Back to Journals » International Journal of General Medicine » Volume 16
Ultrasound Imaging in Subjects with Sickle Cell Disease: The Saudi Arabia Experiences
Authors Adam M , Musa MJ, Al-Qahtani SM, Alelyani M , Musa A , Elzaki M , Alzain AF, Ali S, Medani A, Mukhtar EM, Gareeballah A
Received 4 June 2023
Accepted for publication 17 October 2023
Published 31 October 2023 Volume 2023:16 Pages 4931—4942
DOI https://doi.org/10.2147/IJGM.S419013
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mohamed Adam,1 Mustafa J Musa,2 Saleh M Al-Qahtani,3 Magbool Alelyani,1 Alamin Musa,1 Maisa Elzaki,4 Amel FH Alzain,4 Sarra Ali,5 Afaf Medani,1 Emadeldedin Mohamed Mukhtar,1 Awadia Gareeballah4
1Department of Radiological Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Asir, Saudi Arabia; 2Department of Applied Radiologic Science, University of Jeddah, Kingdom of Saudi Arabia (KSA), Jeddah, Saudi Arabia; 3Department of Child Health, College of Medicine, King Khalid University, Abha, Asir, Saudi Arabia; 4Department of Diagnostic Radiology Technology, College of Applied Medical Sciences, Taibah University, Al-Madianah Al-Munawwarrah, Saudi Arabia; 5Department of Diagnostic Radiography Technology, College of Applied Medical Sciences, Jazan University, Jazan, Saudi Arabia
Correspondence: Mohamed Adam, Department of Radiological Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Asir, Saudi Arabia, Email [email protected]; [email protected]
Background: Abdominal organ sonography is a crucial part of the workup for treating sickle cell disease (SCD) patients.
Objective: The main objective of this study was to evaluate the abdominal organs in SCD patients using ultrasonography.
Methodology: A non-interventional descriptive cross-sectional study was carried out in Asir region Saudi Arabia from April 2019 to July 2020. The study was conducted in 78 patients with sickle cell disease (SCD). Data were gathered using a data collection sheet included demographic information, clinical information including medication types, and complications linked to SCD. Furthermore, the study evaluated abdominal ultrasound findings pertaining to the liver, gall bladder, spleen, and kidneys. The data were analyzed using Statistical Package for Social Sciences (SPSS).
Results: More than half of the study participants 43 (55.1%) were females. About 53.8% of the study participants received blood transfusions, and (11.5%) receive extra-vaccine. Concerning ultrasound findings, hepatomegaly was found in seventeen (21.8%), focal liver lesions in four (5.1%), gallstones in five (6.4%), splenomegaly in fifteen (19.3%), and the presence of splenic focal lesions was found in seven (9.0%). The most frequent complication associated with SCD was osteomyelitis sepsis in six cases (7.7%). The study revealed a significant correlation between the type of crisis and type of medication used and the size of the spleen (P-value < 0.01), and no notable correlation was found between the types of crises and the size of the liver (P-value > 0.05).
Conclusion: Abdominal sonography in SCD patients revealed a wide range of alterations in the liver, gallbladder, and spleen. The most frequently observed complications in SCD were hepatomegaly, splenomegaly, localized lesions in both organs, and the presence of gallstones.
