Back to Journals » Infection and Drug Resistance » Volume 16
Uncorrected Preoperative Infection Causing the Death of a Patient with a Thoracic Aortic Aneurysm
Authors He XQ, Qiu HQ, Wang M, Mao YF, Li XY, Wang XY, Geng YL, Wang L
Received 4 November 2022
Accepted for publication 23 December 2022
Published 12 January 2023 Volume 2023:16 Pages 243—248
DOI https://doi.org/10.2147/IDR.S396269
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xin-Qi He,1,* Hui-Qing Qiu,2,* Meng Wang,3,* Ya-Fei Mao,4 Xin-Yuan Li,4 Xian-Yun Wang,5 Yu-Lan Geng,4 Le Wang6
1Department of Vascular Surgery, The First Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 2Department of Neurology, The First Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 3Department of Radiology and Nuclear Medicine, The First Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 4Department of Laboratory Medicine, The First Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 5Scientific Research Data Center, The First Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 6Department of Cardiology, The First Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu-Lan Geng, Department of Laboratory Medicine, The First Hospital of Hebei Medical University, No. 89 of Donggang Road, Yuhua District, Shijiazhuang, 050031, People’s Republic of China, Tel +86 311 87156567, Fax +86 311 85917029, Email [email protected] Le Wang, Department of Cardiology, The First Hospital of Hebei Medical University, No. 89 of Donggang Road, Yuhua District, Shijiazhuang, 050031, People’s Republic of China, Tel +86 311 87155263, Fax +86 311 85917029, Email [email protected]
Background: A thoracic aortic aneurysm (TAA) is a known condition seen in cardiovascular practice. A TAA rupture and postoperative infection may result in death. Preoperative infections leading to death are extremely rare.
Case Study: A 62-year-old Chinese female was admitted to The First Hospital of Hebei Medical University with a two-day history of abdominal pain. She was diagnosed with a TAA rupture and underwent immediate surgery. The preoperative urine analysis indicated that the positive bacteria and white blood cell count suggested a urinary tract bacterial infection. The patient was administered the empiric antibiotics, cefazolin; however, her blood pressure continued to drop during the perioperative period and she died of uncorrectable acidosis 8 h after the operation. On the second day after death, both the blood and urine cultures were positive for Pseudomonas aeruginosa.
Conclusion: Given that this patient with a TAA rupture died of uncorrected acidosis caused by preoperative infection, it is important to evoke the diagnosis in the context of TAA. Routine laboratory indicators are valuable factors for surgeons and physicians in assessing a patient’s condition and improving their prognosis.
Keywords: thoracic aortic aneurysm, infection, sepsis, acidosis, urine routine, emergency surgery, empirical medication, Pseudomonas aeruginosa
Introduction
A thoracic aortic aneurysm (TAA) is a type of aortic aneurysm (AA) and is a common clinical entity seen in cardiovascular practice. TAAs have a higher prevalence rate of 1.3–8.9% in men than 1.0–2.2% in women.1 And approximately 5–10 people out of 100,000 individuals are diagnosed with a TAA every year, representing a major cause of mortality and morbidity in Western countries.1 The etiology of aneurysmal formation is still not well-understood. Most aortic aneurysms are caused by degenerative, infectious, or genetic conditions that weaken the aortic wall.2 Infective TAA may be caused by fungal, bacterial, spirochetal, viral, or mycobacterium microorganisms.3 Infected AAs are difficult to treat and with a mortality of more than 20%, the main cause being delaying diagnosis and subsequent complications, such as rupture and sepsis.4,5 Common bacterial pathogens include Staphylococcus aureus, Streptococcus pneumoniae, and non-typhoidal Salmonella followed by other gram-negative organisms such as Escherichia coli, Klebsiella, and Pseudomonas spp.6,7 Most TAAs are clinically asymptomatic and are identified incidentally upon imaging examination. The initial symptoms are generally secondary to an aortic dissection or rupture that follows hypertension, Marfan syndrome, and Turner syndrome, often being the leading cause of death.8 While endovascular stent grafts have significantly reduced the TAA death rate, some patients with this diagnosis die due to a postoperative infection.9
TAA rupture is characterized by high death rate and has an urgent surgery required. At the same time of emergency surgery, identification of TAA etiology is also crucial to reduce the risk of complications and death. No published literatures were found to rapidly identifying pathogens. Here, this study reports the case of a 62-year-old Chinese female with a TAA rupture who died of uncorrected severe preoperative infection and acidosis, which is very significant to identify some infective conditions and to be more precisely administered.
