Back to Journals » Journal of Pain Research » Volume 15
Ultrasound Guided Continuous Erector Spinae Plane Block versus Patient Controlled Analgesia in Open Nephrectomy for Renal Malignancies: A Randomized Controlled Study
Authors Abdelgalil AS , Ahmed AM , Gamal RM , Elshal MM, Bakeer AH, Shaker EH
Received 23 June 2022
Accepted for publication 15 September 2022
Published 30 September 2022 Volume 2022:15 Pages 3093—3102
DOI https://doi.org/10.2147/JPR.S379721
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Ahmed Salah Abdelgalil, Ahmed Mansour Ahmed, Reham M Gamal, Mamdouh Mahmoud Elshal, Ahmed Hussein Bakeer, Ehab Hanafy Shaker
Anesthesia, Intensive Care and Pain Management, National Cancer Institute, Cairo University, Cairo, Egypt
Correspondence: Ahmed Salah Abdelgalil, Anesthesia, Intensive Care and Pain Management, National Cancer Institute, Cairo University, Cairo, 11796, Egypt, Email [email protected]
Background: It is critical to manage acute postoperative pain for patient satisfaction and better outcome. Erector spinae plane block (ESPB) can produce sensory blocking on both visceral and somatic levels. This study aimed to evaluate the ESPB efficacy in controlling acute postoperative pain in open nephrectomy for renal malignancies.
Methods: This prospective randomized, controlled, open-label trial included 60 cases scheduled for open nephrectomy for renal malignancy under general anesthesia. Cases were assigned randomly into two equal groups. Group E administered continuous preoperative unilateral ESPB (20mL bupivacaine 0.25% bolus then 6 mL/h 0.1% for 48 hours). Group C administered intravenous (IV) patient-controlled analgesia (PCA) morphine (0.01 mg/kg/h). Postoperative analgesia was managed by morphine (3 mg IV in group E or 0.01 mg/kg bolus with a 15-min lockout in group C) to keep the visual analog scale (VAS) scores < 4.
Results: Intraoperative fentanyl consumption and total morphine consumption in 1st 48 hours postoperatively were significantly lower in group E than group C (P= 0.001 and < 0.001, respectively). The time to first analgesic request was significantly longer in group E than group C (P < 0.001). VAS scores at movement and rest were significantly lower in group E than group C (P < 0.001).
Conclusion: In renal malignancies, ESPB provided better analgesia with prolonged time and lower pain scores at both rest and movement compared to IV PCA following open nephrectomy.
Keywords: erector spinae plane block, PCA, analgesia, nephrectomy, renal malignancy
Introduction
It is critical to manage acute postoperative pain properly for both patient satisfaction and better outcome. Inadequately managed postoperative pain can cause acute effects and may increase risk for chronic post-surgical pain.1
Although intravenous (IV) opioids are the most widely used for managing acute postoperative pain, they are associated with various adverse events, such as respiratory depression, constipation and postoperative nausea and vomiting.2 Hence, alternative modalities are essential to minimize opioid usage such as regional blocks, non-steroidal anti-inflammatory drugs, and multimodal analgesia.3
Erector spinae plane block (ESPB) is a simple approach which relies on easily recognized sonographic landmarks.4 ESPB is carried out by local anesthetic (LA) injection in the fascial plane deeper than the erector spinae muscle (ESM) at the transverse process of the vertebra. Consequently, the craniocaudal fascial plane is dispersed with LA.5 In addition, it anteriorly diffuses into the epidural and paravertebral areas and lateral into the intercostal area at multiple levels. ESPB can produce both the visceral and somatic sensory block.6–8
Just some research had discussed ESPB use in nephrectomy,9–12 but there is lack in studies describing the effect of continuous ESPB in patients undergoing open nephrectomy. So, we aimed to assess the efficacy of continuous ESPB in reducing acute postoperative pain in renal cancer patients undergoing open nephrectomy.