Keywords: sickle cell disease, ultrasonography, abdominal organ, echo-texture
Introduction
Sickle cell disease is a type of hereditarily acquired red blood cell (RBC) disorder, the most typical form of which is sickle cell anaemia (SCD). It is linked to the pathologic consequences of chronic illnesses, such as sickle cell crises, which can manifest in various forms in SCD patients, resulting in vaso-occlusion, ischemia in several organs, and complex illness of the cell.1
Pathophysiology: sickle cause HbSS to polymerases in long filaments (HbSS is 25-fold less soluble than HbAA).2–4 Red blood cells that have undergone polymerization have sickle-shaped cells as a result. The hemoglobin polymers break the cell membrane when deformability is further impaired, allowing free hemoglobin to escape into the bloodstream where it will scavenge circulating nitric oxide and worsen vaso-occlusion.3,5–7 The generation of reticulocytes and the release of early red cell progenitors from the bone marrow-containing adhesion molecules will be stimulated by hemolysis. Sickle cells can cause organ infarcts by adhering to the vascular endothelium, obstructing microcirculation, and producing vaso-occlusion.8,9 The “sickle cell crisis” is represented by this procedure. Inflammatory cytokines boost endothelial cell production of adhesion molecules in the presence of infection, further raising the risk of thrombosis.
In Africa, SCD is the third leading cause of pediatric hospital admissions and mortality10 In West African countries like Ghana and Nigeria, SCD is still widespread in northeastern Nigeria due to poverty, lack of access to healthcare facilities, and limited exposure to health education, about 20–30% of the population suffers.10–14 The disease’s severity varies greatly from person to person. An SCD patient lived on average only fourteen years in 1973; however, a person with SCD in high-income countries has a life expectancy of forty to sixty years. Advances in the detection and treatment of SCD have made this improvement possible. Sickle cell disease was first noted in the Eastern Province of the Kingdom of Saudi Arabia (KSA) in the 1960s.15 Because SCD is a prevalent disorder in KSA, several screening studies at the regional and national level studies have been conducted to discover the clinical features and distribution of SCD genes throughout the country. The KSA Screening Program estimates that the prevalence of SCA is 5/1000 for SCD and 0.38/1000 for sickle cell trait (SCT). Based on new-born screening, 2.6% of neonates had SCD whereas 21% showed SCT.16
The increased frequency of consanguinity is one of the primary reasons for the occurrence of SCT and increasing the risk for homozygous sickle cell patients.17 Premarital screening is the primary preventive intervention in KSA to avert the inheritance of haemoglobinopathies, including SCD. Since there are no existing works, this study intends to investigate.
Sickle cell disease is still widespread in north-eastern Nigeria due to poverty, lack of access to health care facilities, and limited exposure to health education. The most prominent organs affected by SCD are the liver, spleen, and kidneys, which respond by changing their dimensions and parenchyma.11,18 The gallbladder (GB) may develop stones, and its wall thickness may change, as well as the possibility of Gamma Gandy bodies inclusions in the spleen and liver.3 Diagnosis and management in sub-Saharan Africa requires the development of care delivery models focused on continuous monitoring, early crisis detection, and prompt presentation to specialized treatment facilities. Sonography can be used to scan the liver, spleen, kidneys, and GB to improve early monitoring and detection through increased awareness of complications associated with sickle cell disease.
Spleen is the first organ injured in sickle cell disease (SCD) with evidence of hyposplenism present before 12 months in the majority of children. Repeated splenic vaso-occlusion leads to fibrosis and progressive atrophy of the organ (auto splenectomy), which is generally complete by 5 years in SCD. Splenic injury is generally silent, progressive and life-threatening complication in infants.19,20 On hepatobiliary Severe hepatic crises should be suspected in every child with liver pain, increased jaundice, and Cholelithiasis was the most frequent that was responsible in some cases for severe complications.21 Kidney Complications SCD is associated with many structural and functional abnormalities of the kidney, which may progress to chronic renal failure and end-stage renal disease.22 Early signs of complication associated with SCD can determine by ultrasound such as splenomegaly, or hyposplenism. Hyposplenism indirectly increases the risk of vaso-occlusion or other circulatory complications increases the susceptibility of SCA children to infection with encapsulated bacteria, which is notably reduced by penicillin prophylaxis and immunization.23 Hepatobiliary ultrasonography performed in an emergency was sufficient for diagnosis and can guide to prevent the complication with elective cholecystectomy.24 Regarding kidney disorders associated with SCD ultrasound can determine early change and abnormal progress which include renal enlargement, a medullary or diffuse increase renal and medullary echogenicity that can guide to monitoring either case.25
Ultrasound is an easy, inexpensive, non-invasive, and widely available imaging technique. According to multiple reports, there are regional differences in the parenchymal echotexture and organ size of SCD patients.1 To our knowledge, there have been no studies in our area; hence, this research was conducted to evaluate organs in the abdomen, including the GB, spleen, liver, and kidneys.