Case Presentation
A 62-year-old Chinese female was admitted to The First Hospital of Hebei Medical University with a two-day history of abdominal pain, especially in the subxiphoid region, as well as dyspnea, sweating, headache, and dizziness. She had received regular treatment for nine years for a coronary stent and had a nine-year hypertension history.
The initial physical examination returned the following results: temperature = 36.2°C, heart rate = 90 beats per minute, respiratory rate = 18 breaths per minute, and blood pressure = 90/51mmHg. She was week and apathic. She had clammy skin, abdominal distension, and percussion sound on the abdomen. No obvious abnormalities were found in terms of auscultation and palpation of the lung and abdomen or in terms of the nervous system.
The initial laboratory examinations revealed an increased neutrophil percentage (90%) and a decreased platelet count (PLT) (Table 1). The serum biomarkers of glucose, urea, creatinine, alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transpeptidase, alkaline phosphatase, total protein, albumin, and globulin returned values of 7.80 mmol/L (reference range: 3.90–6.10 mmol/L), 12.39 mmol/L (2.60–7.50 mmol/L), 194 μmol/L (41.0–73.0 μmol/L), 78.6 U/L (7.0–40.0 U/L), 111.3 U/L (13.0–35.0 U/L), 159 U/L (7–45 U/L), 354 U/L (50–135 U/L), 44.5 g/L (65.0–85.0 g/L), 29.1 g/L (40.0–55.0 g/L), and 15.4 g/L (20.0–40.0 g/L), respectively. The coagulation tests indicated a prolonged prothrombin time (21.6 s) (reference range: 9.4–12.5 s), an activated partial thromboplastin time (42.1 s) (25.1–36.5 s), and an extremely elevated D-dimer (43.49 mg/L) (0–0.55 mg/L), while the patient’s fibrinogen (3.50 g/L) (2.38–4.98 g/L) and thrombin time (14.9 s) (10.3–16.6 s) were normal. Among the heart biomarkers, the serum N-terminal pro-B type natriuretic peptide (NT-proBNP) level was markedly higher, while the patient returned a mildly high troponin I result and a normal creatine kinase–MB result. The arterial blood gas analysis indicated compensatory metabolic acidosis with a pH of 7.36 (reference range: 7.35–7.45), a partial pressure of carbon dioxide of 33.4 mmHg (35–45 mmHg), and a partial pressure of oxygen of 61.6 mmHg (80–100 mmHg). The routine urine tests indicated increased red blood cell (RBC) count (134 cells/μL) (reference range: 0–17), white blood cell (WBC) count (1264 cells/μL) (0–28 cells/μL), purulent cell count (32 cells/μL) (0–2 cells/μL), bacteria (325/μL) (0–340 cells/μL), fungi (0 cells/μL) (0–1 cells/μL), urine protein (2+) (negative), and occult blood (3+) (negative), with negative nitrite (negative). Hepatitis virus series, human immunodeficiency virus (HIV), and syphilis antibodies were negative. Pneumoclide IgM test showed that the antibodies of Legionella pneumophila type 1, mycoplasma pneumoniae, rickettsia Q, chlamydia pneumoniae, adenovirus, respiratory syncytial virus, influenza A virus, influenza B virus, and parainfluenza virus type 1, 2 and 3 were all negative. All other autoantibodies were negative.
 |
Table 1 Laboratory Findings Before and After Operation |
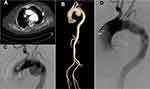
The computed tomography (CT) and CT angiography (CTA) examinations revealed a TAA rupture (Figures 1A–C) and no abnormality on both lungs, while the electrocardiogram (ECG) examination indicated a prolonged corrected QT interval. Finally, the cardiac ultrasound examination indicated left ventricular enlargement, thickening of the basal segment of the ventricular septum, and left ventricular diastolic dysfunction.
Based on the general manifestations and the ECG, cardiac ultrasound, CT, CTA, and BNP results, the patient was diagnosed with a TAA complicated with heart failure, while a urinary tract infection was also considered. Other laboratory examinations, including serum procalcitonin (PCT), interleukin (IL)-6, and lymphocyte subset examinations, were also performed to identify the infection and immune function, along with a bacterial culture of blood and urine (Table 1). Meanwhile, the cefazolin antibiotic was empirically administered as an anti-bacterial agent. An aortography operation, stent implantation involving the aortic branch, and an exploration of the right femoral artery and left brachial artery were performed immediately to target the TAA rupture and to determine the etiology of chest pain. The aortography procedure revealed a TAA 12 cm in diameter, while the bilateral renal arteries and bilateral iliac arteries were observed to be well developed. The surrounding tissue showed no inflammatory findings and no pus was discharged from the aortic aneurysm wall incision. During the operation, 10 units of cryoprecipitate and 200 mL of fresh frozen plasma were transfused. The operation was successful and resulted in a better recovery of blood flow (Figure 1D). There were no clear differences in complete blood count, serum biomarkers, coagulation tests, and heart biomarkers, but the NT-proBNP level increased after the repair operation. During the whole procedure, the patient’s blood pressure continuously dropped, and they experienced acidosis aggravation and shock. The patient died 8 h after the operation. On the second day after death, both serum fungitec G test and serum glactomannan test were negative while both the blood and the urine cultures for bacteria and fungi showed positive for Pseudomonas aeruginosa.
