary disease patients: a ramdomised controlled trial. J Tradit Complement Med. 2020;10(4):354–359. doi:10.1016/j.jtcme.2019.03.008
170. Ehsani H, Mohler MJ, Golden T, Toosizadeh N. Upper-extremity function prospectively predicts adverse discharge and all-cause COPD readmissions: a pilot study. COPD. 2018;14:39–49. doi:10.2147/COPD.S182802
171. Rahman MJ, Nemati E, Rahman M, Vatanparvar K, Nathan V, Kuang J. Toward Early Severity Assessment of Obstructive Lung Disease Using Multi-Modal Wearable Sensor Data Fusion During Walking. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:5935–5938. doi:10.1109/EMBC44109.2020.9176559
172. Gerus P, Rao G, Berton E. Subject-Specific Tendon-Aponeurosis Definition in Hill- Type Model Predicts Higher Muscle Forces in Dynamic Tasks. PLoS One. 2012;7:e44406. doi:10.1371/journal.pone.0044406
173. Davis LC, Baumer TG, Bey MJ. Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography. 2018;38(1):2–12. doi:10.14366/usg.18039
174. Boffino CC, Pereira ACAC, Coelho DB, et al. Age and Disease have a Distinct Influence on Postural Balance of Patients with COPD. COPD. 2019;16(3–4):246–253. doi:10.1080/15412555.2019.1634683
175. de Castro LA, Felcar JM, de Carvalho DR, et al. Effects of land- and water-based exercise programmes on postural balance in individuals with COPD: additional results from a randomised clinical trial. Physiotherapy. 2020;107:58–65. doi:10.1016/j.physio.2019.08.001
176. Chauvin S, Kirkwood R, Brooks D, Goldstein RS, Beauchamp M. Which Balance Subcomponents Distinguish Between Fallers and Non-Fallers in People with COPD? COPD. 2020;15:1557–1564. doi:10.2147/COPD.S253561
177. Karpman C, DePew ZS, LeBrasseur NK, Novotny PJ, Benzo RP. Determinants of Gait Speed in COPD. Chest. 2014;146(1):104–110. doi:10.1378/chest.13-2017
178. Casabona A, Valle MS, Laudani L, et al. Is the Power Spectrum of Electromyography Signal a Feasible Tool to Estimate Muscle Fiber Composition in Patients with COPD? JCM. 2021;10(17):3815. doi:10.3390/jcm10173815
179. de Sá RB, Pessoa MF, Cavalcanti AGL, Campos SL, Amorim C. Immediate effects of respiratory muscle stretching on chest wall kinematics and electromyography in COPD patients. Respir Physiol Neurobiol. 2017;242:1–7. doi:10.1016/j.resp.2017.03.002
180. Bhatt SP, Bodduluri S, Newell JD, et al. CT-derived Biomechanical Metrics Improve Agreement Between Spirometry and Emphysema. Acad Radiol. 2016;23(10):1255–1263. doi:10.1016/j.acra.2016.02.002
181. Binazzi B, Lanini B, Gigliotti F, Scano G. Breathing Pattern and Chest Wall Kinematics during Phonation in Chronic Obstructive Pulmonary Disease Patients. Respiration. 2013;86(6):462–471. doi:10.1159/000346027
182. Bodduluri S, Bhatt SP, Hoffman EA, et al. Biomechanical CT metrics are associated with patient outcomes in COPD. Thorax. 2017;72(5):409–414. doi:10.1136/thoraxjnl-2016-209544
183. Capeletti AM. Can a physical activity similar to activities of daily living cause dynamic hyperinflation and change the thoracoabdominal configuration in patients with chronic obstructive pulmonary disease? COPD. 2019;14:1281–1287. doi:10.2147/COPD.S196223
184. Chynkiamis N, Lane ND, Megaritis D, et al. Effect of portable noninvasive ventilation on thoracoabdominal volumes in recovery from intermittent exercise in patients with COPD. J Appl Physiol. 2021;131(1):401–413. doi:10.1152/japplphysiol.00081.2021
185. Gagliardi E, Innocenti Bruni G, Presi I, Gigliotti F, Scano G. Thoraco-abdominal motion/displacement does not affect dyspnea following exercise training in COPD patients. Respir Physiol Neurobiol. 2014;190:124–130. doi:10.1016/j.resp.2013.10.005
186. Kruapanich C, Tantisuwat A, Thaveeratitham P, et al. The effect of unsupported arm elevations on regional chest wall volumes and thoracoabdominal asynchrony in patients with chronic obstructive pulmonary disease. Physiother Theory Pract. 2021:1–13. doi:10.1080/09593985.2021.1882018
187. Lee CT, Chien JY, Hsu MJ, Wu HD, Wang LY. Inspiratory muscle activation during inspiratory muscle training in patients with COPD. Respir Med. 2021;190:106676. doi:10.1016/j.rmed.2021.106676
188. Mendes LP, Moraes KS, Hoffman M, et al. Effects of Diaphragmatic Breathing With and Without Pursed-Lips Breathing in Subjects With COPD. Respir Care. 2019;64(2):136–144. doi:10.4187/respcare.06319
189. Priori R, Aliverti A, Albuquerque AL, Quaranta M, Albert P, Calverley PMA. The effect of posture on asynchronous chest wall movement in COPD. J Appl Physiol. 2013;114(8):1066–1075. doi:10.1152/japplphysiol.00414.2012
190. Romagnoli I, Gigliotti F, Lanini B, et al. Chest wall kinematics and breathlessness during unsupported arm exercise in COPD patients. Respir Physiol Neurobiol. 2011;178(2):242–249. doi:10.1016/j.resp.2011.06.014
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.