Back to Journals » Drug Design, Development and Therapy » Volume 17
Untapping the Potential of Astragaloside IV in the Battle Against Respiratory Diseases
Authors Tan J, Yi J, Cao X, Wang F, Xie S, Dai A
Received 6 April 2023
Accepted for publication 20 June 2023
Published 3 July 2023 Volume 2023:17 Pages 1963—1978
DOI https://doi.org/10.2147/DDDT.S416091
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jianbo Sun
Junlan Tan,1,2 Jian Yi,2,3 Xianya Cao,1,2 Feiying Wang,2,4 Silin Xie,2,4 Aiguo Dai2,4,5
1School of Integrated Chinese and Western Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, 410208, People’s Republic of China; 2Hunan Provincial Key Laboratory of Vascular Biology and Translational Medicine, Changsha, Hunan, 410208, People’s Republic of China; 3The First Affiliated Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, 410021, People’s Republic of China; 4Department of Respiratory Diseases, School of Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, 410208, People’s Republic of China; 5Department of Respiratory Medicine, the First Affiliated Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, 410021, People’s Republic of China
Correspondence: Aiguo Dai, Department of Respiratory Diseases, School of Medicine, Hunan University of Chinese Medicine, 300 Xueshi Road, Hanpu Science and Education District, Changsha, Hunan, 410208, People’s Republic of China, Tel +86-731-88458128, Fax +86-731-88458111, Email [email protected]
Abstract: Respiratory diseases are an emerging public health concern, that pose a risk to the global community. There, it is essential to establish effective treatments to reduce the global burden of respiratory diseases. Astragaloside IV (AS-IV) is a natural saponin isolated from Radix astragali (Huangqi in Chinese) used for thousands of years in Chinese medicine. This compound has become increasingly popular due to its potential anti-inflammatory, antioxidant, and anticancer properties. In the last decade, accumulated evidence has indicated the AS-IV protective effect against respiratory diseases. This article presents a current understanding of AS-IV roles and mechanisms in combatting respiratory diseases. The ability of the agent to suppress oxidative stress, cell proliferation, and epithelial-mesenchymal transition (EMT), to attenuate inflammatory responses, and modulate programmed cell death (PCD) will be discussed. This review highlights the current challenges in respiratory diseases and recommendations to improve disease management.
Keywords: astragaloside IV, Radix astragali, respiratory diseases, oxidative stress, inflammatory responses
Introduction
Respiratory diseases are one of the leading causes of death and disability globally, becoming a burden for patients and their caregivers.1 Recently, the incidence of respiratory diseases worldwide has risen alarmingly, particularly in developed countries. A global investigation revealed an increase of 39.8% in 2017 from 1990, amounting to 544.9 million patients living with chronic respiratory disease.2 Furthermore, chronic respiratory diseases were the third leading cause of death in 2017, behind cardiovascular diseases and neoplasms.2 Despite recent advances in patient care and intervention, many respiratory diseases, such as pulmonary hypertension (PH) and idiopathic pulmonary fibrosis (IPF), still lack effective treatments and are thus impossible to cure. Therefore, researchers must discover new approaches to alleviate these debilitating diseases.
Natural products are an essential source for discovering and developing new drugs. Consequently, herbal medicine is gaining attention in the scientific community as a respiratory disease treatment.3–13 One of the emerging natural compounds is astragaloside IV (AS-IV), a natural saponin (Figure 1) extracted from Radix astragali (Huangqi in Chinese) with multi-target therapeutic properties. Studies have reported the pharmacological effects of AS-IV, such as antioxidant,14 anti-inflammatory,15 anti-fibrotic,16 and anticancer.17 More importantly, AS-IV is potentially less toxic. For example, an earlier study revealed that oral administration of AS-IV at 10 mg/kg/day for 14 weeks had no adverse effects on rat liver and kidney functions.18 Furthermore, AS-IV is the recommended treatment for various diseases in preclinical models, including cerebral ischemia,19,20 atherosclerosis,21 and cancer.22 Various studies have also highlighted the potential therapeutic effects of AS-IV in various respiratory diseases, such as PH,7 chronic obstructive pulmonary disease (COPD),23 asthma,24 lung cancer,25 pulmonary fibrosis,26 and lung injury.27 Herein, this review discusses the role of AS-IV in respiratory diseases and the potential therapeutic efficacies from the existing literature.
Roles of as-IV as a Treatment for Respiratory Diseases
In the past two decades, AS-IV reportedly improved the symptoms of respiratory diseases, especially PH and pulmonary fibrosis, in cellular and animal models. However, the key pharmacological mechanisms remain unclear and controversial. This section discusses in detail the effects and mechanisms of AS-IV in treating various respiratory diseases. The cellular signaling pathways of PH and pulmonary fibrosis affected by AS-IV are illustrated in Figures 2 and Figure 3, respectively.
AS-IV and PH
PH is a complex disorder characterized by pulmonary vascular remodeling and right ventricular hypertrophy, leading to right heart failure.28 Currently, there are few FDA-approved treatments, and the clinical efficacy of these anti-PH drugs remains limited.29 Plant-derived natural compounds with anti-PH properties offer new opportunities for developing low-toxicity and cost-effective drugs than synthetic alternatives.30 Mean pulmonary artery pressure (mPAP) reduction and improved pulmonary vascular remodeling by AS-IV were first detailed by Zhang et al using a hypoxic PH rat model.31 This finding became a starting point for further exploring of the anti-PH effects and mechanisms of AS-IV.
Inhibition of Pulmonary Arterial Smooth Muscle Cells (PASMCs) Proliferation in PH
Excessive proliferation of PASMCs is critical in the pathogenesis of pulmonary artery remodeling.32 The Zhang group31 reported that AS-IV is a potent PASMCs-proliferation inhibitor that could suppress pulmonary artery remodeling and lead to significant mPAP decline in rats with hypoxia-induced pulmonary hypertension. This study found that 50 μM AS-IV resulted in marked inhibition of hypoxia-induced PASMCs proliferation in vitro. Notch signaling, a highly evolutionarily conserved signaling pathway, is vital in PASMCs proliferation. Notch-3 targets hes family bHLH transcription factor 5 (Hes-5), which is expressed exclusively in smooth muscle cells (SMCs) in adults and might be associated with SMC identity, maturation, and proliferation.33,34 It has been proven in vitro and in vivo that AS-IV can reverse the hypoxia-induced PASMCs proliferation by suppressing Jagged-1, Notch-3, and Hes-5 expressions.35 Furthermore, AS-IV inhibited the proliferation, migration, and adhesion of PASMCs under hypoxic conditions by downregulating RhoA and upregulating p27 at the protein level.7
Promoting PASMCs Apoptosis in PH
Resistance to apoptosis by PASMCs contributes to the pathophysiology of PH. Meanwhile, AS-IV ameliorated apoptosis resistance in PASMCs by downregulating Bcl-2, phospho-ERK, and hypoxia-inducible factor-1α (HIF-1α) expressions.36
Inflammation Regulation in PH
There is increasing evidence concerning the role of inflammation in pulmonary vascular remodeling. For instance, the Nod-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome, comprising the NLRP3, the apoptosis speck-like protein containing a caspase-recruitment domain (ASC), and pro-caspase-1, mediate cytokine and inflammatory responses in PH.37 Sun et al38 reported that AS-IV treatments [(40 and 80 mg/kg/day, intraperitoneally (i.p.)] attenuated inflammatory response mediated by NLRP-3/calpain-1, thereby alleviating pulmonary vascular remodeling in monocrotaline (MCT)-induced PH in rats. In a recent study, AS-IV reportedly reduced the blood levels of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in MCT-induced PH rats.36 Moreover, AS-IV reduced serum levels of endothelin-1 (ET-1), angiotensin II (AngII), TNF-α, and IL-6 in rats with hypoxia-induced PH.31
Summary Section
There are various causative factors of PH and AS-IV targets or pathways in treating the disease (Figure 2). Consequently, the preclinical mechanisms of AS-IV against PH have not been fully elucidated. Nonetheless, the literature indicated the potential of AS-IV as a treatment ingredient or prevention drug for PH, particularly in the absence of good clinical options for group 3 PH patients.
