Back to Journals » Journal of Pain Research » Volume 16
Ultrasound-Guided Bilateral Sequential Thoracic Paravertebral Block for Simultaneous Bilateral Uniportal Video-Assisted Thoracoscopic Surgery: Study Protocol for a Randomized Controlled Trial
Authors Lu Y, Zhou Q, Fu Y , Wen Z , Lv X
Received 1 December 2022
Accepted for publication 30 January 2023
Published 3 February 2023 Volume 2023:16 Pages 373—381
DOI https://doi.org/10.2147/JPR.S398349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ellen M Soffin
Yugang Lu,* Qing Zhou,* Yu Fu,* Zongmei Wen, Xin Lv
Department of Anesthesiology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zongmei Wen; Xin Lv, Department of Anesthesiology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, No. 507 Zhengmin Road, Shanghai, 200433, People’s Republic of China, Tel/Fax +86 21 65115006, Email [email protected]; [email protected]
Purpose: Simultaneous bilateral pulmonary resection via uniportal video-assisted thoracoscopic surgery (UVATS) was safe and feasible for the treatment of bilateral multiple pulmonary nodules. But, it should be noted that considerable postoperative pain at the bilateral surgical site was a crucial issue. The safety and efficacy of bilateral thoracic paravertebral block (TPVB) have been reported for postoperative analgesia. But, whether bilateral sequential TPVB can be safely and effectively used in simultaneous bilateral UVATS remains unknown. Therefore, this study aimed to determine the analgesic efficacy and safety of bilateral sequential TPVB after simultaneous bilateral UVATS.
Study Design and Methods: In this study, 80 participants scheduled for UVATS will be randomly allocated to the bilateral sequential TPVB group (G2) and the control group (G1). The patient of G2 will be performed bilateral TPVB at 2 time-points: before the start of the first side of pulmonary resection and before the start of the contralateral pulmonary resection. G1 will only receive standard analgesia protocol. The primary outcome is the numeric rating scale score during coughing at 24 h postoperatively. The secondary outcomes include the Prince Henry Pain Score scores, sufentanil consumption, postoperative nausea and vomiting, levels of inflammatory factors, and the Quality of Recovery-40 scores at different time points, as well as chronic pain at postoperative day (POD) 90.
Discussion: This is the first prospective trial to determine the safety and effectiveness of ultrasound-guided bilateral sequential TPVB for postoperative analgesia following simultaneous bilateral UVATS. This study also intended to evaluate the effect of this intervention on postoperative quality of recovery and inflammation levels. The final results will provide clinical evidence related to bilateral sequential TPVB, and promote the application of that acting as a more appropriate analgesic method for simultaneous bilateral UVATS.
Keywords: thoracic surgery, video-assisted, multiple pulmonary nodules, nerve block, pain measurement
Introduction
Lung cancer was the most common cancer and the first leading cause of cancer death in China, reported by the latest data from National Cancer Center (NCC) of China.1 With the development of diagnostic radiographic methods and the increasing awareness of early screening, the detection rate of bilateral multiple pulmonary nodules (BMPNs) is increasing.2–4 Currently, minimally invasive surgery has become the main method of the diagnosis and treatment for BMPNs highly suspected to be synchronous multiple primary lung cancers.5,6 A series of studies have demonstrated that simultaneous bilateral pulmonary resection via uniportal video-assisted thoracoscopic surgery (UVATS) was safe and feasible for the treatment of BMPNs with appropriate patient selection.7–10 Furthermore, simultaneous resection has brought better clinical outcomes than staged resection, such as reductions of cost, total operative time and hospital stays as well as better disease-free survival.11 However, it should be noted that considerable postoperative pain at the bilateral surgical site also was a crucial issue affecting postoperative quality of life.