All procedures were performed in accordance with both the ethical standards of the institutional and/or national research committee(s) and the Helsinki Declaration (as revised in 2013). Written informed consent for publication was obtained from the patient’s legal husband.
Discussion
This case involved a patient with a TAA rupture who underwent thoracic endovascular aneurysm repair. The main cause of death was determined to be uncorrected sepsis and acidosis due to preoperative infection.
The repair operation was successful and the patient’s condition was stable during the perioperative period, and there were no clear differences in complete blood cells with differentials, liver and renal function, glucose, and coagulation function. Therefore, operation failure was excluded as a possible cause of death.
The uncorrected preoperative infection was ultimately considered to be the main cause of death. First, the abnormally high WBC and RBC count, and the amount of bacteria in the urine served as key evidence that a bacterial infection had emerged prior to the operation. Second, all other pathogen antibodies included in common hepatitis viruses, HIV, Legionella pneumophila type 1, mycoplasma pneumoniae, Rickettsia Q, Chlamydia pneumoniae were negative while both serum fungitec G test and serum glactomannan test reflecting fungi infection were negative. Third, both PCT and IL-6 are strong evidence of a bacterial infection and are also appropriate detection and monitoring indicators during antibiotic-based therapy.10 The increased levels of serum PCT and IL-6 in this case were important indicators of an uncontrolled bacterial infection.
In the World Health Organization’s “Model List of Essential Medicines”, cefazolin is the representative first-generation cephalosporin classified as an “access” antimicrobial, and countries should ensure its availability on a nationwide level.11,12 Cefazolin is often used to treat methicillin-sensitive Staphylococcus aureus infections and serves as a prophylaxis for many types of surgical operation.13 However, while cefazolin was administered prior to the operation in the present case, it was not as effective as expected. The urine-negative nitrite result suggested that the infectious pathogen could not reduce nitrate to nitrite, with the test results suggesting that the pathogenic bacteria were not S. aureus, a Gram-positive opportunistic pathogen that can reduce nitrate into nitrite. In fact, the final blood and urine bacteria culture confirmed that the pathogen was P. aeruginosa, which could not be effectively killed by the cefazolin used in the treatment.
Pseudomonas aeruginosa, a bacterium characterized by its low antibiotic susceptibility, is one of the most relevant opportunistic pathogens and one of the leading pathogens causing bloodstream infections.14 In a previous study, a P. aeruginosa infection in mice induced a mild but significant state of peripheral thrombocytopenia in addition to pulmonary PLT accumulation,15 which may have been an important cause of the low PLT in the present case. As innate immune cells, WBCs and neutrophils are the first line of defense against pathogens. The present case involved a lower first-line defense ability with normal WBC and higher neutrophil percentages.
Lymphocyte subsets reflect adaptive immunity. In this case, a higher B cell percentage ensured greater protection from bacterial infection and progress. The B cells promoted by IL-6 transform into plasma cells to produce antibodies. Natural killer (NK) cells exert antibody-dependent cytotoxicity to kill bacteria.16 However, in the present case, the abnormally low counts of T, T-helper, cytotoxic T, NK, and NKT cell lymphocytes were manifestations of low immunity and the main cause of the P. aeruginosa infection.
Whether the patient experienced sepsis and sepsis shock was a further issue. On admission, the patient presented altered consciousness and a systolic blood pressure of 90 mmHg, while she also had a suspected infection and met the sepsis criteria.17 Therefore, it was concluded that the patient experienced sepsis and sepsis shock during hospitalization.
Conclusion
In conclusion, this case involved a patient with a TAA rupture who died due to an uncorrected preoperative infection and sepsis. Thus, it is important to evoke the diagnosis within the context of TAA. With emergency surgery, rapid laboratory examinations such as urine routine may be valuable for surgeons and physicians in prevention medication, accurately assessing a patient’s condition and improving their prognosis.