AS-IV and Pulmonary Fibrosis (PF)
PF, specifically IPF, is a highly confounding and fatal pathological process of unknown cause and is characterized by alveolar injury, fibroblast proliferation, and excessive deposition of extracellular matrix (ECM) proteins, progressively resulting in respiratory failure and death.39 Current scientific evidence supports that AS-IV has a prominent anti-fibrotic and protective role against the progression of PF (Figure 3).
Inhibition of EMT in PF
Epithelial-mesenchymal transition (EMT) is a process in which fully differentiated epithelial cells are transformed into a mesenchymal phenotype. Numerous studies indicated EMT is a major driver of fibrosis and is involved in the pathological process of PF.40 Transforming growth factor-β1 (TGF-β1) is an essential pro-fibrotic factor that induces EMT via Smad-dependent or Smad-independent pathways. In 2018, Qian et al41 reported that AS-IV demonstrated protective effects against EMT in bleomycin (BLM)‐induced pulmonary fibrosis in rats by suppressing TGF-β1/phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (Akt)‐induced Forkhead box O3α (FOXO3α) hyperphosphorylation. Subsequently, they demonstrated that AS-IV blocked TGF-β1-induced EMT in alveolar type II epithelial (RLE-6TN) cells by inhibiting metastasis-associated gene 1 (MTA1) expression.42
In another study, NLRP3 inflammasome activation reportedly promoted EMT in alveolar epithelial cells with BLM treatment by upregulating TGF-β1/Smad2/3-mediated transcriptional activity.43 Meanwhile, Hou et al44 indicated that AS-IV could attenuate EMT in alveolar epithelial cells with BLM treatment by regulating NLRP3/TGF-β1 signaling pathway. Finally, AS-IV treatment could also retard pathological progression by inhibiting EMT in other disease models, such as diabetic nephropathy,45 renal fibrosis,46 and gastric cancer.47
Regulation of Inflammation and Oxidative Stress in PF
A recent study48 illustrated that increased levels of malondialdehyde (MDA), total antioxidant capacity (T-AOC), reactive oxygen species (ROS), IL-1β, TNF-α and IL-6 in the bronchoalveolar lavage fluid (BALF) in BLM-induced PF rats were substantially down-regulated by AS-IV treatment (10, 20 and 50 mg/kg/day, i.p.). This outcome indicated the potential of AS-IV in reducing lung tissue injury by inhibiting inflammation and oxidative stress in BLM-induced PF rats. In addition, AS-IV (20 mg/kg/day, i.p.) treatment inhibited inflammation by impeding NF-κB pathway in silica-induced PF rats. Tong et al49 also reported that AS-IV, combined with ferulic acid, protected lung tissue from oxidative stress by upregulating nuclear factor-E2-related factor (Nrf2) in BLM-induced PF mice.
Inhibition of Lung Fibroblast to Myofibroblast Differentiation
It is well-recognized that the uncontrolled proliferation of lung fibroblasts and excessive differentiation of fibroblasts into myofibroblasts produce ECM proteins that contribute to lung fibrosis. In 2019, Li et al50 demonstrated that AS-IV inhibited fibroblast collagen production and myofibroblast transformation via TGF-β1/Smads signaling pathway. Furthermore, it was concluded that AS-IV (10 mg/kg/day, i.p.) reduced blood levels of type III collagen (collagen-III), laminin (LN), and hyaluronic acid (HA) and hydroxyproline (HYP) in lung tissues of BLM-induced PF rats.51
Summary Section
Excessive deposition of ECM proteins, inflammation, oxidative stress, and chemoresistance are the major factors of PF progression. Though pirfenidone and nintedanib are approved for IPF treatment, their clinical utility is limited by toxic side effects such as gastrointestinal problems, photosensitivity and skin reactions. Therefore, it is postulated that AS-IV alone or in combination with other drugs is a promising alternative for IPF treatment, and further research is needed to meet increasing clinical demands.
AS-IV and Lung Cancer
The anticancer properties of AS-IV are observed in the immunomodulatory action in cancer treatment and antagonizing the development of lung cancer through multiple pathways, such as inhibiting cell proliferation, migration, and invasion and enhancing the sensitivity of chemotherapy and targeted therapy.52
Improvement of Tumor Immunosuppressive Microenvironment in Lung Cancer
Cancer is characterized by immune escape and an immunosuppressive microenvironment. Consequently, tumor microenvironment (TME) remodeling has become an important research direction for current lung cancer treatment.53–55 Indoleamine 2.3-dioxygenase (IDO) is a tryptophan catabolic enzyme that induces immune escape in lung cancer cells.56,57 Zhang et al58 reported that AS-IV reduced tumor growth in the Lewis lung cancer model by suppressing IDO expression to upregulate cytotoxic T lymphocytes (CTLs) and downregulate regulatory T cells (Tregs) activities. In addition, GBP1 binds to IDO1 and promotes the extracellular secretion of IDO.59 In vitro and in vivo studies exhibited that AS-IV reduced the extracellular secretion of IDO1 by blocking the interaction between IDO1 and GBP1, reducing T-cell depletion and inhibiting lung cancer progression.59
Tumor-associated macrophages (TAMs) or M2-polarized macrophages are essential immunosuppressive cells in the TME,60–62 and their increased infiltration in tumor tissue is often associated with poor prognosis. Thus, inhibiting M2-polarized macrophage activity becomes a promising therapeutic strategy for lung cancer. Xu et al63 found that AS-IV significantly decreased IL-13- and IL-4-induced M2 macrophage polarization and attenuated M2-CM-induced invasion, migration, and angiogenesis in A549 and H1299 cells by inhibiting adenosine monophosphate (AMP)-activated protein kinase α (AMPKα) activation. Likewise, an in vivo study demonstrated that AS-IV inhibited tumor growth in the Lewis lung cancer mice model and reduced M2 macrophage infiltration in tumor tissue.63 Therefore, TME remodeling could be a new avenue to explore the AS-IV anti-tumor mechanism of action.
Inhibition of Cell Proliferation, Invasion, and Migration in Lung Cancer
A study reported that AS-IV could inhibit the invasion and migration of A549 cells by restraining the protein kinase C-alpha/extracellular signal-related kinases 1 and 2/nuclear factor-κB (PKC-α/ERK1/2/NF-κB) signaling pathway and downregulating matrix level metalloproteinase-2 (MMP-2), MMP-9, integrin β1, transforming growth factor β1 (TGF-β1), TNF-α and IL-6 in A549 cells.64 Moreover, AS-IV inhibited the growth and promoted apoptosis in three NSCLC cell lines (HCC827, H1299, and A549) by suppressing the protein kinase B/glycogen synthase kinase-3β (Akt/GSK-3β)/β-catenin signaling pathway.65
Enhanced the Sensitivity to Chemotherapy and Targeted Therapies in Lung Cancer
Cisplatin resistance is the most critical cause of chemotherapy failure in lung cancer patients.66–69 An in vitro study showed that AS-IV enhanced the sensitivity of non-small cell lung cancer (NSCLC) cell lines to cisplatin by inhibiting autophagy and endoplasmic reticulum (ER) stress.70 Furthermore, AS-IV elevated the sensitivity of A549, HCC827, and H1299 lung cancer cells to cisplatin by inhibiting B7-H3 protein expression.71 Targeted therapy is also an essential treatment for NSCLC and faces a similar challenge to chemotherapy in treatment resistance.72 A recent study stated that AS-IV enhanced A549 cells sensitivity to bevacizumab, potentially by suppressing autophagy and activating the Akt/mTOR signaling pathway.25 Moreover, gefitinib combined with AS-IV treatment was more effective in restraining NSCLC cell proliferation than gefitinib alone.73
Summary Section
It is increasingly recognized that chemoresistance and immune escape pose major obstacles to the therapeutic management of lung cancer. Encouragingly, existing evidence indicated that AS-IV ameliorated chemoresistance, reshaped the tumor immune microenvironment, and may shed the new dawn on the treatment of lung cancer.
