Back to Journals » Journal of Inflammation Research » Volume 16
Unveiling COVID-19 Secrets: Harnessing Cytokines as Powerful Biomarkers for Diagnosis and Predicting Severity
Authors Wolszczak-Biedrzycka B , Dorf J , Wojewódzka-Żelezniakowicz M, Żendzian-Piotrowska M , Dymicka-Piekarska VJ , Matowicka-Karna J , Maciejczyk M
Received 8 October 2023
Accepted for publication 21 November 2023
Published 11 December 2023 Volume 2023:16 Pages 6055—6070
DOI https://doi.org/10.2147/JIR.S439217
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Blanka Wolszczak-Biedrzycka,1 Justyna Dorf,2 Marzena Wojewódzka-Żelezniakowicz,3 Małgorzata Żendzian-Piotrowska,4 Violetta Joanna Dymicka-Piekarska,2 Joanna Matowicka-Karna,2 Mateusz Maciejczyk4
1Department of Psychology and Sociology of Health and Public Health, University of Warmia and Mazury in Olsztyn, Olsztyn, 10-900, Poland; 2Department of Clinical Laboratory Diagnostics, Medical University of Bialystok, Bialystok, 15-089, Poland; 3Department of Emergency Medicine and Disasters, Medical University of Bialystok, Bialystok, 15-089, Poland; 4Department of Hygiene, Epidemiology and Ergonomics, Medical University of Bialystok, Bialystok, 15-089, Poland
Correspondence: Blanka Wolszczak-Biedrzycka, Email [email protected]
Introduction: In coronavirus disease (COVID-19), inflammation takes center stage, with a cascade of cytokines released, contributing to both inflammation and lung damage. The objective of this study is to identify biomarkers for diagnosing and predicting the severity of COVID-19.
Materials and Methods: Cytokine levels were determined in the serum from venous blood samples collected from 100 patients with COVID-19 and 50 healthy controls. COVID-19 patients classified based on the Modified Early Warning (MEWS) score. Cytokine concentrations were determined with a multiplex ELISA kit (Bio-Plex Pro™ Human Cytokine Screening Panel).
Results: The concentrations of all analyzed cytokines were elevated in the serum of COVID-19 patients relative to the control group, but no significant differences were observed in interleukin-9 (IL-9) and IL-12 p70 levels. In addition, the concentrations of IL-1α, IL-1β, IL-1ra, IL-2Rα, IL-6, IL-12 p40, IL-18, and tumor necrosis factor alpha (TNFα) were significantly higher in symptomatic patients with accompanying pneumonia without respiratory failure (stage 2) than in asymptomatic/mildly symptomatic patients (stage 1).
Conclusion: The study revealed that IL-1ra, IL-2Rα, IL-6, IL-8, IL-12 p40, IL-16, and IL-18 levels serve as potential diagnostic biomarkers in COVID-19 patients. Furthermore, elevated IL-1α levels proved to be valuable in assessing the severity of COVID-19.
Keywords: cytokines, COVID-19, MEWS score, biomarkers, severity, diagnosis
Introduction
The pathogenesis of the cytokine storm (CS) in coronavirus disease 2019 (COVID-19), which is observed mainly in patients with severe symptoms of the disease, has attracted considerable research interest.1 Clinical data indicate that most patients who died from COVID-19 and patients with severe symptoms of the disease did not present with serious clinical symptoms in the early stage of infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 virus).2 The clinical state of these patients deteriorated unexpectedly in the subsequent stages of disease, and the acute respiratory distress syndrome (ARDS) and the multiple organ dysfunction syndrome (MODS) led to death within a short period of time.3,4
When a compromised immune system is unable to fight the infection, the inflammation can trigger hypercytokinemia, commonly referred to as the CS, followed by the cytokine release syndrome (CRS), also known as the cytokine storm syndrome (CSS).5,6 The CS, in particular the local overproduction of cytokines, is the key determinant of disease severity and mortality in patients infected with the SARS-CoV-2 virus.7 Numerous studies have shown that ARDS in severe/critical COVID-19 is not directly triggered by the virus’ mechanism of action, but occurs in consequence of CS/CRS.7–9 The spike (S) protein on the surface of the SARS-CoV-2 virus activates alveolar epithelial cells, macrophages, and circulating blood monocytes via Toll-like membrane receptors and stimulates the production of proinflammatory cytokines and chemokines which mobilize immune cells, in particular monocytes and T cells, leading to pneumonia.10 In patients infected with the SARS-CoV-2 virus, an inflammation develops due to an imbalance in the activity of proinflammatory and anti-inflammatory cytokines.11 Cytokines are proteins synthesized by cells that directly participate in the immune response. Based on their function, cytokines can be divided into the following groups: interleukins (IL), tumor necrosis factors (TNF), transforming growth factors (TGF), colony-stimulating factors (CSF), and chemokines.12,13 Cytokines have pleiotropic properties because they act on different cell populations and elicit different effects.14 Cytokines can also enter into synergistic or antagonistic interactions.15 Cytokines are released in response to viral infections, including infections with the SARS-CoV-2 virus.16 When the immune system is compromised, the body cannot fight the infection, which leads to CS and, consequently, CRS.17 The CS increases the severity of disease symptoms and the risk of mortality in patients infected with the SARS-CoV-2.18,19 According to clinical data, most critically ill COVID-19 patients and patients who died from COVID-19 did not present with serious clinical symptoms in the early stage of infection. Their condition deteriorated rapidly, and they died from ARDS and MODS within a short period of time.16,20,21