Methods
A cross-sectional analysis was carried out in Saudi Arabia using a simple sampling method from April 2019 to July 2020. This study involved seventy-eight participants, of whom thirty-five were male (44.9%) and forty-three were female (55.1%), with an age range of 5–20 years. Ethical approval was received from the Ethical Committee of King Khalid University in Abha, Saudi Arabia, under approval number RGP2/384/44. Prior to the study, informed consent was obtained from each patient as well as that of their legal guardians or parents if they were children. In total, seventy-eight SCD patients confirmed to have homozygous hemoglobin were selected intentionally from the sickle cell group in Mahayel Asir General Hospital and Khamis Maternity and Children Hospital.
Patient Recruitment
Sickle cell disease (SCD) patients were recruited from the hematology department. According to patient access, the radiology department scheduled an abdominal scan for each patient agreeing to participate in the study, twice weekly on Tuesdays and Thursdays. Demographic information anthropometric measures, and patient records and files were examined to assess if there is any complication associated with SCA was diagnosed, history of vaccination also was taken, records of laboratory tests, such as hemoglobin electrophoresis and previous operations (splenectomy and/or cholecystectomy), and the results of the abdominal ultrasound scan were recorded using a datasheet. Vaccination history was taken because vaccination and prophylactic treatment prevent some abnormalities caused by infection and vaso-occlusive disorders such as splenic injury and asplenia and other complications, so a relationship between vaccination and sonographic abnormalities of the spleen was expected.
Equipment
A real-time sonogram, General Electric Logiq E10, was used for the research. A Convex probe with a frequency range between 3.5 and 10 megahertz (MHz) was used to assess the internal organs. The departmental medical physicist verified the functionality of the instrument before the measurements. The probe was chosen to provide sufficient resolution and penetration of the internal organs in pediatric patients. The required organ dimension measurements were done by using Caliper/distance. A scale, tested periodically for calibration and accuracy, was used to measure the height and weight of the participants after removing their shoes and while standing upright with their heads in a Frankfurt position. The BMI (kg/m2) was also computed.
Scanning Technique
Before being scanned, all patients fasted the previous day or for a minimum of six hours. No surgery had been performed on the patients at the time of the scan. The patients underwent evaluation while supine, both for comfort and to obtain the best views of the liver, GB, kidneys, and spleen. Right and left oblique orientations were used as alternative positions if the organs could not be viewed clearly in the supine position. A sagittal approach was used to measure the liver span in the mid-clavicular line by lining up the right lobe’s longitudinal center with the right kidney’s longitudinal center on the imaging plane. The liver parenchyma was examined for localized and echo-textural abnormalities. Hepatomegaly was considered when the long axis of the liver measured greater than 150 millimetres.26 The length of the spleen was also measured, and the wall thickness and content of the GB were evaluated at the level of the hilum. An auto-splenectomy was performed when the spleen could not be seen in its usual place15,27 Splenomegaly in adults was described as the long axis of the organ being >130 mm, whereas a shrunken spleen was defined as the long axis being 50 mm. The spleen’s parenchymal echo-textural alterations and localized abnormalities were also evaluated.28–30 The kidneys were examined while the patient lay supine or slightly to their left or right lateral decubitus, with the renal hilum in the sagittal and axial planes. Maximum renal length was measured only once using sonography; then, using the longitudinal image, the thickness and width of the kidney were measured in a section perpendicular to its long axis. The level of this transverse section was intended to be positioned relatively close to the kidney’s hilum after measuring the thickness and width in two perpendicular directions.31 It was proposed that the kidneys were unusually echogenic when the renal cortex was as echogenic or more than the adjacent spleen or liver16 When the cortex was difficult to visually differentiate from the medulla, renal corticomedullary differentiation was thought to be abnormal. Additionally, the presence of renal calculi, cysts, or other localized parenchymal abnormalities was examined in the kidneys. According to the grading method developed by Using the grading method developed by Shetty et al, who classified the echogenicity of the organs descending from high to low as the diaphragm, pancreas, spleen, liver, and kidneys, the evaluation of the organs’ echo-texture was conducted subjectively.32
Ethical Consideration and Procedure
The College of Medicine at King Khalid University’s Research Ethics Committee (REC) awarded ethical authorization (approval number: REC #2016-04-11). The study met with the Helsinki Declaration’s guidelines as they were updated in Edinburgh in 2000. All participants received guarantees that the study would keep their information private, their participation was entirely voluntary, and they could withdraw from participation whenever they chose. Prior to inclusion, written consent was obtained from each.