AS-IV and Lung Injury
Various pathological factors such as fine particulate matter (PM2.5) and paraquat (PQ) can induce lung injury, damaging critical lung functions. Multiple studies have demonstrated that AS-IV could be a protector against lung injury by decreasing inflammatory responses and regulating PCD.
Regulation of Programmed Cell Death in PM2.5-Induced Lung Injury
PM2.5 is currently the most critical factor in lung diseases caused by environmental pollution.74,75 Previous studies indicated that PM2.5-induced pulmonary injury is associated with the activation of multiple PCD pathways, including ferroptosis,76 pyroptosis,77 and autophagy.78
Autophagy is essential for cellular homeostasis as an evolutionarily-conserved intracellular degradation pathway.79 Multiple studies suggested that autophagy is pivotal in the pathogenesis of lung injury.80 Pei et al81 demonstrated that AS-IV exerted a protective role in PM2.5-induced lung injury in rats by inhibiting autophagy via PI3K/Akt/mammalian target of the rapamycin (mTOR) signaling pathway. Conversely, Wang et al82 exhibited that AS-IV mitigated PM2.5-induced lung toxicity in rats by activating autophagy the AMP-activated protein kinase (AMPK)/mTOR signaling pathway. These results suggested that autophagy acts as a double-edged sword in PM-2.5-induced lung injury, and AS-IV exerted therapeutic benefits in lung injury via autophagy induction or autophagy flux inhibition.
Ferroptosis is characterized by the iron-dependent accumulation of lipid hydroperoxides and can induce various respiratory diseases.83 This PCD mechanism is possibly involved in the pathological cell death associated with COPD,84 PH,85 and lung injury.86 Glutathione peroxidase 4 (GPX4) is a known deterrent for ferroptosis. In a PM2.5-induced mouse model, AS-IV suppressed ferroptosis, inflammation, and oxidative stress through Nrf2/SLC7A11/GPX4 signaling pathway, resulting in a protective effect on lung tissue.27
Pyroptosis is a pro-inflammatory form of PCD resulting from the activation of caspase-1 within the inflammasome complex and caspase-11 (caspase-4/5 in humans) following intracellular lipopolysaccharide (LPS) recognition.87 Huang et al88 revealed the protective role of AS-IV against PM2.5-induced lung toxicity by suppressing NLRP3 inflammasome-mediated pyroptosis via NLRP3/caspase-1 axis inhibition. Consequently, PM2.5-induced lung inflammation and oxidative damage were prevented, leading to prolonged survival in mice. Furthermore, Wu et al89 reported that AS-IV could prevent PM2.5-induced lung injury in rats by inhibiting the toll-like receptor 4 (TLR4)/MyD88/NF-κB signaling pathway and inflammation, besides delaying lung tissue injury.
Amelioration of Inflammation in Paraquat-Induced Lung Injury
PQ can cause multi-system injury, particularly severe lung tissue damage. Chen et al90 revealed that AS-IV reduced Txnip/Trx expression and suppressed the Rho/ROCK/NF-κB signaling pathway in PQ-challenged mice, thus, alleviating pulmonary tissue injury.
Hypoxia/reoxygenation (HR) of pulmonary organization can also induce apoptosis in alveolar epithelial cells, resulting in lung impairment. Li et al91 demonstrated that 1nM AS-IV inhibited TLR4/NF-κB pathway through the upregulation of miR-21-5p, thereby attenuating HR injury-induced type II alveolar epithelial cell apoptosis in vitro. Furthermore, pulmonary ischemia/reperfusion (I/R) lung injury can severely limit the postoperative lung function recovery and contribute to complications such as PH, pulmonary edema, and respiratory failure, eventually leading to patient mortality. Notably, AS-IV attenuated I/R lung injury in rats by reducing myeloperoxidase (MPO) levels in lung tissue and lung wet-to-dry (D/W) ratio.92
Summary Section
Impaired lung function due to lung and alveolar injury is a hallmark of many acute and chronic lung diseases. Because of its antioxidant and anti-inflammatory effects, AS-IV could mitigate lung injury triggered by various pathological factors. However, most related studies are based only on animal experiments and relevant clinical data is required in the future.
AS-IV and Asthma
Asthma is a heterogeneous disease with multiple underlying inflammatory pathways and structural airway abnormalities that influence the disease persistence and severity.93 A study has revealed that AS-IV could inhibit airway inflammation and reduce airway hypersensitivity (AHR) by regulating various inflammatory cells and mediators, such as neutrophils, IL-4, and IL-10. Precisely, AS-IV treatment ameliorated airway inflammation and AHR in the ovalbumin (OVA)-sensitized allergic asthma mouse model by inhibiting the Janus kinase 2/signal transducer and activator of transcription 6 (JAK2/STAT6) signaling pathway.24 In addition, AS-IV attenuated allergic inflammation by downregulating IL-4 and IL-10, upregulating interferon-γ (IFN-γ), and enhancing CD4(+) CD25(+) Foxp3 T cells in OVA-induced asthma mouse model.94
Multiple studies have portrayed the importance of inflamed bronchial epithelial cells in the pathological features of asthma exacerbation. An in vitro study indicated that AS-IV suppressed inflammation and oxidative stress in human bronchial epithelial cells by blocking the NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways.95 In contrast, Jin et al96 found that AS-IV improved AHR, attenuated lung inflammation, and reduced the production of inflammatory mediators, such as IL-4, IL-5, and IL-17, by inhibiting the mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway in vivo.
Eosinophils are involved in developing asthmatic characteristics, including airway remodeling, hyper-responsiveness, and initiating allergic inflammation of the airways. Thus, an effective treatment for this disease would be to reduce eosinophil infiltration in the airways. Du et al97 reported that AS-IV inhibited eosinophil infiltration in airways, hence, suppressing mucus hypersecretion, airway inflammation, and hyperreactivity in allergen-sensitized and challenged mice. Likewise, 50 μg/mL AS-IV significantly suppressed eosinophil activation induced by house dust mite allergen Dermatophagoides pteronyssinus (Der p) in vitro.98 In summary, the anti-inflammatory and antioxidant feature of AS-IV offer potential treatment options for asthmatic patients.
AS-IV and COPD
COPD is a common chronic inflammatory disorder of the airways characterized by irreversible airflow limitation, ranking as the third highest cause of death worldwide.99 Despite the incomplete knowledge regarding the pathological mechanism of COPD, airway inflammation and oxidative injury are involved in many aspects of the disease pathophysiology. Prolonged airflow restriction in COPD patients can lead to respiratory muscle fatigue, further aggravating the disease. For example, impaired diaphragm function often induces respiratory failure. Wang et al100 found that AS-IV attenuated IL-8-induced apoptosis and inflammatory response in diaphragm cells by inhibiting AKT phosphorylation, caspase-3, and −9 protein expression, ROS, and inflammatory factor production in vitro.