Materials and Methods
This randomized controlled, open-label trial was conducted on 60 cases between the ages of 18 and 65 years, with body mass index (BMI) between 20 and 40 kg/m2, American Society of Anesthesiologist (ASA) physical status II scheduled for open nephrectomy for malignant renal tumors under general anesthesia (GA). The research received approval by Cairo University’s ethical committee and registered on clinicaltrials.gov (ID: NCT04537598). This trial was conducted in accordance with the Declaration of Helsinki. All participants signed an informed written consent form. The research was performed at the National Cancer Institute, Cairo University, Egypt from March 2020 to February 2021.
Patients with hypersensitivity to local anesthetics, history of psychiatric problems, coagulopathy (International normalized ratio [INR] > 1.4, or platelet count < 80,000/µL) or localized infection at the block site were excluded.
Random numbers were generated and sealed in an opaque envelope through the use of a computer-generated randomization code. Cases were assigned randomly into two equal groups in a parallel way. Group E administered a preoperative unilateral continuous ESPB (20mL bupivacaine 0.25% bolus then 6 mL/h 0.1% for 48 hours). Group C (Control group) administered IV patient-controlled analgesia (PCA) morphine (0.01 mg/kg/h).
Cases were evaluated preoperatively by history taking and routine laboratory tests. Cases were educated on how to use the visual analogue (VAS) scale to estimate pain, in which 0 = ”no pain” and 10 = “worst possible pain”. Premedication with 0.02 mg/kg midazolam was injected intravenously 30 minutes.
Surgical Technique
In all patients, the surgical procedures were performed by the same experienced surgeon, through a 10–15 cm flank incision, between the ninth and the tenth ribs. The intercostal muscle, external and internal oblique muscles, and transversum muscle were dissected and the peritoneum was opened ventrally, exposing Gerota’s fascia and, subsequently, the kidney. The ureter and lower pole of the kidney were isolated and subsequently the renal artery and vein were ligated and divided. Finally, the dissection of the upper pole (together with adrenal gland in the case of upper pole tumours) was completed and the ureter was transected. A drain was left in the retroperitoneal space and surgical procedure was completed by closing muscle layers, s.c. space, and skin, respectively, with running sutures.
Espb
The block was carried out by a skilled anesthesiologist. A Sonosite Edge (Sonosite Inc., USA) machine and an HFL38X linear transducer 13–16 MHz were used to conduct the block. The participant was positioned in the position of sitting, and the probe was protected with a cover sheath and plenty of lubricant gel. The probe was positioned longitudinally, three centimeters lateral to the T7 spinous process. The trapezius and erector spinae and major rhomboid muscles should be superficial to the hyperechoic transverse process shadow. The skin was anesthetized by using 3mL of 1% lidocaine. A 20-gauge block needle (Perifix® complete set; B. Braun Melsungen AG, Melsungen, Germany) was put in the plane from cephalad to caudad, with the tip deposited in the deep (anterior) portion of the erector spinae muscle fascial plane. Then, 20 mL of bupivacaine 0.25% was administrated gradually. Fluid spread lifted the erector spinae muscle away from the transverse process’s bone shadow, validating the needle’s placement.10 The catheter was inserted and bupivacaine 0.1% was administered at 6 mL/h rate for 48 hours. After that, the catheter was removed after 12h from administration of prophylactic enoxaparin. The block’s effectiveness was validated by the absence of pinprick sensation at the block’s dermatomal location after 30 minutes of injection. Cases with failed block were excluded.
Anesthetic Management
Throughout the surgical process, patients were monitored constantly using the Datex-Ohmeda S5 anesthetic monitor, model USE1913A by electrocardiography, peripheral oxygen saturation, temperature probe end-tidal carbon dioxide, and noninvasive blood pressure.
Both groups had general anesthetic induction of 2 mg/kg of IV propofol, fentanyl 2 μg/kg, and 0.5 mg/kg of atracurium. Sevoflurane 2–2.5% MAC in air enriched with oxygen (FiO2 of 0.5) was used to maintain anesthesia. Atracurium top-up doses (0.1 mg/kg IV) were given when necessary. Acetaminophen (1gm IV) was given to each patient. Additional bolus fentanyl doses (0.5 µg/kg) were given when the mean arterial blood pressure (MAP) or heart rate (HR) exceeded 20% of values at baseline. Intraoperative fentanyl consumption was recoded.