Thoracic epidural analgesia (TEA) has traditionally been considered the gold standard of care analgesic modality after thoracotomy. With recent improvements in minimally invasive surgery, it is necessary to search for alternative methods of postoperative pain management with a better risk-benefit profile, since the significant side effects or complications of TEA might be disastrous.12,13 Recently, there has been increasing interest in regional nerve blocks, particularly thoracic paravertebral block (TPVB). TPVB is a well-established technique for regional anesthesia, which refers to the injection of local anesthetics into the thoracic paravertebral space to block the paravertebral nerve to achieve the analgesic effect. For unilateral thoracic surgeries, there is sufficient evidence that TPVB offers noninferior analgesia to that of TEA with lower adverse effects or serious complications.14,15 In addition, ultrasound-guided TPVB has more advantages including the visualization of pleural and lung tissue, and the real-time imaging of the puncture needle, which is simpler, safer, and more effective to perform.16
TPVB can be used unilaterally or bilaterally, which provides ipsilateral, segmental, somatic, and sympathetic nerve blockade in contiguous thoracic dermatomes.14 The safety and efficacy of bilateral TPVB have been reported for postoperative analgesia after cardiac, thoracic, and open abdominal surgery.17–19 But, whether bilateral sequential TPVB can be safely and effectively used in simultaneous bilateral UVATS remains unknown.
Thus, this randomized controlled trial aims to determine the analgesic efficacy and safety of ultrasound-guided bilateral sequential TPVB after simultaneous bilateral UVATS.
Materials and Methods
Study Design
The study was designed as a single-center, prospective double-blind, randomized controlled trial, and completed according to the SPIRIT 2013 statement. This trial was approved by the institutional review board of Shanghai Pulmonary Hospital (No. 2022LY0725) and registered on November 8, 2022 in the Chinese Clinical Trial Registry (identifier: ChiCTR2200065541). This study protocol has been designed in accordance with the Declaration of Helsinki. A flowchart detailing the study design is presented in Figure 1. Further, the SPIRIT figure of enrolment, interventions, and assessments is outlined in Figure 2.
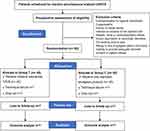 |
Figure 1 Flowchart of the study design. Abbreviations: UVATS, uniportal video-assisted thoracoscopic surgery; TPVB, thoracic paravertebral block. |
Study Objective
This study aims to assess the efficacy of ultrasound-guided bilateral sequential TPVB on postoperative pain and recovery in patients undergoing simultaneous bilateral UVATS.
Participants
Prospective participants will be carefully evaluated based on the following inclusion criteria: (1) age over 18 years, (2) American Society of Anesthesiologists (ASA) score of I-III, (3) participants or their families provided informed consent, and (4) scheduled for elective simultaneous bilateral UVATS.
Participants with any of the following will not be enrolled in the study: (1) contraindication to regional anesthesia (ropivacaine), (2) coagulopathy, (3) pre-existing opiate abuse, (4) local skin infection or eczema, (5) hepatic, renal or cardiorespiratory failure, (6) history of psychiatric or neurologic disorders, (7) history of chronic pain, (8) allergy to any drug/agent used in the study, and (9) inability to provide adequate informed consent or patient refusal.
In the patient recruitment process, patients will be rejected if they are transformed into thoracotomy, or operated on again due to bleeding and anastomotic fistula, or if the technique of TPVB failed.
Preoperative Visit and Evaluation
During the preoperative interview, all patients will receive an explanation of how to assess their pain score adopting the numerical rating scale (NRS) at rest or during coughing. Before the operation, arterial blood gas, pulmonary function and echocardiography will be performed to determine whether they could tolerate simultaneous bilateral pulmonary resection.
Informed Consent
Participants will be pre-selected from the surgical list one day before surgery, according to inclusion/exclusion criteria. The details of this trial will be spelled out to the participant using comprehensible language. After a thorough discussion and consideration of risks and benefits, those who agree to sign the informed consent documents voluntarily will participate in this study officially. The confidentiality of subject records will be protected. Moreover, participants in this study will be allowed to obtain all relevant information and withdraw their consent for any reason at any time.