Cigarette smoking (CS) remains the most critical risk factor for COPD.101 Therefore, long-term exposure to cigarette smoke is currently the best method for establishing COPD rodent models. Studies on mice have depicted that AS-IV alleviated CS-induced pathological injury in lung tissue in a dose-dependent manner by suppressing JAK3/STAT3/NF-κB pathway.23 Likewise, AS-IV significantly repressed the protein levels in JAK3/STAT3/NF-κB pathway in cigarette smoke extract (CSE)-induced human bronchial epithelial cells.23 Furthermore, AS-IV has been proven to be a potent NF-κB pathway inhibitor and an antioxidant by exerting a protective effect against CS-induced airway inflammation in COPD rats.102
AS-IV and Coronavirus Disease 2019 (COVID-19)
COVID-19 remains a global pandemic to this day. Once contaminated with SARS-CoV-2, the patient’s immune cells regularly secrete excessive pro-inflammatory cytokines, also termed “cytokine storms.” The excessive inflammatory response caused by cytokine storms leads to multi-organ functional impairment in COVID-19 patients, often causing them to end up in the intensive care unit (ICU).103 Early clinical research indicated that plasma levels of cytokines (IL-10, TNF-α, IL-2, and IL-10) and monocyte chemoattractant protein 1 (MCP1) were higher in ICU patients than in non-ICU patients.104 Thus, hyperinflammation suppression may improve the prognosis of patients with severe COVID-19.
Network pharmacology and molecular docking have demonstrated the potential of AS-IV as a treatment option that may alleviate excessive inflammation in COVID-19 patients by inhibiting the NOD-like receptor signaling pathway.105 Furthermore, a study has revealed that SARS-CoV-2 virus proliferated human cells via angiotensin-converting enzyme 2 (ACE2), thus, one of the key targets for COVID-19 inhibition. Ye et al106 used molecular docking in combination with the surface plasmon resonance technique to investigate the potential of AS-IV in binding with ACE2, which may impede the invasion of SARS-CoV-2 into host cells. However, no preclinical trials have been performed to confirm the efficacy of AS-IV in treating COVID-19.
AS-IV and Obstructive Sleep Apnoea (OSA)
OSA is characterized by intermittent hypoxia and sleep disruption. Long-term chronic intermittent hypoxia can lead to oxidative stress and inflammatory responses in lung tissues and cells of OSA patients, resulting in a series of complications. In vitro illustrated that AS-IV significantly reduced IL-6, IL-1β, IL-8, MDA, ROS, and LDH levels in intermittent hypoxia-induced Beas-2B cells by inhibiting the TLR4/MAPK/NF-κB signaling pathway.95
Challenges and New Strategies for Application
Despite the promising therapeutic outcomes on lung diseases, several problems limit the clinical application of AS-IV. For instance, this natural saponin is a relatively large molecule (molecular weight = 784.97 g/mol) with poor solubility in water, leading to low bioavailability at targeted sites, particularly through oral administration. Gu et al107 revealed that AS-IV exhibited a low absorption rate in a perfused rat intestinal model, and the oral bioavailability was only 2.2%. Similarly, Zhang et al108 discovered that the absolute oral bioavailability of AS-IV in Beagle dogs is only 7.4%. Although various studies have indicated that the potential toxicity of AS-IV is low, others have reported the reproductive toxicity of this compound. For instance, Zhu et al109 reported that AS-IV was maternally toxic in rats at 1 mg/kg and fetotoxic at > 0.5 mg/kg.
Liposomes are effective carriers for hydrophobic drugs, known for the efficacy enhancement and toxicity reduction. Rajesh et al110 developed a novel multifunctional liposome to load AS-IV for drug delivery, which significantly improved the bioavailability and efficacy of the compound. Furthermore, utilizing nanomaterials in developing novel drugs is trending due to the ability to enhance the native drug efficacy substantially.110 Interestingly, a recent study by Zhou et al111 exhibited that Licorice-derived protein nanoparticles solubilized the insoluble AS-IV via encapsulation. Moreover, Sun et al112 reported that dry age-related macular degeneration can be effectively managed with AS-IV-loaded lipid nanocapsules. Despite that, AS-IV nanoformulations are years away from clinical translation and require the joint efforts of pharmacologists, chemists, and material scientists to develop a stable, scalable, and effective products. Structural modification of a natural product improves drug efficacy and minimizes toxicity. Nonetheless, studies on the structural modification of AS-IV are lacking in existing literature.
Although multi-target modulation is a therapeutic feature of AS-IV in respiratory diseases, this method poses a significant obstacle to clinical translation. Multiple drug side effects may occur if drugs are not accurately targeted owing to the diverse physiological conditions and complex signaling pathways in vivo, thus, reducing drug efficacy. Therefore, identifying the specific therapeutic targets for AS-IV in lung disease is currently one of the crucial research directions. Cao et al113 revealed that AS-IV attenuated renal fibrosis in diabetic nephropathy rats by blocking the NLR signaling through transcriptomic techniques. Furthermore, Fu et al have identified miR-26b-5p/ATF3/JUN as the major mediator of AS-IV’s cardioprotective effect by transcriptome screening and experimental validation.114 In addition, Xia et al115 utilized quantitative proteomics to display that AS-IV inhibited cervical cancer cell invasion by targeting DCP1A and TMSB4X for autophagy induction. Therefore, modern biological techniques such as transcriptomics, proteomics, and metabolomics are reliable for exploring AS-IV-specific therapeutic targets.
Clinical trials have demonstrated that intravenous infusion of 200 mL astragalosides injection (contained 18 mg AS-IV) is safe and well-tolerated in healthy Chinese volunteers,116 suggesting that AS-IV has excellent potential for clinical application. Still, most studies on applying AS-IV for lung disease have focused on preclinical models. Thus, it is essential to conduct clinical studies to confirm the efficacy and safety of AS-IV in preventing and treating lung diseases. Long-term studies have found that applying drug combinations is critical to achieving optimal effectiveness, providing a novel idea for the clinical translation of AS-IV. For example, AS-IV and ferulic acid combination demonstrated synergistic effects in various fibrotic disease models, thus, a promising anti-fibrotic treatment.49,117 Moreover, the combination of AS-IV with atorvastatin,118 bevacizumab,25 tanshinone IIA119 and ginsenoside Rg1120 has been used in preclinical studies to treat various diseases. In summary, the application of AS-IV combination therapy in respiratory diseases is worth investigating in the future.
Conclusion and Perspectives
AS-IV, a natural saponin with several beneficial biological activities, has made major progress in the research on the role and mechanism of respiratory diseases prevention (Table 1 and Figure 4). This natural saponin inhibited respiratory diseases progression through anti-inflammatory, antioxidant, cell proliferation, EMT inhibition, and PCD modulation. Precisely, AS-IV combined with ferulic acid, cisplatin, and bevacizumab demonstrated synergistic effects and significantly improved drug efficacy. More importantly, AS-IV showed low toxicity in vivo, thus, facilitating future clinical translation. Despite the promising therapeutic impacts of AS-IV in multiple preclinical models of respiratory diseases, the mechanism of action and direct targets have yet to be elucidated. Modern biological techniques such as transcriptomics, surface plasmon resonance, and protein microarray technologies offer new strategies to fill knowledge gaps in AS-IV studies. Nevertheless, a major hurdle in AS-IV application in respiratory diseases is the lack of clinical data. Therefore, future research should focus on clinical trials to confirm the efficacy and safety of AS-IV in treating respiratory diseases. In addition, the high molecular weight of AS-IV results in low bioavailability, further limiting the its clinical use. Novel drug delivery systems, such as nanocapsules, have improved AS-IV bioavailability. In conclusion, AS-IV is a potential drug candidate for treating respiratory diseases and should be further explored in future research.
 |
Table 1 The Summary of Mechanisms of as-IV in Respiratory Diseases |
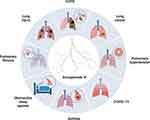 |
Figure 4 The general map of AS-IV in treating various respiratory diseases. Created with BioRender.com. |
Data Sharing Statement
The data used to support the findings of this study are included in the article.
