Back to Journals » Risk Management and Healthcare Policy » Volume 13
U-Shaped Association of High-Density Lipoprotein Cholesterol with All-Cause and Cardiovascular Mortality in Hypertensive Population
Authors Chen C , Liu X , Liu L, Lo K , Yu Y , Huang J, Huang Y, Chen J
Received 17 July 2020
Accepted for publication 12 September 2020
Published 8 October 2020 Volume 2020:13 Pages 2013—2025
DOI https://doi.org/10.2147/RMHP.S272624
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Marco Carotenuto
Chao-lei Chen,1 Xiao-cong Liu,1 Lin Liu,1 Kenneth Lo,2 Yu-ling Yu,1 Jia-yi Huang,1 Yu-qing Huang,1 Ji-yan Chen1
1Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, People’s Republic of China; 2Centre for Global Cardiometabolic Health, Department of Epidemiology, Brown University, Providence, RI, USA
Correspondence: Yu-qing Huang; Ji-yan Chen
Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No. 106, Zhongshan Second Road, Yuexiu District, Guangzhou 510080, People’s Republic of China
Tel/Fax +86-20-83827812
Email [email protected]; [email protected]
Purpose: Whether the paradox of high-density lipoprotein cholesterol (HDL-C) and elevated mortality risk extends to hypertensive patients is unclear. We aimed to investigate the association between HDL-C and all-cause and cardiovascular disease mortality in adults with hypertension.
Methods: In the National Health and Nutrition Examination Surveys, 11,497 hypertensive participants aged ≥ 18years old and examined at baseline between 1999 and 2014 were followed up until December 2015. We categorized the HDL-C concentration as ≤ 30, 31– 40, 41– 50, 51– 60 (reference), 61– 70, > 70 mg/dL and examined their associations with all-cause and cardiovascular mortality, respectively. Multivariate Cox regression was used to calculated hazard ratio (HR) and 95% confidence interval (CI) for mortality risk.
Results: During follow-up (median: 9.2 ± 3.8 years), 3012 deaths and 713 cardiovascular deaths were observed. In the restrictive cubic curves, associations of HDL-C levels and all-cause and cardiovascular mortality were detected to be U-shaped. After multivariable adjustment, HRs for all-cause mortality were for the lowest HDL-C concentration (≤ 30 mg/dL) 1.29 (95% CI, 1.07– 1.56) and the highest (> 70 mg/dL) 1.20 (1.06– 1.37), comparing with the reference group. For cardiovascular mortality, HRs were 1.31 (0.83– 1.48) and 1.09 (0.83– 1.43), respectively. Similar results were obtained in subgroups stratified by age, gender, race, and taking lipid-lowering drugs. The lowest all-cause mortality risk was observed at HDL-C 66 mg/dL (concentration) and 51– 60 mg/dL (range).
Conclusion: Both lower and higher HDL-C concentration appeared to be associated with higher mortality in hypertensive population. Further investigation is warranted to clarify the underlying mechanisms.
Keywords: all-cause mortality, high-density lipoprotein cholesterol, cardiovascular mortality, hypertension
Introduction
High-density lipoprotein cholesterol (HDL-C) levels play a critical role in cardiovascular risk assessment and are recommended by current American and European guidelines for routine measurement in clinical practice.1,2 The recommendation was to some extent based on early epidemiological studies that indicated an inverse linear association between high-density HDL-C concentration and incidence of cardiovascular event (CVD) and all-cause mortality.3–6 These findings supported the hypothesis that raising HDL-C levels could promote prognosis and consolidated the protective role of HDL-C in the field of cardiovascular risk prevention. However, whether extremely high level of HDL-C could maintain its protective role has been questioned in the past few years. In fact, most of the clinical trials targeting an increase in HDL-C have failed to reduce the risk of CVD or mortality compared with patients receiving placebo.7–11 Moreover, studies have found that higher level of HDL-C was not associated with decreased risk of CVD and cardiovascular mortality in some specific populations.12–14 These data from observational and randomized controlled studies indicated a more complicated relationship between HDL-C and mortality than the traditional HDL-C hypothesis.