Randomization and Blinding
Once participants sign the informed consent, they will be randomized to the ultrasound-guided bilateral sequential TPVB group (G2) and the control group (G1) at a 1:1 ratio, using sequentially numbered opaque envelopes. The allocation will be sealed and created by an independent researcher who will not involve in the investigation. All patients in G2 will receive ultrasound-guided bilateral sequential TPVB, whereas patients in G1 will receive no intervention. The anesthesiologist who performs bilateral sequential TPVB will not be involved in the data collection, and the health-care worker who evaluates postoperative pain, vital parameters, and other outcomes will be blinded to the group assignment. Patients will receive blocks after anesthesia induction, with unclear group assignments themselves. To provide blinding of group allocation for nurses, we will use a specific dressing to cover the injection site in all participants, regardless of whether they are injected with local anesthetics or not.
Induction and Maintenance of Anesthesia
Before anesthesia, a catheter will be arranged in the right internal jugular vein for infusion. Blood pressure (BP), heart rate (HR), electrocardiogram (ECG), pulse oximetry (SpO2) and other vital signs will be monitored closely during the surgery. Anesthesia will be induced with 0.05 mg/kg midazolam, 1.5–2.5 mg/kg propofol, 0.5–0.7 μg/kg sufentanil and 0.6 mg/kg rocuronium bromide. And then double-lumen tracheal intubation will be practiced for mechanical ventilation. During the one-lung ventilation, the tidal volume will be set to 5 to 6 mL/kg and the breathing frequency was adjusted to maintain PETCO2 at 35 to 45 cmH2O.
General anesthesia will be maintained with remifentanil, propofol, and rocuronium. When suffering severe cardiovascular responsiveness to noxious stimuli, 0.1 μg/kg additional sufentanil will be administered intravenously to reach a stable hemodynamics state. Moreover, the fluid volume, infusion speed, and transfusion will be adjusted based on hemodynamic monitoring conditions to keep the stable hemodynamic parameters.
Surgical Procedure
For simultaneous bilateral pulmonary resection, the procedure will be started at the side with a smaller resection range, and then rotate the patient to the opposite side to complete the contralateral operation. The patient will be placed in a lateral decubitus position throughout the operation. The specific surgical plan will be based on the size, location, computed tomography characteristics of tumors, the pulmonary function reserve and the intraoperative frozen examination results. At the end of the operation, a pleural drainage tube will be placed in each pleural cavity respectively.
Ultrasound-Guided Bilateral Sequential TPVB Procedure
Patients in G2 will receive ultrasound-guided bilateral sequential TPVB in the lateral decubitus position after anesthesia induction. The paravertebral block will be started at the priority side of the operation. After the completion of the first side of pulmonary resection, the patient will be rotated to the opposite side, and then receive the contralateral paravertebral block before the start of the contralateral operation. In general, ultrasound-guided bilateral sequential TPVB will be performed at 2 time-points: before the start of the first side of pulmonary resection and before the start of the contralateral pulmonary resection.
Using a transversal transducer position, lateral techniques,14 a curved array transducer (2–5 MHz) will be used to scan from the midline laterally to identify the transverse processes, the desired paravertebral spaces (at the T4-T5 vertebral level), the pleura and lung tissue. Under ultrasound guidance, a block needle (22G, 80mm, UniPlex®, NanoLine®, Germany) will be inserted from lateral to medial and advanced until it enters the desired paravertebral spaces, a triangular area in-between the parietal pleura (anterior) and the internal intercostal membrane and intercostal muscles (posterior), under aseptic conditions using an in-plane approach. After negative aspiration for blood or air, 20 mL 0.375% ropivacaine will be injected at the desired paravertebral spaces under real-time imaging. A successful spread of local anesthetic is defined as anterior movement of the pleura and widening of the paravertebral space. The contralateral paravertebral block will be performed on the contralateral side using the same technique. Finally, a total of 150 mg of ropivacaine will be injected into each subject. In patients whose weight is less than 50 kg, this amount will be decreased so as not to exceed the dose of 3 mg/kg.