Proinflammatory cytokines include interleukins (ILs) IL-1α, IL-1β, IL-2Ra, IL-6, IL-8, IL-17, IL-18, and IL-23, as well as the tumor necrosis factors α and β (TNFα and TNFβ).12,22 The main anti-inflammatory cytokines include the IL-1 receptor antagonist (IL-1ra), IL-4, IL-9, and IL-10.23
Research has confirmed that the SARS-CoV-2 virus selectively induces high IL-6 levels and that IL-6 levels are significantly correlated with disease severity.24 A similar correlation has been reported for IL-8, IL-10, and IL-17.25,26 High levels of these cytokines, in particular IL-6, are unfavorable prognostic markers because they shorten survival.27
Infections caused by the SARS-CoV-2 virus with a similar severity of early symptoms have a high risk of complications and, in extreme cases, can lead to death, especially among the elderly.28 This study aims to pinpoint specific biomarkers that can serve both as diagnostic indicators and predictors of the severity of COVID-19. The role of various cytokines in the progression of COVID-19 was evaluated by analyzing the activity of proinflammatory and anti-inflammatory cytokines in patients infected with the SARS-CoV-2 virus and experiencing different severity of disease symptoms.
Materials and Methods
Experimental Design
The study was approved by the Bioethics Committee of the Medical University in Białystok (decision No. APK.002.353.2021–28.01.2021 r.). The study was conducted in accordance with the World Medical Association Declaration of Helsinki for ethical principles for medical research involving human subjects. All research participants gave their written consent to participate in the study.
Studied COVID-19 Population
The studied population consisted of non-vaccinated 100 patients with a positive result of a COVID-19 PCR test (nasopharyngeal swab) who were admitted to the Emergency Department of the University Clinical Hospital in Białystok between 20 January and 20 November 2021.
The severity of COVID-19 was assessed based on the Modified Early Warning Score (MEWS) which is recommended by the Polish Society of Epidemiology and Infectious Diseases and relies on the following parameters: systolic blood pressure, heart rate, respiratory rate, body temperature, and neurological symptoms. Four stages of COVID-19 progression were described based on the above parameters: 1) asymptomatic and mildly symptomatic infection, 2) symptomatic infection with pneumonia without symptoms of ARDS, 3) symptomatic infection with pneumonia and symptoms of ARDS, 4) symptomatic infection with MODS (Table S1).
The studied population was divided into two groups. Group 1 consisted of asymptomatic and mildly symptomatic patients (stage 1), whereas group 2 consisted of symptomatic patients with pneumonia without symptoms of ARDS (stage 2) and symptomatic patients with pneumonia and symptoms of ARDS (stage 3). None of the studied patients presented with disease symptoms characteristic of stage 4 based on the MEWS score.29
The studied patients were subjected to body mass index (BMI), imaging examinations (radiography and computed tomography of the chest) and laboratory tests, including complete blood count (CBC), coagulation parameters (PT, APTT, D-dimers), kidney function tests (creatinine levels with estimated glomerular filtration rate (eGFR), urea), electrolyte levels (Na+, K+), and lactate dehydrogenase (LDH) activity, oxygen saturation and C-reactive protein (CRP). Demographic parameters (sex, age), length of hospital stay (days), comorbidities (present, absent), hematological disorders (present, absent), diabetes (present, absent), hypertension (present, absent), obesity (present, absent), heart disease (present, absent), history of cancer (present, absent), and clinical symptoms, including fever (present, absent), cough (present, absent), dyspnea (present, absent) and ARDS (present, absent), were analyzed.
In both groups, blood for analyses was collected form the basilic vein into clot activator tubes. The serum was separated by centrifugation (1000 × g, 20 minutes), and the samples were stored at a temperature of −80°C until analysis.
Control Group
The control group consisted of 50 healthy subjects without a history of COVID-19 or reported comorbidities.
Cytokine Detection
Interleukins IL-1α, IL-1β, IL-1ra, IL-2Rα, IL-4, IL-6, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-16, IL-17, IL-18, TNFα, and TNFβ were identified with the use of the Bio-Plex Pro™ Human Cytokine Screening Panel (Biorad) and the Bio-Plex Multiplex system based on the Luminex xMAP technology. The Bio-Plex technology relies on the ELISA method. Antibodies targeting specific biomarkers form covalent bonds with magnetic beads. In the next stage, magnetic beads react with the samples containing these biomarkers. The sample is rinsed to remove unbound protein, and a biotinylated detection antibody is added to produce a sandwich compound. The final product (complex) is obtained by adding streptavidin phycoerythrin (SA-PE) conjugate. The results are read in the Bio-Plex 200 system. The efficacy of the applied method is comparable to that of a standard ELISA assay.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 9.0 (GraphPad Software, La Jolla, USA). The Shapiro–Wilk test was used to determine the normality of distribution.