Acknowledgments
The authors acknowledge the BioRender (www.biorender.com), as figures in this review were created with the BioRender platform. We thank Home for Researchers editorial team (www.home-for-researchers.com) for improving the English language in this manuscript.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the Youth Program of National Natural Science Foundation of China (82200066), the Natural Science Foundation of Hunan Province (2021JJ30017), the China Postdoctoral Science Foundation (2021M690982), the Hunan Scientific Research Program of traditional Chinese Medicine (C2022001), and the Postgraduate Scientific Research Innovation Project of Hunan Province (CX20220823).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Li X, Cao X, Guo M, et al. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: systematic analysis for the Global Burden of Disease Study 2017. BMJ. 2020;368:m234. doi:10.1136/bmj.m234
2. GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi:10.1016/S2213-2600(20)30105-3
3. Liu Y, Tong C, Tang Y, et al. Tanshinone IIA alleviates blast-induced inflammation, oxidative stress and apoptosis in mice partly by inhibiting the PI3K/Akt/FoxO1 signaling pathway. Free Radic Biol Med. 2020;152:52–60. doi:10.1016/j.freeradbiomed.2020.02.032
4. Huang B, Hao M, Li C, et al. Acetyltanshinone IIA reduces the synthesis of cell cycle-related proteins by degrading p70S6K and subsequently inhibits drug-resistant lung cancer cell growth. Pharmacol Res. 2022;179:106209. doi:10.1016/j.phrs.2022.106209
5. Li H, Wu M, Guo C, et al. Tanshinone IIA regulates Keap1/Nrf2 signal pathway by activating sestrin2 to restrain pulmonary fibrosis. Am J Chin Med. 2022;50(8):2125–2151. doi:10.1142/S0192415X22500914
6. Li L, Dong P, Hou C, et al. Hydroxysafflor yellow A (HSYA) attenuates hypoxic pulmonary arterial remodelling and reverses right ventricular hypertrophy in rats. J Ethnopharmacol. 2016;186:224–233. doi:10.1016/j.jep.2016.04.004
7. Li C, Zhu H, Zhang S, et al. Astragaloside IV ameliorates pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension by restraining the T follicular helper cell response and expanding T follicular regulatory cell response. Phytomedicine. 2022;102:154171. doi:10.1016/j.phymed.2022.154171
8. Lee MK, Kim HD, Lee SH, et al. Curcumin ameliorates particulate matter-induced pulmonary injury through bimodal regulation of macrophage inflammation via NF-κB and Nrf2. Int J Mol Sci. 2023;24(3):1858. doi:10.3390/ijms24031858
9. Chuang HM, Ho LI, Huang MH, et al. Non-canonical regulation of type I collagen through promoter binding of SOX2 and its contribution to ameliorating pulmonary fibrosis by butylidenephthalide. Int J Mol Sci. 2018;19(10):3024. doi:10.3390/ijms19103024
10. Que H, Hong W, Lan T, et al. Tripterin liposome relieves severe acute respiratory syndrome as a potent COVID-19 treatment. Signal Transduct Target Ther. 2022;7(1):399. doi:10.1038/s41392-022-01283-6
11. Li C, Han P, Mao H, et al. Glycyrrhizic acid-based carbonized dots boost antiviral activity against influenza A virus via multisite inhibition mechanisms. ACS Appl Mater Interfaces. 2023. doi:10.1021/acsami.2c21319
12. Zhang P, Zhang Y, Wang L, et al. Reversal of NADPH oxidase-dependent early oxidative and inflammatory responses in chronic obstructive pulmonary disease by puerarin. Oxid Med Cell Longev. 2022;2022:5595781. doi:10.1155/2022/5595781
13. Teng C, Li B, Lin C, et al. Targeted delivery of baicalein-p53 complex to smooth muscle cells reverses pulmonary hypertension. J Control Release. 2022;341:591–604. doi:10.1016/j.jconrel.2021.12.006
14. Li X, Wang X, Han C, et al. Astragaloside IV suppresses collagen production of activated hepatic stellate cells via oxidative stress-mediated p38 MAPK pathway. Free Radic Biol Med. 2013;60:168–176. doi:10.1016/j.freeradbiomed.2013.02.027
15. Song MT, Ruan J, Zhang RY, et al. Astragaloside IV ameliorates neuroinflammation-induced depressive-like behaviors in mice via the PPARγ/NF-κB/NLRP3 inflammasome axis. Acta Pharmacol Sin. 2018;39(10):1559–1570. doi:10.1038/aps.2017.208
16. Lu J, Wang QY, Zhou Y, et al. Astragaloside IV against cardiac fibrosis by inhibiting TRPM7 channel. Phytomedicine. 2017;30:10–17. doi:10.1016/j.phymed.2017.04.002
17. Zheng Y, Dai Y, Liu W, et al. Astragaloside IV enhances taxol chemosensitivity of breast cancer via caveolin-1-targeting oxidant damage. J Cell Physiol. 2019;234(4):4277–4290. doi:10.1002/jcp.27196
18. Yu SY, Ouyang HT, Yang JY, et al. Subchronic toxicity studies of Radix astragali extract in rats and dogs. J Ethnopharmacol. 2007;110(2):352–355. doi:10.1016/j.jep.2006.09.024
19. Kang X, Su S, Hong W, et al. Research progress on the ability of astragaloside IV to protect the brain against ischemia-reperfusion injury. Front Neurosci. 2021;15:755902. doi:10.3389/fnins.2021.755902
20. Hl W, Qh Z, Mb X, et al. Astragaloside IV for experimental focal cerebral ischemia: preclinical evidence and possible mechanisms. Oxid Med Cell Longev. 2017;2017. doi:10.1155/2017/8424326
21. Zhang Y, Du M, Wang J, et al. Astragaloside IV relieves atherosclerosis and hepatic steatosis via MAPK/NF-κB signaling pathway in LDLR−/− mice. Front Pharmacol. 2022;13:828161. doi:10.3389/fphar.2022.828161
22. Chen T, Yang P, Jia Y. Molecular mechanisms of astragaloside‑IV in cancer therapy (Review). Int J Mol Med. 2021;47(3):13. doi:10.3892/ijmm.2021.4846
23. Meiqian Z, Leying Z, Chang C. Astragaloside IV inhibits cigarette smoke-induced pulmonary inflammation in mice. Inflammation. 2018;41(5):1671–1680. doi:10.1007/s10753-018-0811-x
24. Yang X, Wang F. The effect of astragaloside IV on JAK2-STAT6 signalling pathway in mouse model of ovalbumin-induced asthma. J Anim Physiol Anim Nutr (Berl). 2019;103(5):1578–1584. doi:10.1111/jpn.13114
25. Li L, Li G, Chen M, et al. Astragaloside IV enhances the sensibility of lung adenocarcinoma cells to bevacizumab by inhibiting autophagy. Drug Dev Res. 2022;83(2):461–469. doi:10.1002/ddr.21878
26. Gong F, Qu R, Li Y, et al. Astragalus mongholicus: a review of its anti-fibrosis properties. Front Pharmacol. 2022;13:976561. doi:10.3389/fphar.2022.976561
27. Wang X, Wang Y, Huang D, et al. Astragaloside IV regulates the ferroptosis signaling pathway via the Nrf2/SLC7A11/GPX4 axis to inhibit PM2.5-mediated lung injury in mice. Int Immunopharmacol. 2022;112:109186. doi:10.1016/j.intimp.2022.109186