Before scanning minor patients, the RAs identified the co-patients (a parent) and explained the study’s goal, information, risks, and advantages. The co-patient was then asked to complete the surveys and sign the informed consent form, which took 10 to 20 minutes. The nurse reviewed everything and requested the co-patient complete any missing information by reviewing data from the medical records and confirming it with the co-patient.
Data Analysis
Data were analyzed using SPSS Sciences version 23.0. Armonk, NY: IBM Corp. Descriptive statistics were performed for continuous variables and presented as mean ± STD. Categorical data were presented as frequency and percentage, and then cross-tabulation using a chi-square test was performed to assess the correlation between the study variables. P ≤ 0.05 was considered to have statistical significance.
Results
In this study, the largest groups were 5–10 years old and 11–5 years old (38.5%) and (24.4%), respectively. More than half were female (55.1%), and more than half were 121 cm or taller (53.8%), and in the weight group of more than “30Kg” (39.7%). Most had not received a blood transfusion (46.2%), and (20.5%) had received blood transfusions four times. Only (11.5%) received extra-vaccines regularly, and (38.5%) received governmental support (see Table 1).
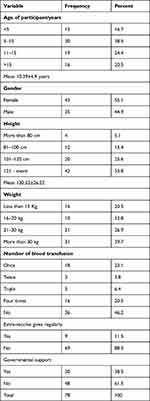 |
Table 1 Demographic Characteristics and Clinical Data of SCA Paediatrics |
Concerning abdominal ultrasound findings, in (21.8%) of pediatrics presented with SCD, the liver was enlarged, and in all cases, it was homogeneous in echo-texture (100%). The liver was hyperechoic in only two cases (2.6%), hypo-echoic in one case (1.3%), and in four cases (5.1%) there was a focal lesion in the liver. When scanning the biliary system, there were biliary stones in (6.4%) of cases, while in one case it was post-cholecystectomy. The spleen was enlarged in fifteen cases (19.3%), shrunken in 1 case (1.3%), and the presence of focal splenic lesions was found in 7 cases (9.0%). In all cases, the renal system was normal (100%). The other complications associated with SCA in the included sample where osteomyelitis sepsis were 6 cases (7.7%), stroke in 1 case (1.3%), acute chest syndrome in 3 cases (3.8%), and meningitis 1 case (1.3%) (see Table 2).
 |
Table 2 Frequency Distribution of Abdominal Ultrasound Findings and Complication of SCA |
The study found a significant correlation between spleen findings and type of crises, p-value <0.01, as the markedly enlarged spleen was found in hemolytic and vaso-occlusive crises and mildly enlarged in hemolytic crises patients, also significant correlation between the type of treatment used and spleen size, and no significant correlation between liver size and type of crises (see Figures 1–3). A significant relationship was noted between the number of blood transfusions and the spleen size in SCA patients, p-value <0.05 (see Table 3).