In the case of the lack of normal distribution, the Mann–Whitney U-test was used. The results were presented as median (minimum-maximum), and the value of p<0.05 was considered statistically significant. Using the corresponding receiver operating characteristic (ROC) curve approach and computing the area under the curve (AUC), the diagnosis and prediction of the severity indices were evaluated. Youden’s index was used to establish the best cutoff values for biomarkers.
Results
Characteristics of the Study Group
A total of 100 COVID-19 patients aged 36 to 87 were analyzed. The studied population consisted of 35% males and 65% females. The patients were divided into two groups based on their MEWS scores (Table S1): stage 1 (asymptomatic/mildly symptomatic) – 53%, and stages 2+3 (pneumonia without/with ARDS) – 47%. Approximately 67% of the patients were hospitalized for less than 10 days, 12% – for 10–20 days, and 21% – for more than 20 days. Comorbidities, mainly hypertension (36%), ischemic heart disease (26%) and diabetes (17%), were reported by 57% of the studied subjects. The most prevalent symptoms were fever and dyspnea which were noted in 37% and 35% of the patients, respectively. The studied population with laboratory test results is presented in Table 1.
 |
Table 1 Characteristics of the Studied Population with Selected Laboratory Test Results |
A Comparison of Proinflammatory and Anti-Inflammatory Cytokine Levels in COVID-19 Patients and the Control Group
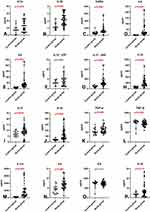
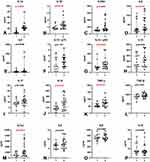
The cytokine analysis revealed that the concentrations of all evaluated proteins were higher in COVID-19 patients than in the control group. With the exception of IL-9 and IL-12 p 70, the observed differences were statistically significant (p<0.05) (Figure 1 and Table 2).
 |
Table 2 Serum Levels of Proinflammatory and Anti-Inflammatory Cytokines in COVID-19 Patients and the Control Group |
A Comparison of Proinflammatory and Anti-Inflammatory Cytokine Levels in COVID-19 Patients with Different MEWS Scores
The concentrations of IL-1α, IL-1β, IL-1ra, IL-2Rα, IL-6, IL-12 p40, IL-18, and TNFα were also significantly higher in symptomatic patients with accompanying pneumonia without symptoms of ARDS (group 2) than in asymptomatic or mildly symptomatic patients (group 1) (Figure 2 and Table 3).
A ROC Analysis of Pro- and Anti-Inflammatory Cytokine Levels for Differentiating Between COVID-19 Patients and the Control Group
The ROC analysis revealed that the evaluated cytokines are potentially useful parameters for differentiating between COVID-19 patients and healthy subjects (Table 4). The optimal cut-off values were calculated, and the ROC curves are presented in Figure 3. The area under the curve (AUC) >0.9 calculated for IL-1ra, IL-6, IL-8, IL-12 p40, IL-16 was determined at 0.9633, 0.9864, 0.9642, 0.9304, 0.9468 respectively.
 |
Table 4 Area Under the Curve (AUC) for the Analyzed Cytokines for Differentiating Between COVID-19 Patients and the Control Group |
 |
Figure 3 Receiver operating characteristic (ROC) curves of proinflammatory (A–F) and anti- inflammatory (G) cytokines for differentiating between COVID-19 patients and the control group. |
A ROC Analysis of Proinflammatory and Anti-Inflammatory Cytokines for Differentiating COVID-19 Patients with Different MEWS Scores
The ROC analysis demonstrated that the evaluated cytokines are potentially useful biomarkers for differentiating COVID-19 patients with different disease severity (Table 5). The optimal cut-off values were calculated, and the ROC curves are presented in Figure 4. The AUC value >0.9 calculated for AUC IL-1α and were determined at 0.9611.
 |
Table 5 Area Under the Curve (AUC) for the Analyzed Cytokines for Differentiating COVID-19 Patients with Different MEWS Scores |
 |
Figure 4 Receiver operating characteristic (ROC) curves of proinflammatory (A–E) and anti- inflammatory (F) cytokines for differentiating COVID-19 patients with different MEWS scores. |
Discussion
New biomarkers for effective diagnosis of COVID-19 are needed. Our previous study of inflammatory markers in patients infected with the SARS-CoV-2 virus demonstrated that IL-6 levels increase with the severity of COVID-19 symptoms classified based on the MEWS score.30 The present study was undertaken to examine the concentrations of other proinflammatory and anti-inflammatory cytokines in COVID-19 patients and to assess, for the first time, the relationship between changes in cytokine levels and the MEWS score. The MEWS score is calculated based on systolic blood pressure, heart rate, respiratory rate, body temperature, and neurological parameters, and it is used to classify patients into four groups/stages of COVID-19 severity. Diagnostic scales are useful tools for assessing disease progression in clinical practice.31 In the literature, the correlations between selected cytokine levels and the MEWS score have been examined in various diseases, including acute ischemic stroke21 and cirrhosis,32 but these parameters have never been assessed in patients infected with the SARS-CoV-2 virus.