The paravertebral block will be performed under the close supervision of experienced doctors, and all patients will be continuously cardiac and respiration monitored. In addition, vasoactive drugs can be used in patients with unstable hemodynamics (including any undesirable effects related to sympathetic block) as appropriate, in order to maintain a mean arterial pressure of 70 to 100 mm Hg, and a heart rate of 50 to 100 beats/min during surgery.
Postoperative Management
After completing surgery, neuromuscular blockade will be reversed with neostigmine (0.04 mg/kg) and atropine (0.02 mg/kg), and tracheal extubation occurs when adequate muscle strength will be established. After extubation, the blinded anesthesia team will transfer the patient to the post-anesthesia care unit (PACU). When the Aldrete score reaches 9 points or more, the patient can be transferred from the PACU to a surgical ward.
Standard Analgesia Protocol
To prescribe flurbiprofen axetil 50 mg intravenously 30 minutes before the surgical incision and immediately after arriving in PACU. In addition, all patients will receive intravenous analgesia through patient-controlled intravenous analgesia (PCIA) during the first 2 days after the operation. The PCIA protocol is programmed with 2 μg/kg sufentanil diluted to 100 mL (bolus, 2 mL; background infusion, 2 mL/hour; lockout time interval, 15 min; 1 h limit, 8 mL without any baseline infusion). PCIA will be administered when the NRS score is ≥4 or at the request of the patient. When the pain relief is inadequate, a rescue analgesic will be provided through flurbiprofen axetil 50 mg intravenously, and it could be repeated if necessary. Note that the maximum daily dose of flurbiprofen axetil is 200 mg.
Follow-Up
A blinded investigator is responsible for the follow-up. All subjects will be visited from the day before the surgery to 90 postoperative days (POD).
Assessment Tools
The pain intensity at rest and during coughing will be measured using a NRS of 0–10, where 0 is no pain and 10 is the worst pain ever experienced. The pain symptoms during basic physical activity will be evaluated by the Prince Henry Pain Score (PHPS) (0, no pain on cough; 1, pain on cough, no pain while taking deep breaths; 2, pain on deep breathing, no pain at rest; 3, slight pain at rest; 4, severe pain at rest).20 Postoperative nausea and vomiting (PONV) will be documented using a categorical scale (none = no nausea or vomiting; mild = nausea but not vomiting; moderate = vomiting one to three times; severe = vomiting more than four times).21 Quality of recovery (QoR) will be assessed using Quality of Recovery 40 (QoR-40), which scores range from 40 to 200.22 There are 40 items in QoR-40 to assess five dimensions of recovery, including emotions, physical comfort, psychological support, physical independence, and pain.
Measurement of Perioperative Plasma Inflammatory Markers
For this study, blood samples of all patients will be collected at 4 time-points: before the start of the first side of pulmonary resection (T1), before the start of the contralateral pulmonary resection (T2), immediately after the end of the bilateral pulmonary resection (T3), and POD 1 (T4). Samples will be labelled, centrifuged, frozen, and stored at −80° for subsequent measurement of interleukin 6 (IL-6) and high-sensitivity C-reactive protein (hs-CRP). IL-6 and hs-CRP levels will be determined by enzyme-linked immunosorbent assay (ELISA).
Primary Outcome
The primary study outcome is the NRS score during coughing at 24 h postoperatively. An investigator blinded to the group allocation will visit the patients at 24 h after surgery and complete the assessment of pain using the NRS score (where 0 means no pain and 10 is the worst imaginable pain).
Secondary Outcomes
The secondary outcomes are as follows:
- NRS scores at rest and during coughing at 6 h, 12 h and 48 h postoperatively.
- NRS score at rest at 24 h postoperatively.
- PHPS scores at 6 h, 12 h, 24 h and 48 h postoperatively.
- Cumulative opioid consumption at 6h, 12h, 24h and 48h postoperatively.
- Total number of times that patients pressed the PCIA button at POD 1 and POD 2, including effective presses and ineffective presses.
- Total consumption of analgesic drugs (type and dosage) except opioid within POD 1 and POD 2.
- The degree of PONV at POD 1 and POD 2.
- QoR-40 scores at POD 1, 2, 30, and 90.