HR and MAP were recorded at baseline before the block, just before the surgical incision, 30, 60, 90 and 120 min intraoperative and at the completion of surgery. After full recovery, extubation was done. Patients were then sent to the post-anesthesia care unit (PACU). VAS (at rest and at movement), HR and MAP were recorded upon entrance to PACU and at 2, 4, 6, 12, 24, 36, and 48 h following the surgery. In both groups, IV acetaminophen 1 gm every 8 hours and 30mg ketorolac IV each twelve hours were administered.
Postoperative analgesia was provided by morphine (3 mg IV in group E or 0.01 mg/kg bolus with a 15-min lockout in group C) to keep the VAS at rest less than 4. The initial request for analgesia was recorded.
Side effects [postoperative nausea and vomiting (PONV), hypotension, bradycardia, oversedation, and hematoma] were documented. Those patients with moderate or severe PONV received ondansetron 0.1 mg/kg IV.
The primary outcome was VAS scores in the 1st 48 hours, and the secondary outcomes were fentanyl and morphine consumption, and postoperative complications.
Sample Size Justification
The sample size was calculated using G*Power 3.1.9.2 (University of Kiel, Germany). We conducted a pilot trial with 10 patients per group and the mean VAS at rest in the first 48 hours was 2.14 ± 1.3 in group E and 3.34 ± 1.68 in group C. With 80% power and a 5% confidence interval, 26 patients per group were enough to reject the null hypothesis. In order to avoid dropouts, four patients were added to each group.
Statistical Analysis
SPSS v27 (IBM, Chicago, IL, USA) was used to conduct the statistical analysis. The normality of the data distribution was assessed by the Shapiro–Wilks test and histograms. Quantitative parametric data were presented as mean and standard deviation (SD) and were compared using unpaired t-test. Quantitative non-parametric data was reported as median and interquartile range (IQR) and were compared by Mann Whitney-test. For qualitative variables, the frequency and percentage were presented and the Chi-square test or Fisher’s exact test was used where suitable for comparison. Statistical significance was defined as a two-tailed P value ≤0.05.
Results
In this trials, 86 cases were evaluated for eligibility; 19 did not match the requirements, and seven patients refused to join. The remaining 60 cases were recruited randomly into two groups of equal size. All cases were then followed and statistically analyzed (Figure 1).
 |
Figure 1 Consort flow diagram of patients that were enrolled. |
The characteristics of the patients, duration of operation, and side of operation were insignificantly different between both groups (Table 1).
 |
Table 1 Patient Characteristics, Duration of Surgery and Site of Operation of the Studied Groups |
Intraoperative HR and MAP were significantly decreased in group E compared to group C at 30, 60, 90 and 120 min and at the end of surgery (P <0.001) and were insignificantly different at baseline and before skin incision. Postoperative HR and MAP were significantly decreased in group E compared to group C at 2, 6, and 12h (P <0.05) (Figures 2 and 3).
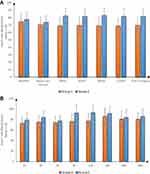 |
Figure 2 Heart rate (beats/min) in both groups (A) intraoperative, (B) postoperative. |
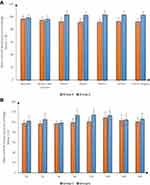 |
Figure 3 Mean arterial blood pressure (mmHg) in both groups (A) intraoperative, (B) postoperative. |
Intraoperative fentanyl consumption was decreased significantly in group E compared to group C (P <0.001). In group E, the time to first analgesic request was significantly longer than in the control group. Total morphine consumption in the 1st 48 hours was significantly lower in group E than in controls (P <0.001). (Table 2).
 |
Table 2 Intraoperative Fentanyl Consumption, Time to First Request of Analgesia and Postoperative Morphine Consumption Between Both Groups |
VAS at rest had significantly decreased in group E than controls at PACU, 2, 6, and 12h (P <0.001) and was insignificantly between both groups at 4, 24, 36, and 48h. (Table 3) VAS at movement had significantly decreased in group E when compared to controls at all times of readings (P <0.05). (Table 4).
 |
Table 3 VAS at Rest Between Both Groups |
 |
Table 4 VAS at Movement Between Both Groups |
There is no significance between the two groups according to PONV, hypotension, and bradycardia. In ESPB group, the local anesthetic toxicity and block placement complications did not occur in any patient. (Table 5).