In the present study, the concentrations of all cytokines and TNF were significantly higher in patients infected with the SARS-CoV-2 virus than in healthy subjects, which confirms that cytokines are released in COVID-19 patients. Based on their MEWS scores, the studied COVID-19 patients were classified into stage 1 (asymptomatic or mildly symptomatic) or stage 2 (pneumonia without ARDS) of disease progression. Therefore, a significant increase in cytokine levels (mainly IL1-ra, IL-2ra, IL-6, IL-8, IL-12p40, IL-16, IL-18) in the early stage of infection can be an important diagnostic factor, in particular in mildly symptomatic patients, that can be helpful in selecting the appropriate treatment, preventing disease progression and worsening of symptoms. It should also be noted that most of the analyzed cytokines were also highly useful markers for diagnosing COVID-19 (mainly IL-1ra, IL-6, IL-8, IL-16) and differentiating between patients with different disease severity (IL-1α). The AUC for this proteins were >0.9, sensitivity and specificity >90%.
According to research, IL-1, IL-6, and TNF-α play a particularly important role in the initiation of CS in patients infected with the SARS-CoV-2 virus.33–35 Liu et al36 analyzed the cytokine profiles of COVID-19 patients and found that cytokine levels were significantly higher in the blood plasma of infected patients than in healthy subjects. They also reported a strong correlation between severe lung damage and elevated concentrations of IFN-γ, IFN-α2, IL-1ra, IL-2, 4, 6, 10, 12 and 17.
Interleukin-1 is a group of cytokines that includes IL-1α, IL-1β, and IL-1ra. These cytokines stimulate the immune system and induce inflammatory processes by activating T cells, eosinophils, basophils, dendritic cells, and natural killer (NK) cells.35,37 This observation which explains the significant increase in IL-1α, IL-1β, and IL-1ra concentrations in the current study.
The concentration of IL-18 also was also significantly higher in COVID-19 patients than in healthy subjects, and this parameter increased with a rise in the severity of disease symptoms. This cytokine is produced by macrophages, dendritic cells, and epithelial cells, and it induces Th1-, Th2-, and Th17-mediated immune responses, which increases the synthesis of IL-4 and IL-13.36,38,39 Interleukin-18 can trigger a strong inflammatory response, which suggests that this cytokine may have a pathophysiological function in diseases associated with inflammation, including COVID-19.40–43 According to many researchers, IL-6 plays a key role in immune system regulation, which is why this cytokine should be regarded as a priority biomarker in SARS-CoV-2 infection.24,25,27 This cytokine is produced mainly by monocytes and macrophages, and, to a smaller extent, by T and B cells, vascular endothelial cells, keratinocytes, and chondrocytes.44 Interleukin-6 has pleiotropic activity, and it exerts various effects on innate and adaptive immune system cells.25 This cytokine activates the differentiation of B cells, stimulates cytotoxic T cells, stimulates the secretion of IL-2 by T cells and the production of acute-phase proteins (APPs) by liver cells.44–47 Interleukin-6 binds to the transmembrane IL-6 receptor (IL-6R) to produce the IL-6/IL-6R complex,48 which results in the formation of the gp130 protein homodimer and the activation of the intercellular signal transduction pathway involving TYK-2, JAK1, and JAK2 kinase → transcription factor STAT3 → IL-6 transcription (Chonov et al 2019; Hwang et al 2019; Zheng et al 2021). Research has shown that elevated IL-6 levels can activate the clotting system and increase vascular permeability, which promotes the spread of pro-inflammatory mediators and numerous inflammatory cells.49,50 In the present study, D-dimer levels were significantly higher in patients with pneumonia without ARDS (stage 2) than in asymptomatic or mildly symptomatic patients (stage 1), which points to intensified coagulation and fibrinolysis in this group of patients. It has been previously investigated that D-dimer is associated with COVID-19 mortality.51,52 Elevated IL-6 levels are associated with lymphopenia, decreased cytotoxicity of Tc-CD8+ cells, and activation of the vascular endothelium.53–55 The concentration of IL-6 also increases significantly as a result of inflammatory damage to the lungs.56 In the present study and in our previous research, IL-6 levels were significantly elevated in patients with SARS-CoV-2 infection.
Tumor necrosis factor α also plays an important role in SARS-CoV-2 infection and CS.57 The concentration of TNF-α increases at the beginning of infection, remains elevated during disease progression, and increases considerably when the patient’s condition deteriorates.58 This cytokine is produced by macrophages, monocytes, neutrophils, activated lymphocytes, mast cells, astrocytes, adipocytes, and smooth muscle cells. Similarly to IL-6, TNF-α stimulates the secretion of APPs.59,60 These observations explain the observed increase in TNF-α concentration in COVID-19 patients in the current study.