 |
Table 3 Correlation Between the Spleen Size and Number of Blood Transfusions |
 |
Figure 1 Bar chart shows the relation between the type of crises at presentation for ultrasound and splenic size. |
 |
Figure 2 Bar chart shows the relation between the medication used and splenic size. |
 |
Figure 3 Bar chart shows the correlation between the liver size and type of crises. |
Discussion
Sickle cell disease “SCD” is a hereditary condition in which the usual globin chain is altered, causing the RBC to produce aberrant hemoglobin chains. This sickling of the cell causes arterial blockage and ischemia in several organs.1,33 These result in changes in the organs, some of which may have long-term effects on the spleen, kidneys, liver, and GB, and others, based on race and ethnicity.34 Crucial factor in the early diagnosis of these alterations for ongoing therapy and follow-up is provided by ultrasound, which is an uncomplicated, relatively inexpensive, and widely available imaging modality. Table 1In this study, the most affected age groups with SCA were 11-15 years and 5-10 years. The female sampling presented to the ultrasound department for ultrasound scanning was greater than the male sampling, the result is inconsistent to the previous study whom found the male sampling were more than females owing to the fact that episodes of pain in crises occur more frequently in males. Extravacine is taken regularly in this study in only 11.5 %, it was nessecary to take the vaccine regulary to avoid the complications, one study found that the incidence of pnumococcal diseases decreased after introduction of vaccine in SCD patients.35,36
The most common finding in the livers of SCD patients in this investigation was hepatomegaly. Earlier researchers reported similar results.11,20,36,37 Several complications contribute to the development of liver disease related to both SCD and its treatment. Blood transfusions often place the patient at risk of viral infection and iron overload in combination with chronic hemodialysis, and those patients can develop hepatic malignancies, infiltrative and granulomatous illnesses11,28 In two cases (2.6%), patients had an increase in the echogenicity of the liver, similar to Balci et al, who found that in (5.9%) of their cases, the liver was hyperechoic. Variations might result from the patient’s economic state, way of life, or diet, which can potentially impact the liver’s echogenicity. Several liver histological characteristics in SCD patients, such as periportal fibrosis and sinusoid distension with sickle cell, may be responsible for the brightening of the liver echo-texture.28,31,38 In SCD patients, abdominal sonography revealed a variety of notable abnormalities in the liver, GB, and spleen, as well as in the size, echo-texture, and intraluminal accumulation. A similar study showed little alteration in the liver in patients with SCD.34 This study found GBs in five (6.4%) of the SCD patients. Previous studies found a progressive age-related increase in the prevalence of GBs; however, a higher prevalence was reported by abdominal sonography in SCD patients, which included remarkable changes in the size, echo-texture, and liver, GB, spleen, and intraluminal deposit.1,38 These differences may be due to the combination of different age groups, races, symptomatic patients, diet, access to and availability of diagnostic and therapeutic drugs, and other health services, which may be insufficient in impoverished countries such as Nigeria.