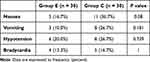 |
Table 5 Adverse Effects Between Both Groups |
Discussion
Acute pain management following open nephrectomy is difficult due to the wide subcostal flank incision required for a wide operating field and extensive cutting of the muscles.13,14
ESPB was found as a promising therapy for treating thoracic neuropathic pain for the first time.15 Subsequent studies established that ESPB conducted from the thoracic vertebral levels was a successful analgesic technique for bariatric surgery, and major abdominal surgery.15–17
Local anaesthetic diffusion into the paravertebral space, with sympathetic fiber blockage of the ventral and rami dorsal, resulting in visceral and somatic pain relief, is thought to be the mechanism of action for ESPB.18
The findings of this investigation demonstrated that ESPB provided better analgesia than PCA as shown by lower intraoperative fentanyl consumption, postoperative morphine consumption at 1st 48 hours and VAS score at movement and rest and delayed first rescue analgesia with no significance difference regarding side effects.
In line with our results, Abd Ellatif et al12 found that first-time rescue of analgesic request was significantly longer in the ESPB group than controls (without ESPB) after open nephrectomy (p < 0.001). VAS score in ESPB group was significantly lower at rest and during movement. Additionally, there were no complications signs regarding local anesthetic with ESPB or the block placement.
Our results came in line with Siam et al19 who observed that patients underwent ESPB consumed significantly lower intraoperative fentanyl and had significantly better hemodynamic stability than those who received conventional GA with multimodal analgesia during lumbar spine surgery.
Additionally, Ghanem et al20 revealed that The ESPB group experienced less pain during rest and activity, and breakthrough pain was more readily managed than in the quadratus lumborum block.(QLB) group in patients undergoing open colorectal cancer surgery. After the first 24 hours postoperative, cumulative morphine usage was 7.24 ± 0.987 and 3.62 ± 0.493 in the ESPB group, but 14.26 ± 2.206 and 7.32 ± 1.007 in QLB group, respectively. The ESPB group had a significantly lower overall PONV intensity score than the QLB group, but this difference was not statistically significant.
Our study was in line with Wasfy et al,21 who found that ESPB had lower intraoperative fentanyl (403.75 ± 44.63) than the multimodal intravenous analgesia group (685 ± 99.47) in patients undergoing coronary bypass surgery (P < 0.001). Also, they reported a lower VAS score, and shorter ICU stay with no clinically reported significant side effects in ESPB group than the other group.
Also, Zhang et al22 found that intraoperative and postoperative opioid consumption were significantly lower in cases who received ESPB than those who underwent GA only with lower HR and MAP in cases undertaking open posterior lumbar surgery.
A case series23 showed that continuous ESPB (20 mL Levo-bupivacaine 0.375% bolus accompanied with 10 mL/h 0.25% bupivacaine) following acute abdominal surgeries (such as open nephrectomy), produced good somatic and visceral analgesia with a significant reduction in opioid consumption and pain score over 48 hours postoperatively.
Similarly, Ozdemir et al24 revealed that pain score at rest and movement was significantly lower in ESPB group at PACU and at 2, 4, 6, and 12 h postoperatively compared to subcostal transversus abdominis plane block in patients underwent laparoscopic cholecystectomy. The ESPB group had lower intraoperative and postoperative fentanyl demand, longer time to first analgesia request, lower PACU rescue analgesic requirement with no complications.
Further, Chin et al16 documented that opioid intake and pain scores were lower in ESPB group following laparoscopic ventral hernia repair and other abdominal surgeries.
Also, Wahdan et al25 revealed that the pain scores in the 1st 12 h were significantly decreased in ESPB group (with 20 mL levobupivacaine 0.25%) than in the controls (20 mL normal saline) after lumbar spine surgeries.