The statistical analysis revealed a significant (p<0.05) increase in the concentrations of IL-1α, IL-1β, IL-1ra, IL-2Rα, IL-6, IL-12 p40, IL-18, and TNFα with a rise in disease severity classified based on the MEWS score. Diagnostic scales are highly useful in clinical practice because they facilitate the assessment of a patient’s condition and the selection of the optimal treatment.61–64 In Poland, most COVID-19 patients were classified based on the MEWS score65 because a diagnostic scale specific for this disease has not been developed. Potential biomarkers of significant differences between groups of patients characterized by minor differences in disease symptoms are needed. Significant differences in cytokine levels between asymptomatic/mildly symptomatic patients and patients with moderate symptoms of COVID-19 have not been described in the literature to date. In studies where cytokine levels were analyzed, only high levels of IL-6, TNFα, and IL-2ra were reported in patients with severe COVID-19.17,66–70 An analysis of 10 cohort studies involving a total of 1798 patients with severe COVID-19 revealed elevated IL-6 concentration in all patients.35,71–76 The serum levels of IL-6 were determined at 517–796 pg/mL in patients with ARDS, and they decreased to 42.9–94.7pg/mL in convalescents. In another study, IL-6 levels were also markedly higher in 86.8% patients with severe COVID-19.74
The results of this study can be useful for differentiating patients in the early stage of SARS-CoV-2 infection and identifying patients whose health condition can deteriorate rapidly due to the excessive production of inflammatory cytokines. It should be noted that IL-6 has been included in the panel of laboratory tests for assessing the condition of COVID-19 patients, and similarly to TNFα, IL-6 concentration is determined routinely in many laboratories.77,7879–82The changes of serum concentrations of those proteins are really useful and help to diagnose and monitor the course of the disease patients with COVID-19.
In view of the above, new prognostic markers should be developed and included in routine clinical practice to effectively diagnose patients infected with the SARS-CoV-2 virus in different stages of the disease. The present findings indicate that the analyzed cytokines have potential diagnostic value for identifying patients with mild symptoms in the initial stage of COVID-19 and downregulating CS which has potentially life-threatening consequences. The research showed that IL-1ra, IL-2Rα, IL-6, IL-8, IL-12 p40, IL-16, and IL-18 levels serve as potential diagnostic biomarkers in COVID-19 patients. What is more, increased IL-1α levels proved to be valuable in assessing the severity of COVID-19.
Finally, some of limitations of our study are also worth considering. Our study has been conducted on a small group of patients (100 patients) divided into two smaller groups according to the MEWS scale. Moreover, our patients participating in the study were diagnosed with only stage 1 and stage 2 of COVID- 19. Further research should be carried out on a greater group of patients with COVID-19 including 4 stages of COVID-19. Our study may be a pre-eliminary step for further clinical trials evaluating the chemokines and growth factors levels and their diagnostic utility in a larger population of patients with COVID-19.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:11. doi:10.3389/fimmu.2020.01708
2. Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54. doi:10.1016/j.cytogfr.2020.06.001
3. Soy M, Keser G, Atagündüz P. Pathogenesis and treatment of cytokine storm in covid-19. Turk J Biol. 2021;45(SI–1):372–389. doi:10.3906/biy-2105-37
4. Kim JS, Lee JY, Yang JW, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2020;11(1). doi:10.7150/thno.49713
5. Valizadeh H, Abdolmohammadi-vahid S, Danshina S, et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int Immunopharmacol. 2020:89. doi:10.1016/j.intimp.2020.107088
6. Choi JH, Lee YH, Kwon TW, Ko SG, Nah SY, Cho IH. Can Panax ginseng help control cytokine storm in COVID-19? J Ginseng Res. 2022;46(3):337–347. doi:10.1016/j.jgr.2022.02.006
7. Tang L, Yin Z, Hu Y, Mei H, Zhu L. Controlling Cytokine Storm Is Vital in COVID-19. Front Immunol. 2020;11:11. doi:10.3389/fimmu.2020.570993
8. Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39(7):2085–2094. doi:10.1007/s10067-020-05190-5