28. Poch D, Mandel J. Pulmonary hypertension. Ann Intern Med. 2021;174(4):ITC49–ITC64. doi:10.7326/AITC202104200
29. Weatherald J, Boucly A, Peters A, et al. The evolving landscape of pulmonary arterial hypertension clinical trials. Lancet. 2022;400(10366):1884–1898. doi:10.1016/S0140-6736(22)01601-4
30. Xue Z, Li Y, Zhou M, et al. Traditional herbal medicine discovery for the treatment and prevention of pulmonary arterial hypertension. Front Pharmacol. 2021;12:720873. doi:10.3389/fphar.2021.720873
31. Zhang X, Chen J, Xu P, et al. Protective effects of astragaloside IV against hypoxic pulmonary hypertension. Medchemcomm. 2018;9(10):1715–1721. doi:10.1039/c8md00341f
32. Thenappan T, Ormiston ML, Ryan JJ, et al. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. doi:10.1136/bmj.j5492
33. Bodas M, Subramaniyan B, Karmouty-Quintana H, et al. The emerging role of NOTCH3 receptor signalling in human lung diseases. Expert Rev Mol Med. 2022;24:e33. doi:10.1017/erm.2022.27
34. Zhang Y, Hernandez M, Gower J, et al. JAGGED-NOTCH3 signaling in vascular remodeling in pulmonary arterial hypertension. Sci Transl Med. 2022;14(643):eabl5471. doi:10.1126/scitranslmed.abl5471
35. Yao J, Fang X, Zhang C, et al. Astragaloside IV attenuates hypoxia‑induced pulmonary vascular remodeling via the Notch signaling pathway. Mol Med Rep. 2021;23(1):89. doi:10.3892/mmr.2020.11726
36. Tian X, Zhang X, Feng Y, et al. Astragaloside IV in hypoxic pulmonary hypertension: an in vivo and in vitro experiments. Appl Biochem Biotechnol. 2022;194(12):6319–6334. doi:10.1007/s12010-022-04027-y
37. Lee S, Suh GY, Ryter SW, et al. Regulation and function of the nucleotide binding domain leucine-rich repeat-containing receptor, pyrin domain-containing-3 inflammasome in lung disease. Am J Respir Cell Mol Biol. 2016;54(2):151–160. doi:10.1165/rcmb.2015-0231TR
38. Sun Y, Lu M, Sun T, et al. Astragaloside IV attenuates inflammatory response mediated by NLRP-3/calpain-1 is involved in the development of pulmonary hypertension. J Cell Mol Med. 2021;25(1):586–590. doi:10.1111/jcmm.15671
39. Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941–1952. doi:10.1016/S0140-6736(17)30866-8
40. Mackinnon AC, Gibbons MA, Farnworth SL, et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med. 2012;185(5):537–546. doi:10.1164/rccm.201106-0965OC
41. Qian W, Cai X, Qian Q, et al. Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med. 2018;22(9):4354–4365. doi:10.1111/jcmm.13725
42. Qian W, Cai X, Qian Q, et al. Metastasis-associated protein 1 promotes epithelial-mesenchymal transition in idiopathic pulmonary fibrosis by up-regulating Snail expression. J Cell Mol Med. 2020;24(11):5998–6007. doi:10.1111/jcmm.15062
43. Tian R, Zhu Y, Yao J, et al. NLRP3 participates in the regulation of EMT in bleomycin-induced pulmonary fibrosis. Exp Cell Res. 2017;357(2):328–334. doi:10.1016/j.yexcr.2017.05.028
44. Hou Y, Zhen Y, Xue Q, et al. Astragaloside IV attenuates TGF-β-mediated epithelial-mesenchymal transition of pulmonary fibrosis via suppressing NLRP3 expression in vitro. Pharmazie. 2021;76(2):97–102. doi:10.1691/ph.2021.0933
45. Wang E, Wang L, Ding R, et al. Astragaloside IV acts through multi-scale mechanisms to effectively reduce diabetic nephropathy. Pharmacol Res. 2020;157:104831. doi:10.1016/j.phrs.2020.104831
46. Yu X, Xiao Q, Yu X, et al. A network pharmacology-based study on the mechanism of astragaloside IV alleviating renal fibrosis through the AKT1/GSK-3β pathway. J Ethnopharmacol. 2022;297:115535. doi:10.1016/j.jep.2022.115535
47. Zhu J, Wen K. Astragaloside IV inhibits TGF-β1-induced epithelial-mesenchymal transition through inhibition of the PI3K/Akt/NF-κB pathway in gastric cancer cells. Phytother Res. 2018;32(7):1289–1296. doi:10.1002/ptr.6057
48. Yu WN, Sun LF, Yang H. Inhibitory effects of astragaloside IV on bleomycin-induced pulmonary fibrosis in rats via attenuation of oxidative stress and inflammation. Inflammation. 2016;39(5):1835–1841. doi:10.1007/s10753-016-0420-5
49. Tong J, Wu Z, Wang Y, et al. Astragaloside IV synergizing with ferulic acid ameliorates pulmonary fibrosis by TGF-β1/Smad3 signaling. Evid Based Complement Alternat Med. 2021;2021:8845798. doi:10.1155/2021/8845798
50. Li N, Feng F, Wu K, et al. Inhibitory effects of astragaloside IV on silica-induced pulmonary fibrosis via inactivating TGF-β1/Smad3 signaling. Biomed Pharmacother. 2019;119:109387. doi:10.1016/j.biopha.2019.109387
51. Li LC, Xu L, Hu Y, et al. Astragaloside IV improves bleomycin-induced pulmonary fibrosis in rats by attenuating extracellular matrix deposition. Front Pharmacol. 2017;8:513. doi:10.3389/fphar.2017.00513
52. Zhang J, Wu C, Gao L, et al. Astragaloside IV derived from Astragalus membranaceus: a research review on the pharmacological effects. Adv Pharmacol. 2020;87:89–112. doi:10.1016/bs.apha.2019.08.002
53. Pitt JM, Marabelle A, Eggermont A, et al. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27(8):1482–1492. doi:10.1093/annonc/mdw168
54. Otano I, Ucero AC, Zugazagoitia J, et al. At the crossroads of immunotherapy for oncogene-addicted subsets of NSCLC. Nat Rev Clin Oncol. 2023;20(3):143–159. doi:10.1038/s41571-022-00718-x
55. Wang S, Rong R, Yang DM, et al. Features of tumor-microenvironment images predict targeted therapy survival benefit in patients with EGFR-mutant lung cancer. J Clin Invest. 2023;133(2):e160330. doi:10.1172/JCI160330
56. Smith C, Chang MY, Parker KH, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2(8):722–735. doi:10.1158/2159-8290.CD-12-0014
57. Wang K, Ye K, Zhang X, et al. Dual nicotinamide phosphoribosyltransferase (NAMPT) and indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors for the treatment of drug-resistant nonsmall-cell lung cancer. J Med Chem. 2023;66(1):1027–1047. doi:10.1021/acs.jmedchem.2c01954
58. Zhang A, Zheng Y, Que Z, et al. Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J Cancer Res Clin Oncol. 2014;140(11):1883–1890. doi:10.1007/s00432-014-1744-x
59. Meng Y, Wang W, Chen M, et al. GBP1 facilitates indoleamine 2,3-dioxygenase extracellular secretion to promote the malignant progression of lung cancer. Front Immunol. 2020;11:622467. doi:10.3389/fimmu.2020.622467
60. Xiang X, Wang J, Lu D, et al. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6(1):75. doi:10.1038/s41392-021-00484-9
61. Iglesias-Escudero M, Arias-González N, Martínez-Cáceres E. Regulatory cells and the effect of cancer immunotherapy. Mol Cancer. 2023;22(1):26. doi:10.1186/s12943-023-01714-0