- Assessment of chronic pain at POD 90.
Other Outcomes
The other outcomes include:
- Complications related to bilateral sequential TPVB, such as respiratory depression and wound infection.
- Neurological symptoms related to local anesthetic systemic toxicity, such as tinnitus, metallic taste, and altered sensorium.
- Opioid-related adverse effects such as astriction, respiratory depression, itchy skin, and mental status changes.
- Other adverse effects, including any other adverse reaction complained of by the subject.
Assessment of Safety
The ultrasound-guided bilateral sequential TPVB will be performed to patients under standard hemodynamic monitoring in the operating room. This ensures detection and treatment of adverse events immediately. When adverse events associated with the ropivacaine occur, the administration of ropivacaine will be stopped immediately. Moreover, any study-related adverse event will be managed, documented, and reported in particular.
Sample Size Calculation
The primary outcome was the NRS on movement at 24 postoperative hours. In our preliminary study conducted in 10 adult patients (5 in each group), the mean (SD) pain score on movement at 24 postoperative hours in the BTPV group was 3.9 (1.9) and in the control group was 5.7 (2.1). On the basis of these means and SDs, we calculated that a sample of 34 patients per group would provide 95% power at a two-sided alpha level of 0.05. Therefore, we plan to enroll 40 patients in each group considering the possibility of dropout and loss of follow-up. The final sample size was a total of n = 80 participants. The sample size was calculated with the free software G*Power.
Statistical Analysis
Data will be analyzed using Statistical Product and Service Solutions (SPSS) version 26.0 (IBM SPSS Inc., Chicago, IL, USA). The results will be presented as frequency (percentage), mean (standard deviation, SD), or median (interquartile range, IQR) as appropriate. Binary outcomes will be compared using the Chi-squared test or Fisher’s exact test. Continuous outcomes will be compared using the t-test (Student’s t-test) or one-way ANOVA if normally distributed, while non-normally distributed data will be analyzed using the Mann–Whitney U-test. A two-tailed p < 0.05 will be considered significant.
Data Management and Monitoring
Data will be collected into the case report forms (CRF) and monitored timely and correctly by Clinical Research Ethics Committee in our institution. After data entry and confirmation, the CRF will be filed in numerical order and stored in a secure repository at Shanghai Pulmonary Hospital. If necessary, the datasets will be available from the chief investigator (Xin Lv) upon reasonable request.
Discussion
Owing to the popularity of lung cancer screening and the development of imaging technology, an increasing rate of detection of multiple pulmonary nodules has been demonstrated, especially pulmonary nodules with components of ground-glass opacities (GGO).4 It looks beyond dispute that surgery early is currently the best treatment for the pulmonary nodules with higher risk of malignancy. Studies have shown that patients with bilateral lung cancer can benefit more from one-stage bilateral resection than those receiving one side surgery combined with contralateral chemotherapy or radiotherapy.23,24 What is more, several studies have shown that simultaneous bilateral UVATS is safe and feasible, as well as does not increase the risk of perioperative surgery.7,8 As one of the largest centers for thoracoscopic surgery, our center has accumulated rich experience in simultaneous bilateral UVATS, including wedge resection, segmentectomy or lobectomy on each side. Although simultaneous bilateral UVATS can solve the bilateral lesions in one operation with minimally invasive nature, considerable postoperative pain, related to the bilateral surgical incision and longer procedure time, is still a challenge.
TEA is quite an effective analgesic method for the bilateral thoracic surgery traditionally, but application of which is limited since the potential significant side effects or complications and strict requirements on coagulation function.25 It has been confirmed that in open thoracotomy lung surgery, unilateral TPVB has the same analgesic effect and postoperative pulmonary function as TEA but with much less analgesia-related complications.26 For bilateral TPVB, Richardson et al17 have concluded that it is safe and effective for thoracic and abdominal surgery, via reviewed 12 published studies with a total of 538 patients. However, whether bilateral sequential TPVB can be safely and effectively used in simultaneous bilateral UVATS remains unknown.