Abbreviations
TAA, Thoracic aortic aneurysm; AA, aortic aneurysms; PLT, platelet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; PT, prothrombin time; APTT, activated partial thromboplastin time; TT, thrombin time; NT-proBNP, N-terminal pro-B type natriuretic peptide; TnI, troponin I; CK, creatine kinase; CT, computed tomography; CTA, computed tomography angiography; PCT, procalcitonin; IL, interleukin.
Data Sharing Statement
The data generated or analyzed during this study are available in this published article.
Ethics Approval and Consent to Participate
All procedures performed were in accordance with the Declaration of Helsinki. This report was approved by the Ethics Board of The First Hospital of Hebei Medical University (No.2022414). Written informed consent to participate in this report was obtained from the patient’s legal husband because she was died.
Consent for Publication
The patient’s legal husband provided written informed consent for publication because she was died.
Funding
Natural Science Foundation of Hebei Province (No. H202120603).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Silvay G, Lurie JM, Casale M. The anaesthetic management of patients with thoracic ascending aortic aneurysms, A review. J Perioper Pract. 2021;31(7–8):281–288. PMID 32648837. doi:10.1177/1750458920936064
2. Quintana RA, Taylor WR. Cellular mechanisms of aortic aneurysm formation. Circ Res. 2019;124(4):607–618. PMID 30763207; PMCID PMC6383789. doi:10.1161/CIRCRESAHA.118.313187
3. Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease, a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
4. Oderich GS, Panneton JM, Bower TC, et al. Infected aortic aneurysms, aggressive presentation, complicated early outcome, but durable results. J Vasc Surg. 2001;34:900–908. doi:10.1067/mva.2001.118084
5. Hsu R-B, Tsay Y-G, Wang S-S, Chu S-H. Surgical treatment for primary infected aneurysm of the descending thoracic aorta, abdominal aorta, and iliac arteries. J Vasc Surg. 2002;36:746–750. doi:10.1067/mva.2002.126557
6. Marques da Silva R, Caugant DA, Erk E, et al. Bacterial diversity in aortic aneurysms determined by 16S ribosomal RNA gene analysis. J Vasc Surg. 2006;44:1055–1060. doi:10.1016/j.jvs.2006.07.021
7. Brossier J, Lesprit P, Marzelle J, Allaire E, Becquemin J-P, Desgranges P. New bacteriological patterns in primary infected aorto-iliac aneurysms, a single-Centre experience. Eur J Vasc Endovasc Surg. 2010;40:582–588. doi:10.1016/j.ejvs.2010.07.020
8. Aru RG, Tyagi SC, Minion DJ, Orr NT, Bounds MC. Carotid-carotid transposition for zone 1 thoracic endovascular aortic repair. Ann Vasc Surg. 2021;76:325–329. doi:10.1016/j.avsg.2021.04.025
9. Shiraev T, Barrett S, Heywood S, et al. Incidence, management, and outcomes of aortic graft infection. Ann Vasc Surg. 2019;59:73–83. doi:10.1016/j.avsg.2019.01.027
10. Cong S, Ma T, Di X, Tian C, Zhao M, Wang K. Diagnostic value of neutrophil CD64, procalcitonin, and interleukin-6 in sepsis, a meta-analysis. BMC Infect Dis. 2021;21(1):384. doi:10.1186/s12879-021-06064-0
11. World Health Organization. Model list of essential medicines 21st list 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf?ua=1.
12. World Health Organization. List of antibiotics. Available from: https://aware.essentialmeds.org/list.
13. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195–283. doi:10.2146/ajhp120568
14. Kern WV, Rieg S. Burden of bacterial bloodstream infection-A brief update on epidemiology and significance of multidrug-resistant pathogens. Clin Microbiol Infect. 2020;26(2):151–157. doi:10.1016/j.cmi.2019.10.031
15. Amison RT, O’Shaughnessy BG, Arnold S, et al. Platelet depletion impairs host defense to pulmonary infection with pseudomonas aeruginosa in mice. Am J Respir Cell Mol Biol. 2018;58(3):331–340. doi:10.1165/rcmb.2017-0083OC
16. Prager I, Watzl C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J Leukoc Biol. 2019;105(6):1319–1329. doi:10.1002/JLB.MR0718-269R
17. Egi M, Ogura H, Yatabe T, et al. The Japanese clinical practice guidelines for management of sepsis and septic shock 2020 (J-SSCG 2020). Acute Med Surg. 2021;8(1):e659. doi:10.1002/ams2.659
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.