62. Liu PS, Chen YT, Li X, et al. CD40 signal rewires fatty acid and glutamine metabolism for stimulating macrophage anti-tumorigenic functions. Nat Immunol. 2023;24(3):452–462. doi:10.1038/s41590-023-01430-3
63. Xu F, Cui WQ, Wei Y, et al. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J Exp Clin Cancer Res. 2018;37(1):207. doi:10.1186/s13046-018-0878-0
64. Cheng X, Gu J, Zhang M, et al. Astragaloside IV inhibits migration and invasion in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB pathway. Int Immunopharmacol. 2014;23(1):304–313. doi:10.1016/j.intimp.2014.08.027
65. Jia L, Lv D, Zhang S, et al. Astragaloside IV inhibits the progression of non-small cell lung cancer through the Akt/GSK-3β/β-Catenin pathway. Oncol Res. 2019;27(4):503–508. doi:10.3727/096504018X15344989701565
66. Li J, Wang Y, Song Y, et al. miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Mol Cancer. 2014;13:193. doi:10.1186/1476-4598-13-193
67. Moro M, Caiola E, Ganzinelli M, et al. Metformin enhances cisplatin-induced apoptosis and prevents resistance to cisplatin in co-mutated KRAS/LKB1 NSCLC. J Thorac Oncol. 2018;13(11):1692–1704. doi:10.1016/j.jtho.2018.07.102
68. Rampioni Vinciguerra GL, Capece M, Distefano R, et al. Role of the miR-301a/Fra-2/GLIPR1 axis in lung cancer cisplatin resistance. Signal Transduct Target Ther. 2023;8(1):37. doi:10.1038/s41392-022-01228-z
69. Zhu C, Xie Y, Li Q, et al. CPSF6-mediated XBP1 3’UTR shortening attenuates cisplatin-induced ER stress and elevates chemo-resistance in lung adenocarcinoma. Drug Resist Updat. 2023;68:100933. doi:10.1016/j.drup.2023.100933
70. Lai ST, Wang Y, Peng F. Astragaloside IV sensitizes non-small cell lung cancer cells to cisplatin by suppressing endoplasmic reticulum stress and autophagy. J Thorac Dis. 2020;12(7):3715–3724. doi:10.21037/jtd-20-2098
71. He CS, Liu YC, Xu ZP, et al. Astragaloside IV enhances cisplatin chemosensitivity in non-small cell lung cancer cells through inhibition of B7-H3. Cell Physiol Biochem. 2016;40(5):1221–1229. doi:10.1159/000453175
72. Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer. 2017;17(11):637–658. doi:10.1038/nrc.2017.84
73. Dai PC, Liu DL, Zhang L, et al. Astragaloside IV sensitizes non-small cell lung cancer cells to gefitinib potentially via regulation of SIRT6. Tumour Biol. 2017;39(4):1010428317697555. doi:10.1177/1010428317697555
74. Xu X, Wang H, Liu S, et al. TP53-dependent autophagy links the ATR-CHEK1 axis activation to proinflammatory VEGFA production in human bronchial epithelial cells exposed to fine particulate matter (PM2.5). Autophagy. 2016;12(10):1832–1848. doi:10.1080/15548627.2016.1204496
75. Li D, Li Y, Li G, et al. Fluorescent reconstitution on deposition of PM2.5 in lung and extrapulmonary organs. Proc Natl Acad Sci U S A. 2019;116(7):2488–2493. doi:10.1073/pnas.1818134116
76. Guohua F, Tieyuan Z, Xinping M, et al. Melatonin protects against PM2.5-induced lung injury by inhibiting ferroptosis of lung epithelial cells in a Nrf2-dependent manner. Ecotoxicol Environ Saf. 2021;223:112588. doi:10.1016/j.ecoenv.2021.112588
77. Xiong R, Jiang W, Li N, et al. PM2.5-induced lung injury is attenuated in macrophage-specific NLRP3 deficient mice. Ecotoxicol Environ Saf. 2021;221:112433. doi:10.1016/j.ecoenv.2021.112433
78. Li Y, Fu S, Li E, et al. Modulation of autophagy in the protective effect of resveratrol on PM2.5-induced pulmonary oxidative injury in mice. Phytother Res. 2018;32(12):2480–2486. doi:10.1002/ptr.6187
79. D J, G N, Km R. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023. doi:10.1038/s41580-023-00585-z
80. Racanelli AC, Kikkers SA, Choi AMK, et al. Autophagy and inflammation in chronic respiratory disease. Autophagy. 2018;14(2):221–232. doi:10.1080/15548627.2017.1389823
81. Pei C, Wang F, Huang D, et al. Astragaloside IV protects from PM2.5-induced lung injury by regulating autophagy via inhibition of PI3K/Akt/mTOR signaling in vivo and in vitro. J Inflamm Res. 2021;14:4707–4721. doi:10.2147/JIR.S312167
82. Wang Z, Wu Y, Pei C, et al. Astragaloside IV pre-treatment attenuates PM2.5-induced lung injury in rats: impact on autophagy, apoptosis and inflammation. Phytomedicine. 2022;96:153912. doi:10.1016/j.phymed.2021.153912
83. Zhao J, Jia Y, Mahmut D, et al. Human hematopoietic stem cell vulnerability to ferroptosis. Cell. 2023;186(4):732–747.e16. doi:10.1016/j.cell.2023.01.020
84. Xia H, Wu Y, Zhao J, et al. N6-Methyladenosine-modified circSAV1 triggers ferroptosis in COPD through recruiting YTHDF1 to facilitate the translation of IREB2. Cell Death Differ. 2023. doi:10.1038/s41418-023-01138-9
85. Hu P, Xu Y, Jiang Y, et al. The mechanism of the imbalance between proliferation and ferroptosis in pulmonary artery smooth muscle cells based on the activation of SLC7A11. Eur J Pharmacol. 2022;928:175093. doi:10.1016/j.ejphar.2022.175093
86. Shi X, Li C, Cheng L, et al. Mycobacterium tuberculosis Rv1324 Protein Contributes to Mycobacterial Persistence and Causes Pathological Lung Injury in Mice by Inducing Ferroptosis. Microbiol Spectr. 2023;11(1):e0252622. doi:10.1128/spectrum.02526-22
87. Privitera G, Rana N, Armuzzi A, et al. The gasdermin protein family: emerging roles in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol. 2023. doi:10.1038/s41575-023-00743-w
88. Huang D, Shi S, Wang Y, et al. Astragaloside IV alleviates PM2.5-caused lung toxicity by inhibiting inflammasome-mediated pyroptosis via NLRP3/caspase-1 axis inhibition in mice. Biomed Pharmacother. 2022;150:112978. doi:10.1016/j.biopha.2022.112978
89. Wu Y, Xiao W, Pei C, et al. Astragaloside IV alleviates PM2.5-induced lung injury in rats by modulating TLR4/MyD88/NF-κB signalling pathway. Int Immunopharmacol. 2021;91:107290. doi:10.1016/j.intimp.2020.107290
90. Chen T, Wang R, Jiang W, et al. Protective effect of astragaloside IV against paraquat-induced lung injury in mice by suppressing Rho signaling. Inflammation. 2016;39(1):483–492. doi:10.1007/s10753-015-0272-4
91. Li H, Yao C, Shi K, et al. Astragaloside IV attenuates hypoxia/reoxygenation injury-induced apoptosis of type II alveolar epithelial cells through miR-21-5p. Bioengineered. 2021;12(1):7747–7754. doi:10.1080/21655979.2021.1982845
92. Xiong P, Jiang LZ, Liao XQ. 黄芪甲苷保护大鼠肺缺血再灌注肺损伤的形态学研究 [Morphological investigation of the protective effect of astragaloside preconditioning against ischemia-reperfusion lung injury in rats]. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30(8):1864–1867.