9. Kutleša M. The cytokine storm and COVID-19. Medicus. 2020;29:2.
10. Rodda LB, Netland J, Shehata L, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2020;184(1):169–183.e17. doi:10.1016/J.CELL.2020.11.029
11. Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–331. doi:10.23812/CONTI-E
12. Xiao T, Yan Z, Xiao S, Xia Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res Ther. 2020;11(1). doi:10.1186/s13287-020-01755-y
13. Bradding P, Roberts JA, Britten KM, et al. Interleukin-4, −5, and −6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10(5):471–480. doi:10.1165/AJRCMB.10.5.8179909
14. Zanza C, Romenskaya T, Manetti AC, et al. Cytokine Storm in COVID-19: immunopathogenesis and Therapy. Medicina. 2022;58(2). doi:10.3390/medicina58020144
15. Niles MA, Gogesch P, Kronhart S, et al. Macrophages and dendritic cells are not the major source of pro-inflammatory cytokines upon SARS-CoV-2 infection. Front Immunol. 2021:12. doi:10.3389/fimmu.2021.647824
16. Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta. 2020;509. doi:10.1016/j.cca.2020.06.017
17. Olbei M, Hautefort I, Modos D, et al. SARS-CoV-2 Causes a different cytokine response compared to other cytokine storm-causing respiratory viruses in severely ill patients. Front Immunol. 2021:12. doi:10.3389/fimmu.2021.629193
18. Pum A, Ennemoser M, Adage T, Kungl AJ. Cytokines and chemokines in SARS-CoV-2 infections—therapeutic strategies targeting cytokine storm. Biomolecules. 2021;11(1):91. doi:10.3390/biom11010091
19. Ngo B, Lapp SA, Siegel B, et al. Cerebrospinal fluid cytokine, chemokine, and SARS-CoV-2 antibody profiles in children with neuropsychiatric symptoms associated with COVID-19. Mult Scler Relat Disord. 2021:55. doi:10.1016/j.msard.2021.103169
20. Sherwani S, Khan MWA. Cytokine response in SARS-CoV-2 infection in the Elderly. J Inflamm Res. 2020;13. doi:10.2147/JIR.S276091
21. Zhang L, Xu D, Zhang T, Hou W, Yixi L. Correlation between interleukin-6, interleukin-8, and modified early warning score of patients with acute ischemic stroke and their condition and prognosis. Ann Palliat Med. 2021;10(1). doi:10.21037/apm-20-2200
22. Kosidło JW, Wolszczak-Biedrzycka B, Dymicka-Piekarska V, Dorf J, Matowicka-Karna J. Clinical significance and diagnostic utility of NLR, LMR, PLR and SII in the Course of COVID-19: a Literature Review. J Inflamm Res. 2023;16:539–562. doi:10.2147/JIR.S395331
23. Hasanvand A. COVID-19 and the role of cytokines in this disease. Inflammopharmacology. 2022;30(3):789–798. doi:10.1007/s10787-022-00992-2
24. Potere N, Batticciotto A, Vecchié A, et al. The role of IL-6 and IL-6 blockade in COVID-19. Expert Rev Clin Immunol. 2021;17(6):601–618. doi:10.1080/1744666X.2021.1919086
25. Wang X, Tang G, Liu Y, et al. The role of IL-6 in coronavirus, especially in COVID-19. Front Pharmacol. 2022:13. doi:10.3389/fphar.2022.1033674
26. Smail SW, Babaei E, Amin K, Abdulahad WH. Serum IL-23, IL-10, and TNF-α predict in-hospital mortality in COVID-19 patients. Front Immunol. 2023;14:1145840. doi:10.3389/FIMMU.2023.1145840/BIBTEX
27. Du P, Geng J, Wang F, Chen X, Huang Z, Wang Y. Role of IL-6 inhibitor in treatment of COVID-19-related cytokine release syndrome. Int J Med Sci. 2021;18(6):1356–1362. doi:10.7150/ijms.53564
28. Kunnumakkara AB, Rana V, Parama D, et al. COVID-19, cytokines, inflammation, and spices: how are they related? Life Sci. 2021:284. doi:10.1016/j.lfs.2021.119201
29. Flisiak R, Parczewski M, Horban A, et al. Management of SARS-CoV-2 infection: recommendations of the Polish association of epidemiologists and infectiologists. Annex no. 2 as of October 13, 2020. Pol Arch Intern Med. 2020;130(10):915–918. doi:10.20452/PAMW.15658
30. Wolszczak-Biedrzycka B, Dorf J, Milewska A, et al. The diagnostic value of inflammatory markers (CRP, IL6, CRP/IL6, CRP/L, LCR) for assessing the severity of COVID-19 symptoms based on the MEWS and predicting the risk of mortality. J Inflamm Res. 2023;16:2173–2188. doi:10.2147/jir.s406658
31. Dymicka-Piekarska V, Dorf J, Milewska A, et al. Neutrophil/Lymphocyte Ratio (NLR) and Lymphocyte/Monocyte Ratio (LMR) – risk of death inflammatory biomarkers in patients with COVID-19. J Inflamm Res. 2023;16:2209–2222. doi:10.2147/jir.s409871
32. Shawcross DL, Davies NA, Williams R, Jalan R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol. 2004;40(2):247–254. doi:10.1016/j.jhep.2003.10.016
33. Lu Q, Zhu Z, Tan C, et al. Changes of serum IL-10, IL-1β, IL-6, MCP-1, TNF-α, IP-10 and IL-4 in COVID-19 patients. Int J Clin Pract. 2021;75(9). doi:10.1111/ijcp.14462