Despite ESPB is widely used in many regions to treat perioperative pain, its benefits are still somewhat controversial. Several meta-analyses have shown that ESPB can provide sufficient analgesic effects and reduce postoperative opioid consumption; however, the results are not convincing enough due to the small number of cases included and significant heterogeneity among studies.26,27 Besides, the mechanism of ESPB is still indeterminate. In the cadaveric study, no spreading of the dye into the paravertebral space was observed to involve the origin of the ventral and dorsal branches of the thoracic vertebral nerve,28 indicating the extent of blockage was not as wide as that observed in the initial clinical finding.8 Besides, ESPB was performed in six male volunteers, and the authors found that cutaneous sensory loss varied greatly between individuals.29
Also, Chin et al,16 observed radiologically cadaveric distribution of ESPB local anesthetic extending from the injection site to 3 or 4 levels cranially and caudally therefore, we opted to apply a single-level injection at T7 level to block both supra-umbilical and infra-umbilical dermatomes that are inconsistent with the dermatomes affected by open nephrectomy rendering it as effective alternative analgesic technique in such surgeries. Moreover, it could be performed at different levels for different purposes for other clinical situations.
There are limitations to our study. It was a single-center study. The study also lacked blinding due to ethical issues as performing a block that has no expected benefit other than the placebo effect violates the ethical and regulatory principles. We did not assess the effect on chronic pain after 3–6 months postoperatively.
Conclusions
Ultrasound-guided continuous ESPB holds promise as a simple and safe technique for postoperative pain management following open nephrectomy in renal malignancies surgeries. ESPB provided better analgesia for a longer duration, with lower pain score and intraoperative and postoperative opioid consumption than IV PCA.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–2298. doi:10.2147/jpr.S144066
2. Hsu JR, Mir H, Wally MK, Seymour RB, Force TAMPT. Clinical practice guidelines for pain management in acute musculoskeletal injury. J Orthop Trauma. 2019;33(5):e158–e182. doi:10.1097/bot.0000000000001430
3. Bohringer C, Astorga C, Liu H. The benefits of opioid free anesthesia and the precautions necessary when employing it. Transl Perioper Pain Med. 2020;7(1):152–157.
4. Kwon WJ, Bang SU, Sun WY. Erector spinae plane block for effective analgesia after total mastectomy with sentinel or axillary lymph node dissection: a report of three cases. J Korean Med Sci. 2018;33(45):e291. doi:10.3346/jkms.2018.33.e291
5. De Cassai A, Tonetti T. Local anesthetic spread during erector spinae plane block. J Clin Anesth. 2018;48:60–61. doi:10.1016/j.jclinane.2018.05.003
6. Adhikary SD, Bernard S, Lopez H, Chin KJ. Erector spinae plane block versus retrolaminar block: a magnetic resonance imaging and anatomical study. Reg Anesth Pain Med. 2018;43(7):756–762. doi:10.1097/aap.0000000000000798
7. Ueshima H, Hiroshi O. Spread of local anesthetic solution in the erector spinae plane block. J Clin Anesth. 2018;45:23. doi:10.1016/j.jclinane.2017.12.007
8. Vidal E, Giménez H, Forero M, Fajardo M. A cadaver study to determine its mechanism of action. Bloqueo del Plano del músculo erector espinal: estudio anatómico-cadavérico para determinar su mecanismo de acción. Rev Esp Anestesiol Reanim. 2018;65(9):514–519. doi:10.1016/j.redar.2018.07.004
9. Kim S, Bang S, Kwon W. Intermittent erector spinae plane block as a part of multimodal analgesia after open nephrectomy. Chin Med J. 2019;132(12):1507–1508. doi:10.1097/cm9.0000000000000269
10. Aksu C, Gürkan Y. Ultrasound guided erector spinae block for postoperative analgesia in pediatric nephrectomy surgeries. J Clin Anesth. 2018;45:35–36. doi:10.1016/j.jclinane.2017.12.021