93. Georas SN, Wright RJ, Ivanova A, et al. The Precision Interventions for Severe and/or Exacerbation-Prone (PrecISE) Asthma Network: an overview of Network organization, procedures, and interventions. J Allergy Clin Immunol. 2022;149(2):488–516.e9. doi:10.1016/j.jaci.2021.10.035
94. Huang X, Tang L, Wang F, et al. Astragaloside IV attenuates allergic inflammation by regulation Th1/Th2 cytokine and enhancement CD4(+)CD25(+)Foxp3 T cells in ovalbumin-induced asthma. Immunobiology. 2014;219(7):565–571. doi:10.1016/j.imbio.2014.03.005
95. Chen JK, Guo MK, Bai XH, et al. Astragaloside IV ameliorates intermittent hypoxia-induced inflammatory dysfunction by suppressing MAPK/NF-κB signalling pathways in Beas-2B cells. Sleep Breath. 2020;24(3):1237–1245. doi:10.1007/s11325-019-01947-8
96. Jin H, Wang L, Li B, et al. Astragaloside IV ameliorates airway inflammation in an established murine model of asthma by inhibiting the mTORC1 signaling pathway. Evid Based Complement Alternat Med. 2017;2017:4037086. doi:10.1155/2017/4037086
97. Du Q, Chen Z, Zhou LF, et al. Inhibitory effects of astragaloside IV on ovalbumin-induced chronic experimental asthma. Can J Physiol Pharmacol. 2008;86(7):449–457. doi:10.1139/y08-053
98. Gu X, Jiang D, Wang Y, et al. Effects of astragaloside IV on eosinophil activation induced by house dust mite allergen. Mol Med Rep. 2012;6(1):115–120. doi:10.3892/mmr.2012.869
99. Christenson SA, Smith BM, Bafadhel M, et al. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi:10.1016/S0140-6736(22)00470-6
100. Wang L, Gu W, Shi Y, et al. Protective effects of astragaloside IV on IL-8-treated diaphragmatic muscle cells. Exp Ther Med. 2019;17(1):519–524. doi:10.3892/etm.2018.6940
101. Yang IA, Jenkins CR, Salvi SS. Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir Med. 2022;10(5):497–511. doi:10.1016/S2213-2600(21)00506-3
102. Chen L, Sun BB, Wang T, et al. Cigarette smoke enhances {beta}-defensin 2 expression in rat airways via nuclear factor-{kappa}B activation. Eur Respir J. 2010;36(3):638–645. doi:10.1183/09031936.00029409
103. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607–613. doi:10.1016/j.jinf.2020.03.037
104. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
105. Ge C, He Y. In silico prediction of molecular targets of astragaloside IV for alleviation of COVID-19 hyperinflammation by systems network pharmacology and bioinformatic Gene Expression Analysis. Front Pharmacol. 2020;11:556984. doi:10.3389/fphar.2020.556984
106. Ye M, Luo G, Ye D, et al. Network pharmacology, molecular docking integrated surface plasmon resonance technology reveals the mechanism of Toujie Quwen Granules against coronavirus disease 2019 pneumonia. Phytomedicine. 2021;85:153401. doi:10.1016/j.phymed.2020.153401
107. Gu Y, Wang G, Pan G, et al. Transport and bioavailability studies of astragaloside IV, an active ingredient in Radix astragali. Basic Clin Pharmacol Toxicol. 2004;95(6):295–298. doi:10.1111/j.1742-7843.2004.t01-1-pto950508.x
108. Zhang Q, Zhu LL, Chen GG, et al. Pharmacokinetics of astragaloside iv in beagle dogs. Eur J Drug Metab Pharmacokinet. 2007;32(2):75–79. doi:10.1007/BF03190995
109. Jiangbo Z, Xuying W, Yuping Z, et al. Effect of astragaloside IV on the embryo-fetal development of Sprague-Dawley rats and New Zealand White rabbits. J Appl Toxicol. 2009;29(5):381–385. doi:10.1002/jat.1422
110. Kuo YC, Chen IY, Rajesh R. Astragaloside IV- and nesfatin-1-encapsulated phosphatidylserine liposomes conjugated with wheat germ agglutinin and leptin to activate anti-apoptotic pathway and block phosphorylated tau protein expression for Parkinson’s disease treatment. Mater Sci Eng C Mater Biol Appl. 2021;129:112361. doi:10.1016/j.msec.2021.112361
111. Zhou J, Zhang J, Gao G, et al. Boiling licorice produces self-assembled protein nanoparticles: a novel source of bioactive nanomaterials. J Agric Food Chem. 2019;67(33):9354–9361. doi:10.1021/acs.jafc.9b03208
112. Sun R, Zhang A, Ge Y, et al. Ultra-small-size astragaloside-IV loaded lipid nanocapsules eye drops for the effective management of dry age-related macular degeneration. Expert Opin Drug Deliv. 2020;17(9):1305–1320. doi:10.1080/17425247.2020.1783236
113. Zhang Y, Tao C, Xuan C, et al. Transcriptomic analysis reveals the protection of astragaloside IV against diabetic nephropathy by modulating inflammation. Oxid Med Cell Longev. 2020;2020:9542165. doi:10.1155/2020/9542165
114. Wang M, Shi Y, Yao L, et al. Identification of hub genes in protective effect of astragaloside IV on aconitine-induced cardiac damage in zebrafish based on bioinformatics analysis. Front Pharmacol. 2020;11:957. doi:10.3389/fphar.2020.00957
115. Xia C, He Z, Cai Y. Quantitative proteomics analysis of differentially expressed proteins induced by astragaloside IV in cervical cancer cell invasion. Cell Mol Biol Lett. 2020;25:25. doi:10.1186/s11658-020-00218-9
116. Xu M, Yin J, Xie L, et al. Pharmacokinetics and tolerance of toal astragalosides after intravenous infusion of astragalosides injection in healthy Chinese volunteers. Phytomedicine. 2013;20(12):1105–1111. doi:10.1016/j.phymed.2013.05.004
117. Meng LQ, Tang JW, Wang Y, et al. Astragaloside IV synergizes with ferulic acid to inhibit renal tubulointerstitial fibrosis in rats with obstructive nephropathy. Br J Pharmacol. 2011;162(8):1805–1818. doi:10.1111/j.1476-5381.2011.01206.x
118. Sun B, Rui R, Pan H, et al. Effect of combined use of astragaloside IV (AsIV) and atorvastatin (AV) on expression of PPAR-γ and inflammation-associated cytokines in atherosclerosis rats. Med Sci Monit. 2018;24:6229–6236. doi:10.12659/MSM.908480
119. Wang D, Liu Y, Zhong G, et al. Compatibility of tanshinone IIA and astragaloside IV in attenuating hypoxia-induced cardiomyocytes injury. J Ethnopharmacol. 2017;204:67–76. doi:10.1016/j.jep.2017.03.053
120. Du N, Xu Z, Gao M, et al. Combination of ginsenoside Rg1 and astragaloside IV reduces oxidative stress and inhibits TGF-β1/Smads signaling cascade on renal fibrosis in rats with diabetic nephropathy. Drug Des Devel Ther. 2018;12:3517–3524. doi:10.2147/DDDT.S171286
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.