In the recent past, numerous studies that intended to investigate the relationship between HDL-C levels and mortality risk have shown inconsistent results. For example, a J-shaped or U-shaped association between HDL-C and all-cause mortality or cardiovascular mortality was observed in the general population.15–19 In addition, a cohort study from China found that both lower and higher HDL-C were associated with increased risk of CVDs in the general rural China population, but results for cardiovascular mortality were not significant.20 In patients with type 2 diabetes, participants with relatively high HDL-C concentration had higher risk of CVDs and all-cause mortality.21 No significant association between HDL-C and all-cause mortality was found in some other studies.14,22,23 These inconclusive data emphasized the importance of classifying the potential relationship between HDL-C and risk of mortality, especially in hypertensives, considering the limited data in this large population. To address this knowledge gap, the current study aimed to test the hypothesis that both very low (≤30 mg/dL) and high (>70 mg/dL) HDL-C levels were associated with greater risk of all-cause and cardiovascular mortality in patients with hypertension by leveraging data from the National Health and Nutrition Examination Surveys (NHANES).
Methods
Study Population
The NHANES is a nationally representative survey designed and conducted by the Center for Disease Control and Prevention for evaluating the health status of US citizens. In the present study, we used data from the 1999–2014 NHNAES (N = 47,356 for subjects aged ≥18 years old) and set the end of follow-up time on 31 December 2015. We excluded participants who had incomplete HDL-C data (N = 5161), missing covariate data (other laboratory test, medical history, and clinical data; N = 15,822), without available mortality status (N = 49), and without hypertension at baseline (N = 14,647). A total of 11,497 participants were enrolled for the final analysis (Figure 1). The NAHNES was approved by the Institutional Review Board of the Centers for Disease Control and Prevention and informed consents were obtained from all participants.
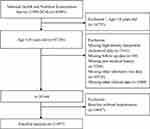 |
Figure 1 Flowchart of study participants. |
Exposure Assessment
The exposure variable was HDL-C concentration. Blood sample collection and lipid measurement were based on a standardized protocol according to Centers for Disease Control and Prevention criteria. Fasting samples were obtained from peripheral venous blood and stored on dry ice until they were shipped to Johns Hopkins University Lipoprotein Analytical Lab. All blood samples were measured through a Hitachi 704 Analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN).24 HDL-C was measured using a heparin-manganese precipitation method or a direct immunoassay technique,25 while triglycerides (TG) and total cholesterol (TC) were measured enzymatically. Low-density lipoprotein cholesterol (LDL-C) was calculated according to the Friedewald formula [LDL-C=TC−HDL-C–(TG/5)] if TG was ≤4.5 mmol/L (400 mg/dL).26
Outcome Assessment
Mortality status of participants in the NHANES 1999–2014 was ascertained through probabilistic record matching with the National death Index. The intend outcomes of the current study were death from all causes and cardiovascular mortality. The International Classification of Diseases (ICD), 10th Revision was used for the identification of underlying causes of death. Cardiovascular mortality was defined as death caused by cardiovascular disease (ICD −10 codes I00 to I09, I11, I13, I20 to I51) or cerebrovascular disease (I60 to I69). Study participants were followed up through December 2015 if they did not meet the intended outcome.
Covariates for Analysis
For each survey in the NHANES, standardized questionnaires and examinations were conducted to assess covariates at baseline based on established association with mortality. These included age, sex, race (dichotomized into White versus Non-white), education (dichotomized into high school or above versus others), had at least 100 cigarettes in lifetime (yes or no), systolic and diastolic blood pressure (average value of four measurements), body mass index (BMI), estimated glomerular filtration rate (eGFR), C-reactive protein (CRP), dietary energy in kcal, self-report of comorbidities (diabetes and cardiovascular disease), and current medication usage (including antihypertensive drugs, hypoglycemic agents, lipid-lowering drugs, and antiplatelet drugs). BMI was calculated by weight divided by height squared [kg/m2]. The Modification of Diet in Renal Disease formula was used to calculate eGFR.27 Hypertension in our analysis was defined as self-report of prior diagnosis of hypertension by a doctor, or taking any antihypertensive drugs, or SBP ≥ 140 mmHg or DBP ≥ 90 mmHg.28 Diabetes was defined as self-reported history of diabetes, or taking anti-diabetes medications, or a fasting blood glucose ≥126 mg/dL (7·0 mmol/L), or a hemoglobin A1c ≥6·5% (48 mmol/mol).29 Antihypertensive drugs included angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), β-blocker, calcium channel blocker (CCB), and diuretics.