11. Kim E, Kwon W, Oh S, Bang S. The erector spinae plane block for postoperative analgesia after percutaneous nephrolithotomy. Chin Med J. 2018;131(15):1877–1878. doi:10.4103/0366-6999.237408
12. Abd Ellatif SE, Abdelnaby SM. Ultrasound guided erector spinae plane block versus quadratus lumborum block for postoperative analgesia in patient undergoing open nephrectomy: a randomized controlled study. Egypt J Anaesth. 2021;37(1):123–134. doi:10.1080/11101849.2021.1894661
13. Marija T, Aleksandar D. Erector spinae plane block in various abdominal surgeries: a case series. Saudi J Anaesth. 2020;14(4):528–530. doi:10.4103/sja.SJA_31_20
14. Chapman E, Pichel A. Anaesthesia for nephrectomy. BJA Educ. 2015;16. doi:10.1093/bjaceaccp/mkv022
15. Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41(5):621–627. doi:10.1097/aap.0000000000000451
16. Chin KJ, Adhikary S, Sarwani N, Forero M. The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair. Anaesthesia. 2017;72(4):452–460. doi:10.1111/anae.13814
17. Bonvicini D, Tagliapietra L, Giacomazzi A, Pizzirani E. Bilateral ultrasound-guided erector spinae plane blocks in breast cancer and reconstruction surgery. J Clin Anesth. 2018;44:3–4. doi:10.1016/j.jclinane.2017.10.006
18. Bang S. Erector spinae plane block: an innovation or a delusion? Korean J Anesthesiol. 2019;72(1):1–3. doi:10.4097/kja.d.18.00359
19. Siam EM, Abo Aliaa DM, Elmedany S, Abdelaa ME. Erector spinae plane block combined with general anaesthesia versus conventional general anaesthesia in lumbar spine surgery. Egypt J Anaesth. 2020;36(1):201–226. doi:10.1080/11101849.2020.1821501
20. Ghanem MA, Attieh AA, Mohasseb AM, Badr ME. A randomized comparative study of analgesic effect of erector spinae plane block versus quadratus lumborum block for open colorectal cancer surgeries. Egypt J Anaesth. 2021;37(1):483–490. doi:10.1080/11101849.2021.1984735
21. Wasfy SF, Kamhawy GA, Omar AH, Abd El Aziz HF. Bilateral continuous erector spinae block versus multimodal intravenous analgesia in coronary bypass surgery. A randomized trial. Egypt J Anaesth. 2021;37(1):152–158. doi:10.1080/11101849.2021.1904548
22. Zhang TJ, Zhang JJ, Qu ZY, Zhang HY, Qiu Y, Hua Z. Bilateral erector spinae plane blocks for open posterior lumbar surgery. J Pain Res. 2020;13:709–717. doi:10.2147/jpr.S248171
23. Tariq GN, Tariq Z, G N. Continuous Erector Spinae Plane (ESP) analgesia in different open abdominal surgical procedures: a case series. J Anesth Surg. 2018;5(1):57–60. doi:10.15436/2377-1364.18.1853
24. Ozdemir H, Araz C, Karaca O, Turk E. Comparison of ultrasound-guided erector spinae plane block and subcostal transversus abdominis plane block for postoperative analgesia after laparoscopic cholecystectomy: a randomized, controlled trial. J Invest Surg. 2021;34:1–8. doi:10.1080/08941939.2021.1931574
25. Wahdan AS, Radwan TA, Mohammed MM, Abdalla Mohamed A, Salama AK. Effect of bilateral ultrasound-guided erector spinae blocks on postoperative pain and opioid use after lumbar spine surgery: a prospective randomized controlled trial. Egypt J Anaesth. 2021;37(1):100–106. doi:10.1080/11101849.2021.1893984
26. Leong RW, Tan ESJ, Wong SN, Tan KH, Liu CW. Efficacy of erector spinae plane block for analgesia in breast surgery: a systematic review and meta-analysis. Anaesthesia. 2021;76(3):404–413. doi:10.1111/anae.15164
27. Ma J, Bi Y, Zhang Y, et al. Erector spinae plane block for postoperative analgesia in spine surgery: a systematic review and meta-analysis. Eur Spine J. 2021;30(11):3137–3149. doi:10.1007/s00586-021-06853-w
28. Ivanusic J, Konishi Y, Barrington MJ. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med. 2018;43(6):567–571. doi:10.1097/aap.0000000000000789
29. Byrne K, Smith C. Human volunteer study examining the sensory changes of the thorax after an erector spinae plane block. Reg Anesth Pain Med. 2020;45(10):761–762. doi:10.1136/rapm-2019-101019
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