At the time of this study conception, no prospective randomized trials looked at the safety or efficacy of bilateral sequential TPVB for simultaneous bilateral UVATS. Therefore, we are conducting this prospective double-blind, randomized control trial to determine the safety and effectiveness of ultrasound-guided bilateral sequential TPVB for postoperative analgesia following simultaneous bilateral UVATS. We will perform ultrasound-guided bilateral sequential TPVB at 2 time-points: before the start of the first side of pulmonary resection and before the start of the contralateral pulmonary resection, which will decrease unnecessary analgesia time on the contralateral side. Furthermore, this study also intended to evaluate the effect of bilateral sequential TPVB on postoperative quality of recovery and inflammation levels. Our results may provide clinical evidence relevant to bilateral sequential TPVB, and promote the application of that acting as a more appropriate analgesic method for simultaneous bilateral UVATS.
Trial Status
This trial was approved by the Clinical Research Ethics Committee of Shanghai Pulmonary Hospital (approval No. 2022LY0725). And, the study protocol was registered at the Chinese Clinical Trial Registry on November 8, 2022 (identifier: ChiCTR2200065541). The first participant was recruited on 10 November 2022, and the patient recruitment is expected to be finished by 1 November 2024.
Acknowledgments
The authors acknowledge all the patients for participation in this project at Shanghai Pulmonary Hospital, Tongji University School of Medicine.
Author Contributions
All authors made a significant contribution to this work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Yugang Lu, Qing Zhou, and Yu Fu contributed equally to this work.
Funding
This work was supported by grants from the following: National Natural Science Foundation of China (82170107), Shanghai Municipal Health Commission (202140406), Excellent Subject Leader Program of Shanghai Municipal Health Commission (2022XD007), and Development Fund of Department of Anesthesiology, Shanghai Pulmonary Hospital, Tongji University School of Medicine 2022.
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2(1):1–9. doi:10.1016/j.jncc.2022.02.002
2. Church TR, Black WC, Aberle DR, et al.; National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980–1991.
3. Jiang L, He J, Shi X, et al. Prognosis of synchronous and metachronous multiple primary lung cancers: systematic review and meta-analysis. Lung Cancer. 2015;87(3):303–310. doi:10.1016/j.lungcan.2014.12.013
4. Bak S, Lee H, Kim J, et al. Quantitative CT scanning analysis of pure ground-glass opacity nodules predicts further CT scanning change. Chest. 2016;149(1):180–191. doi:10.1378/chest.15-0034
5. Hattori A, Matsunaga T, Takamochi K, et al. Surgical management of multifocal ground-glass opacities of the lung: correlation of clinicopathologic and radiologic findings. Thorac Cardiovasc Surg. 2017;65(2):142–149. doi:10.1055/s-0036-1572437
6. Leventakos K, Peikert T, Midthun DE, et al. Management of multifocal lung cancer: results of a survey. J Thorac Oncol. 2017;12(9):1398–1402. doi:10.1016/j.jtho.2017.05.013
7. Huang C, Sun Y, Wu Q, et al. Simultaneous bilateral pulmonary resection via single-utility port VATS for multiple pulmonary nodules: a single-center experience of 16 cases. Thorac Cancer. 2021;12(4):525–533. doi:10.1111/1759-7714.13791
8. Qu R, Hao Z, Zhang Y, et al. Single-center experience of simultaneous bilateral uni-portal video-assisted thoracoscopic surgery for multiple ground-glass opacities. J Cardiothorac Surg. 2020;15(1):1–7. doi:10.1186/s13019-020-01107-0