34. Potere N, Del Buono MG, Caricchio R, et al. Interleukin-1 and the NLRP3 inflammasome in COVID-19: pathogenetic and therapeutic implications: IL-1 and NLRP3 inflammasome in COVID-19. EBioMedicine. 2022:85. doi:10.1016/j.ebiom.2022.104299
35. Della-Torre E, Lanzillotta M, Campochiaro C, et al. Respiratory impairment predicts response to IL-1 and IL-6 Blockade in COVID-19 patients with severe pneumonia and hyper-inflammation. Front Immunol. 2021:12. doi:10.3389/fimmu.2021.675678
36. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020:55. doi:10.1016/j.ebiom.2020.102763
37. Cavalli G, Dagna L. The right place for IL-1 inhibition in COVID-19. Lancet Respir Med. 2021;9(3):223–224. doi:10.1016/S2213-2600(21)00035-7
38. Satış H, Özger HS, Aysert Yıldız P, et al. Prognostic value of interleukin-18 and its association with other inflammatory markers and disease severity in COVID-19. Cytokine. 2021:137. doi:10.1016/j.cyto.2020.155302
39. Kerget B, Kerget F, Aksakal A, Aşkın S, Sağlam L, Akgün M. Evaluation of alpha defensin, IL-1 receptor antagonist, and IL-18 levels in COVID-19 patients with macrophage activation syndrome and acute respiratory distress syndrome. J Med Virol. 2021;93(4):2090–2098. doi:10.1002/jmv.26589
40. Li S, Jiang L, Beckmann K, et al. A novel anti-human IL-1R7 antibody reduces IL-18-mediated inflammatory signaling. J Biol Chem. 2021:296. doi:10.1016/J.JBC.2021.100630
41. Coutinho LLC, Oliveira CN, Albuquerque PLMM, et al. Elevated IL-18 predicts poor prognosis in critically ill COVID-19 patients at a Brazilian hospital in 2020–21. Future Microbiol. 2022;17(16):1287–1294. doi:10.2217/fmb-2022-0057
42. Tao W, Zhang G, Wang X, et al. Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18. Med Microecol. 2020:5. doi:10.1016/j.medmic.2020.100023
43. Smail SW, Babaei E, Amin K. Ct, IL-18 polymorphism, and laboratory biomarkers for predicting chemosensory dysfunctions and mortality in COVID-19. Future Sci OA. 2023;9(2). doi:10.2144/FSOA-2022-0082/ASSET/IMAGES/LARGE/FIGURE4.JPEG
44. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295–a016295. doi:10.1101/CSHPERSPECT.A016295
45. Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2021;33(3):127–148. doi:10.1093/intimm/dxaa078
46. Chen J, Wei Y, Yang W, et al. IL-6: the link between inflammation, immunity and breast cancer. Front Oncol. 2022:12. doi:10.3389/fonc.2022.903800
47. Maddaloni E, Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020;36(7). doi:10.1002/dmrr.3321
48. Ma M, Sun Q, Li X, et al. Blockade of IL-6/IL-6R signaling attenuates acute antibody-mediated rejection in a mouse cardiac transplantation model. Front Immunol. 2021:12. doi:10.3389/fimmu.2021.778359
49. Kruglova TS, Fomina DS, Poteshkina NG, et al. Criteria for the optimal use of interleukin-6 receptor blockers in patients with COVID-19. Ter Arkh. 2021;93(11):1316–1324. doi:10.26442/00403660.2021.11.201248
50. University Hospital Inselspital B, AG RP. Tocilizumab in the treatment of coronavirus induced disease (COVID-19). ClinicalTrials. 2020;2020:1.
51. Smail SW, Babaei E, Amin K. Hematological, inflammatory, coagulation, and oxidative/antioxidant biomarkers as predictors for severity and mortality in COVID-19: a prospective cohort-study. Int J Gen Med. 2023;16:565–580. doi:10.2147/IJGM.S402206
52. Smail SW, Babaei E, Amin K. Demographic, clinical and genetic factors associated with COVID-19 disease susceptibility and mortality in a Kurdish population. Ann Saudi Med. 2023;43(3):125–142. doi:10.5144/0256-4947.2023.125
53. Huang J, Li J, Zou Z, et al. Clinical characteristics of 3 patients infected with COVID-19: age, interleukin 6 (IL-6), lymphopenia, and variations in chest computed tomography (CT). Am J Case Rep. 2020:21. doi:10.12659/AJCR.924905
54. Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Investig. 2020;130(5):2202–2205. doi:10.1172/JCI137647
55. Zhou X, Ye G, Lv Y, et al. IL-6 drives T cell death to participate in lymphopenia in COVID-19. Int Immunopharmacol. 2022:111. doi:10.1016/j.intimp.2022.109132
56. Hanan N, Doud RL, Park IW, Jones HP, Mathew SO. The many faces of innate immunity in SARS-CoV-2 infection. Vaccines. 2021;9(6). doi:10.3390/vaccines9060596
57. Kokkotis G, Kitsou K, Xynogalas I, et al. Systematic review with meta-analysis: COVID-19 outcomes in patients receiving anti-TNF treatments. Aliment Pharmacol Ther. 2022;55(2):154–167. doi:10.1111/apt.16717