As regards the spleen, this study found fifty cases (64.1%) “SCD” patients with a normal spleen. It should be stated that normal sonographic findings of the spleen in sickle cell patients do not rule out the presence of other complications, such as intravascular sickling, which may not be visible at the time of the scan, and the quality of the sonogram operator. Different classifications of splenomegaly in this study were found in fifteen cases (19.3%), which is consistent with a Nigerian study conducted by Ma’aji et al11 who found splenomegaly in fifteen cases (21.1%). Several studies have found splenomegaly is an extremely common abdominal symptom in SCD patients.18,39 Splenomegaly occurs in the first year of life and should be observed in children if the spleen is longer than the adjacent normal kidney by more than 1.25 times.40 This study found one reduced spleen (1.3%), which is consistent with prior research that found shrunken spleen,29 and could last into the third and fourth decades of life. Sequestration syndrome, a frequent splenic complication, is considered the source of splenomegaly in SCD patients. A child or adult with SCA may undergo larger-than-expected blood pooling within the spleen, producing intravascular volume depletion, then quickly progressing to cardiovascular collapse and mortality (Murakami & Shimizu). Infectious or granulomatous disease, cancer, congestive disorders, and other hematologic diseases are additional illnesses that can result in splenomegaly41 Auto-splenectomy in this study was found in one (1.3%) of the SCD patients, and another study conducted in Nigeria found it in three (4.2%) cases.11 At the time of the scan, none of our consents had received any surgery. Auto-splenectomy has been identified in previous research8,16,27,29 as a prevalent occurrence in SCD patients. These results may be caused by the spleen’s tortuous vasculature, which stimulates RBC sickling and leads to a high frequency of splenic infarctions. With time, recurrent infarctions may result to an auto splenectomy.
The study found two (2.6%) SCD patients with decreased echo-texture of the spleen, two (2.6%) with multiple punctuates of the spleen, and seven (9.0%) had focal lesions of the spleen. Similar findings were reported in previous studies.31 This could be due to the similar methodology used. Previous studies have reported shrunken spleen28 which could be attributed to sickle cell nephropathy, a chronic condition that may progress to end-stage renal disease.
The other complications which were diagnosed based on clinical and other radiological examination such as MRI and taken from patients record and associated with SCD in the included sample were osteomyelitis sepsis in six cases (7.7%), stroke in two cases (2.6%), acute chest syndrome in three cases (3.8%), and meningitis in one case (1.3%). Previous studies have reported that among those with SCD, acute and chronic osteomyelitis is one of the most frequent infections, and it ranges from 12% to 17.8%.29
The study found that all cases (100%) had a normal renal system. In contrast, Balci et al1 found that a substantial proportion of patients had renal abnormalities, including renal enlargement in (30.4%) and increased renal echogenicity in 15.7% (133). On the other hand, our results are consistent with the findings of Attala et al,40 who also reported a low prevalence of renal abnormalities in patients with SCD. However, it is important to note that the absence of sonographic abnormalities does not necessarily rule out the presence of other complications, such as intravascular sickling, which may not be visible on ultrasound. Additionally, the accuracy of ultrasound findings can depend on the operator’s skills.42
The study found a significant correlation between the type of crisis and spleen size p-value <0.01, and there was no significant correlation between liver size and type of crisis, P-value >0.05, and significant correlation between the type of treatment used and size of the spleen.
Limitations of the Study
The results of the abdominal sonography were not specific because no biopsy or other imaging technique was utilized to corroborate the results. Since this was a single center study, the sample size was insufficient because most of the individuals we contacted failed to attend the appointments for their scheduled scans.
Conclusion
The study concluded that SCA can result in several abdominal findings, the most frequently observed being an enlarged liver and spleen. In addition, the presence of focal lesions in both organs is also a common finding, and also GBs (gall stones).
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through large group Research Project under grant Number RGP2/384/44.
Funding
This research was funded by Deanship of Scientific Research at King Khalid University (Grant No. RGP2/384/44).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Balci A, Karazincir S, Sangün O, et al. Prevalence of abdominal ultrasonographic abnormalities in patients with sickle cell disease. Diagn Interv Radiol. 2008;14(3):133.
2. Sundd P, Gladwin MT, Novelli EM. Pathophysiology of sickle cell disease. Annu Rev Pathol. 2019;14(1):263–292. doi:10.1146/annurev-pathmechdis-012418-012838
3. Kato GJ, Beck LA, Bieber T, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4(1):1–22. doi:10.1038/s41572-018-0001-z
4. Eaton WA, Hofrichter J. Sickle cell hemoglobin polymerization. Adv Protein Chem. 1990;40:63–279. doi:10.1016/s0065-3233(08)60287-9
5. Zhang D, Xu C, Manwani D, et al. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood J Am Soc Hematol. 2016;127(7):801–809.
6. Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life. 2012;64(1):72–80. doi:10.1002/iub.584
7. Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med. 2004;36(6):707–717. doi:10.1016/j.freeradbiomed.2003.11.032
8. Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. doi:10.1016/S0140-6736(10)61029-X
9. Okpala I. The intriguing contribution of white blood cells to sickle cell disease–a red cell disorder. Blood Rev. 2004;18(1):65–73. doi:10.1016/S0268-960X(03)00037-7
10. Makani J, Cox SE, Soka D, et al. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS One. 2011;6(2):e14699. doi:10.1371/journal.pone.0014699
11. Ma’aji SM, Jiya N, Saidu S, et al. Transabdominal ultrasonographic findings in children with sickle cell anemia in Sokoto, North-Western Nigeria. Niger J Clin Sci. 2012;9(1):14. doi:10.4103/0331-8540.102106
12. Uzoegwu PN, Onwurah A. Prevalence of haemoglobinopathy and malaria diseases in the population of old Aguata Division, Anambra State, Nigeria. Biokemistri. 2003;15(2):57–66.
13. Fleming A, Storey J, Molineaux L, et al. Abnormal haemoglobins in the Sudan savanna of Nigeria: i. Prevalence of haemoglobins and relationships between sickle cell trait, malaria and survival. Ann Trop Med Parasitol. 1979;73(2):161–172. doi:10.1080/00034983.1979.11687243
14. World Health Organization. Sickle-Cell Anaemia: Report by the Secretariat, in Sickle-Cell Anaemia: Report by the Secretariat. World Health Organization; 2005.
15. Alsaeed ES, Farhat GN, Assiri AM, et al. Distribution of hemoglobinopathy disorders in Saudi Arabia based on data from the premarital screening and genetic counseling program, 2011–2015. J Epidemiol Glob Health. 2018;7(S1):S41–S47. doi:10.1016/j.jegh.2017.12.001
16. Al-Qurashi MM, El-Mouzan MI, Al-Herbish AS, et al. The prevalence of sickle cell disease in Saudi children and adolescents. A community-based survey. Saudi Med J. 2008;29(10):1480–1483.
17. Jastaniah W. Epidemiology of sickle cell disease in Saudi Arabia. Ann Saudi Med. 2011;31(3):289–293. doi:10.4103/0256-4947.81540
18. Eze CU, Offordile GC, Agwuna KK, et al. Sonographic evaluation of the spleen among sickle cell disease patients in a teaching hospital in Nigeria. Afr Health Sci. 2015;15(3):949–958. doi:10.4314/ahs.v15i3.32
19. Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick (led) spleen. Br J Haematol. 2014;166(2):165–176. doi:10.1111/bjh.12950
20. Gale HI, Bobbitt CA, Setty BN, et al. Expected sonographic appearance of the spleen in children and young adults with sickle cell disease: an update. J Ultrasound Med. 2016;35(8):1735–1745. doi:10.7863/ultra.15.09023
21. Allali S, de Montalembert M, Brousse V, et al. Hepatobiliary complications in children with sickle cell disease: a retrospective review of medical records from 616 patients. J Clin Med. 2019;8(9):1481. doi:10.3390/jcm8091481
22. Walker T, Serjeant G. Increased renal reflectivity in sickle cell disease: prevalence and characteristics. Clin Radiol. 1995;50(8):566–569. doi:10.1016/S0009-9260(05)83194-0
23. Suell MN, Horton TM, Dishop MK, et al. Outcomes for children with gallbladder abnormalities and sickle cell disease. J Pediatr. 2004;145(5):617–621. doi:10.1016/j.jpeds.2004.06.071