9. Zhang Y, Wang Y, Lv C, et al. Clinical analysis of 56 cases of simultaneous bilateral video-assisted thoracoscopic surgery for bilateral synchronous multiple primary lung adenocarcinoma. J Thorac Dis. 2018;10(12):6452–6457. doi:10.21037/jtd.2018.11.10
10. Yao F, Yang H, Zhao H. Single-stage bilateral pulmonary resections by video-assisted thoracic surgery for multiple small nodules. J Thorac Dis. 2016;8(3):469–475. doi:10.21037/jtd.2016.02.66
11. Zheng H, Peng Q, Xie D, et al. Simultaneous bilateral thoracoscopic lobectomy for synchronous bilateral multiple primary lung cancer—single center experience. J Thorac Dis. 2021;13(3):1717–1727. doi:10.21037/jtd-20-3325
12. Freise H, Van Aken H. Risks and benefits of thoracic epidural anaesthesia. Br J Anaesth. 2011;107(6):859–868. doi:10.1093/bja/aer339
13. Zeltsman M, Dozier J, Vaghjiani RG, et al. Decreasing use of epidural analgesia with increasing minimally invasive lobectomy: impact on postoperative morbidity. Lung Cancer. 2020;139:68–72. doi:10.1016/j.lungcan.2019.11.001
14. Krediet AC, Moayeri N, Van Geffen G-J, et al. Different approaches to ultrasound-guided thoracic paravertebral block: an illustrated review. Anesthesiology. 2015;123(2):459–474. doi:10.1097/ALN.0000000000000747
15. D’ercole F, Arora H, Kumar PA. Paravertebral block for thoracic surgery. J Cardiothorac Vasc Anesth. 2018;32(2):915–927. doi:10.1053/j.jvca.2017.10.003
16. Seidel R, Wree A, Schulze M. Thoracic-paravertebral blocks: comparative anatomical study with different injection techniques and volumes. Reg Anesth Pain Med. 2020;45(2):102–106. doi:10.1136/rapm-2019-100896
17. Richardson J, Lönnqvist P, Naja Z. Bilateral thoracic paravertebral block: potential and practice. Br J Anaesth. 2011;106(2):164–171. doi:10.1093/bja/aeq378
18. Sondekoppam RV, Uppal V, Brookes J, et al. Bilateral thoracic paravertebral blocks compared to thoracic epidural analgesia after midline laparotomy: a pragmatic noninferiority clinical trial. Anesth Analg. 2019;129(3):855–863. doi:10.1213/ANE.0000000000004219
19. Sun L, Li Q, Wang Q, et al. Bilateral thoracic paravertebral block combined with general anesthesia vs. general anesthesia for patients undergoing off-pump coronary artery bypass grafting: a feasibility study. BMC Anesthesiol. 2019;19(1):1–7. doi:10.1186/s12871-019-0768-9
20. Zubrzycki M, Liebold A, Skrabal C, et al. Assessment and pathophysiology of pain in cardiac surgery. J Pain Res. 2018;11:1599–1611. doi:10.2147/JPR.S162067
21. Qiu Y, Wu J, Huang Q, et al. Acute pain after serratus anterior plane or thoracic paravertebral blocks for video-assisted thoracoscopic surgery: a noninferiority randomised trial. Eur J Anaesthesiol. 2021;38:S97–S105. doi:10.1097/EJA.0000000000001450
22. Myles P, Weitkamp B, Jones K, et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84(1):11–15. doi:10.1093/oxfordjournals.bja.a013366
23. Zhou H, Kang X, Dai L, et al. Efficacy of repeated surgery is superior to that of non‐surgery for recurrent/second primary lung cancer after initial operation for primary lung cancer. Thorac Cancer. 2018;9(8):1062–1068. doi:10.1111/1759-7714.12790
24. Watanabe T, Tanahashi M, Suzuki E, et al. Surgical treatment for synchronous multiple primary lung cancer: is it possible to achieve both curability and preservation of the pulmonary function? Thorac Cancer. 2021;12(22):2996–3004. doi:10.1111/1759-7714.14164
25. Pöpping DM, Elia N, Van Aken HK, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2014;259(6):1056–1067. doi:10.1097/SLA.0000000000000237
26. Ren P, Du Y, He G, et al. Efficacy and safety of general anesthesia combined with paravertebral blockade on postoperative recovery in patients undergoing pulmonary surgery: a systematic review and meta-analysis. J Thorac Dis. 2022;14(2):431–442. doi:10.21037/jtd-22-103
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