58. Robinson PC, Liew DFL, Liew JW, et al. The potential for repurposing anti-TNF as a therapy for the treatment of COVID-19. Med. 2020;1(1):90–102. doi:10.1016/j.medj.2020.11.005
59. Mohd Zawawi Z, Kalyanasundram J, Mohd Zain R, Thayan R, Basri DF, Yap WB. Prospective roles of tumor necrosis factor-alpha (TNF-α) in COVID-19: prognosis, therapeutic and management. Int J Mol Sci. 2023;24(7):6142. doi:10.3390/ijms24076142
60. Guo Y, Hu K, Li Y, et al. Targeting TNF-α for COVID-19: recent advanced and controversies. Front Public Health. 2022:10. doi:10.3389/fpubh.2022.833967
61. Ozpolat C, Altunbas E, Zhu A. Diagnostic utility of the Covichem score in predicting COVID-19 disease. Am J Emerg Med. 2022;57:60. doi:10.1016/j.ajem.2022.07.025
62. Sun J, Peng L, Li T, et al. Performance of a chest radiograph AI diagnostic tool for COVID-19: a prospective observational study. Radiol Artif Intell. 2022;4(4). doi:10.1148/ryai.210217
63. Kardos AS, Simon J, Nardocci C, et al. The diagnostic performance of deep-learning-based CT severity score to identify COVID-19 pneumonia. Brit J Radiol. 2022;95(1129). doi:10.1259/bjr.20210759
64. Godfred-Cato S, Abrams JY, Balachandran N, et al. Distinguishing multisystem inflammatory syndrome in children from COVID-19, Kawasaki disease and toxic shock syndrome. J Pediatr Infect Dis. 2022;41(4):315–323. doi:10.1097/INF.0000000000003449
65. Wang L, Lv Q, Zhang X, et al. The utility of MEWS for predicting the mortality in the elderly adults with COVID-19: a retrospective cohort study with comparison to other predictive clinical scores. PeerJ. 2020;8. doi:10.7717/PEERJ.10018/SUPP-5
66. Ghahramani S, Tabrizi R, Lankarani KB, et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):1–10. doi:10.1186/S40001-020-00432-3/TABLES/2
67. Velavan TP, Meyer CG. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis. 2020;95:304–307. doi:10.1016/J.IJID.2020.04.061
68. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi:10.1172/JCI137244
69. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3. doi:10.1016/J.CHOM.2020.04.009
70. Liu T, Zhang J, Yang Y, et al. The role of interleukin‐6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12(7). doi:10.15252/EMMM.202012421
71. Azmi A, Rismani M, Pourmontaseri H, Mirzaii E, Niknia S, Miladpour B. The role of vitamin D receptor and IL −6 in COVID-19. Mol Genet Genomic Med. 2023;11. doi:10.1002/mgg3.2172
72. Sanli DET, Altundag A, Kandemirli SG, et al. Relationship between disease severity and serum IL-6 levels in COVID-19 anosmia. Am J Otolaryngol. 2021;42(1). doi:10.1016/j.amjoto.2020.102796
73. Eljilany I, Elzouki AN. D-dimer, fibrinogen, and il-6 in covid-19 patients with suspected venous thromboembolism: a narrative review. Vasc Health Risk Manag. 2020;16:455–462. doi:10.2147/VHRM.S280962
74. Halim C, Mirza AF, Sari MI. The association between TNF-α, IL-6, and Vitamin D Levels and COVID-19 severity and mortality: a systematic review and meta-analysis. Pathogens. 2022;11(2):195. doi:10.3390/pathogens11020195
75. Scherger S, Henao-Martínez A, Franco-Paredes C, Shapiro L. Rethinking interleukin-6 blockade for treatment of COVID-19. Med Hypotheses. 2020;144. doi:10.1016/j.mehy.2020.110053
76. Tharmarajah E, Buazon A, Patel V, et al. IL-6 inhibition in the treatment of COVID-19: a meta-analysis and meta-regression. J Infect. 2021;82(5):178–185. doi:10.1016/j.jinf.2021.03.008
77. Schultheiß C, Willscher E, Paschold L, et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022;3(6). doi:10.1016/j.xcrm.2022.100663
78. Fishchuk L, Rossokha Z, Pokhylko V, et al. Modifying effects of TNF-α, IL-6 and VDR genes on the development risk and the course of COVID-19. Pilot study. Drug Metab Pers Ther. 2022;37(2). doi:10.1515/dmpt-2021-0127
79. Zheng S, Zeng C, Liu M, Xiang P, Wang L, Li Z. Study on the role of IL-6/IL-6R/GP130 signal transduction pathway in neuroimmunomodulation. Acta Med Mediterr. 2021;37(4). doi:10.19193/0393-6384_2021_4_355
80. Chonov DC, Ignatova MMK, Ananiev JR, Gulubova MV. IL-6 activities in the tumour microenvironment. part 1. Open Access Maced J Med Sci. 2019;7(14):2391–2398. doi:10.3889/oamjms.2019.589
81. Hwang MS, Strainic MG, Pohlmann E, et al. Research article vegfr2 survival and mitotic signaling depends on joint activation of associated C3ar1/C5ar1 and IL-6R-gp130. J Cell Sci. 2019;132(6). doi:10.1242/jcs.219352
82. Vatansever HS, Becer E. Relationship between IL-6 and COVID-19: to be considered during treatment. Future Virol. 2020;15(12):817–822. doi:10.2217/fvl-2020-0168
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.