24. Kell M, Aherne NJ, Coffey C, et al. Emergency surgeon-performed hepatobiliary ultrasonography. J Br Surg. 2002;89(11):1402–1404. doi:10.1046/j.1365-2168.2002.02297.x
25. Scheinman JI. Sickle cell disease and the kidney. Nat Clin Pract Nephrol. 2009;5(2):78–88. doi:10.1038/ncpneph1008
26. Attalla BI. Sonographic findings in Sudanese children with sickle cell anemia. J Diagn Med Sonogr. 2010;26(6):276–280. doi:10.1177/8756479310386788
27. Babadoko A, Ibinaye PO, Hassan A, et al. Autosplenectomy of sickle cell disease in Zaria, Nigeria: an ultrasonographic assessment. Oman Med J. 2012;27(2):121. doi:10.5001/omj.2012.25
28. Hazzazi AA, Ageeli M, Alfaqih A, et al. Epidemiology and characteristics of sickle cell patients admitted to hospitals in Jazan region, Saudi Arabia. J Appl Hematol. 2020;11(1):10. doi:10.4103/joah.joah_67_19
29. Singer K, Chernoff AI, Singer L. Studies on abnormal hemoglobins: i. Their demonstration in sickle cell anemia and other hematologic disorders by means of alkali denaturation. Blood. 1951;6(5):413–428. doi:10.1182/blood.V6.5.413.413
30. Riccabona M. Spleen and Pancreas. In: Pediatric Ultrasound: Requisites and Applications. Springer; 2014:257–288.
31. Luntsi G, Eze C, Ahmadu M, et al. Sonographic evaluation of some abdominal organs in sickle cell disease patients in a tertiary health institution in Northeastern Nigeria. J Med Ultrasound. 2018;26(1):31. doi:10.4103/JMU.JMU_5_17
32. Lonergan GJ, Cline DB, Abbondanzo SL. Sickle cell anemia. Radiographics. 2001;21(4):971–994. doi:10.1148/radiographics.21.4.g01jl23971
33. Malowany JI, Butany J. Pathology of sickle cell disease. In: Seminars in Diagnostic Pathology. Elsevier; 2012.
34. Banerjee S, Owen C, Chopra S. Sickle cell hepatopathy. Hepatology. 2001;33(5):1021–1028. doi:10.1053/jhep.2001.24114
35. Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44(11):1428–1433. doi:10.1086/516781
36. Ceglie G, Di Mauro M, Tarissi De Jacobis I, et al. Gender-related differences in sickle cell disease in a pediatric cohort: a single-center retrospective study. Front Mol Biosci. 2019;6:140. doi:10.3389/fmolb.2019.00140
37. Schubert TT. Hepatobiliary system in sickle cell disease. Gastroenterology. 1986;90(6):2013–2021. doi:10.1016/0016-5085(86)90276-3
38. Agholor C, Akhigbe A, Atalabi O. The prevalence of cholelithiasis in Nigerians with sickle cell disease as diagnosed by ultrasound. Br J Med Med Res. 2014;4(15):2866. doi:10.9734/BJMMR/2014/8645
39. Olaniyi J, Abjah U. Frequency of hepatomegaly and splenomegaly in Nigerian patients with sickle cell disease. West Afr J Med. 2007;26(4):274–277. doi:10.4314/wajm.v26i4.28326
40. Attalla BI. Abdominal sonographic findings in children with sickle cell anemia. J Diagn Med Sonogr. 2010;26(6):281–285. doi:10.1177/8756479310386786
41. Al-Salem AH, Al-Aithan S, Bhamidipati P, et al. Sonographic assessment of spleen size in Saudi patients with sickle cell disease. Ann Saudi Med. 1998;18(3):217–220. doi:10.5144/0256-4947.1998.217
42. Rusheke H. Abdominal Ultrasonographic Abnormalities in Patients with Sickle Cell Anaemia at Muhimbili National Hospital. Muhimbili University of Health and Allied Science; 2010.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