Statistical Analysis
Data were presented as mean standard deviation (SD) for continuous variables and percentages for categorical variables. Baseline characteristics between HDL-C groups were reported and compared using the One-Way ANOVA, Kruskal–Wallis H-test and chi-square tests, as appropriate. We initially performed survival analysis using standardized Kaplan–Meier curves and Log rank test. The shape of association between HDL-C levels and all-cause and cardiovascular mortality was then examined by multivariate adjusted Cox restricted cubic spline regression models. We further applied a two-piecewise linear regression model using a smoothing function to test whether there was a non-linear relationship between HDL-C and all-cause and cardiovascular mortality. Trial and error were used to determine the threshold level, including selecting turning points along predetermined intervals and then selecting the turning point that gave the maximum model likelihood. If a nonlinear relationship was detected, a two-piecewise Cox proportional hazards model on both sides of the inflection point, and log likelihood ratio test were performed. Next, we conducted Cox proportional hazards models to estimate HR with 95% confidence interval (CI) of outcomes of interest for HDL-C categories (≤30, 31–40, 41–50, 51–60 (reference), 61–70, >70 mg/dL). Model I was univariate. Age, sex, and race were included in Model II. Fully adjusted model (Model III) incorporated covariates including age, gender, race, education level, smoking, BMI, energy, systolic blood pressure, eGFR, CRP, TC, diabetes, cardiovascular disease, lipid-lowering agents, antidiabetic drugs, antihypertensive drugs, and antiplatelet drugs. Finally, we conducted subgroup analyses including age (<65 or ≥65 years), sex (men or women), race (White or Non-white), cardiovascular disease (yes or no), diabetes (yes or no), BMI (<25 or ≥25 kg/m2), and taking lipid-lowering drugs (yes or no). P < 0.05 was considered statistically significant. R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
Results
Baseline Characteristics
The baseline characteristics of all the participants according to HDL-C levels are summarized in Table 1. The present study included 11,497 participants (male: 49.7% and mean age: 60.7 years). The mean concentration of HDL-C was 52.34 mg/dl (1.35 mmol/l). HDL-C ≥70mg/dL was identified in 13.0% of participants, with a higher proportion of women in this group. Among HDL-C groups, we observed significant differences in all baseline covariates except for eGFR and usage of antihypertensive medications including ARB, CCB, and diuretics.
 |
Table 1 Demographic and Clinical Characteristics According to High-Density Lipoprotein Cholesterol Levels at Baseline |
Incidence of All-Cause and Cardiovascular Death
As provided in Table 1, 26.20% (n= 3012) of participants died due to all causes and 713 (6.20%) cardiovascular deaths were recorded during an average follow-up of 109.87 ± 46.12 months. There was no significant difference in the incident all-cause and cardiovascular mortality among HDL-C categories due to the results of chi-square tests (P > 0.05). However, including follow-up time as a variable in the survival curve analyses, Figure S1 showed that participants with higher HDL-C had significantly lower all-cause event-free survival (Log rank P =0.025). The difference among HDL-C groups for cardiovascular survival probability, however, was not significant.
Hazard Ratios for Total and Cardiovascular Mortality
In the restricted cubic spline regression models with full adjustment for age, gender, race, education level, smoking, BMI, energy, systolic blood pressure, eGFR, CRP, TC, diabetes, cardiovascular disease, lipid-lowering agents, antidiabetic drugs, antihypertensive drugs, and antiplatelet drugs, the relationships between HDL-C and all-cause and cardiovascular mortality were both U-shaped in participants with hypertension (Figure 2). The results of two-piecewise linear regression model are demonstrated in Table 2. After adjusting for potential confounders, the cut-off values of all-cause, and cardiovascular mortality were 1.71 mmol/L (66mg/dl) and 1.19mmol/L (46mg/dl), respectively. When HDL-C was less than the cut-off value, the HRs for all-cause and cardiovascular mortality were 0.83 (95% CI: 0.72–0.96, P = 0.011) and 0.46 (0.25–0.83, P = 0.010) for every 1 mmol/L increase in HDL-C, respectively. On the right of the cut-off value, the HRs for of all-cause and cardiovascular mortality were 1.71 (1.42–2.07, P < 0.001) and 1.20 (0.93–1.55, P = 0.163), respectively. The multivariate HRs for all-cause mortality for HDL-C levels of ≤30, 31–40, 41–50, 51–60 (reference), 61–70, and >70 mg/dL were 1.29 (1.07–1.56, P = 0.009), 1.10 (0.98–1.24, P = 0.100), 1.07 (0.97–1.19, P = 0.086), 1.00, 1.04 (0.91–1.18, P = 0.576), and 1.20 (1.06–1.37, P = 0.005), respectively (P for trend = 0.869). In addition, the multivariable-adjusted HRs for cardiovascular mortality were 1.31 (0.90–1.91, P = 0.153), 1.13 (0.89–1.42, P = 0.313), 0.97 (0.78–1.21, P = 0.808), 1.00, 0.97 (0.74–1.27, P = 0.843), and 1.09 (0.83–1.43, P = 0.541), respectively (P for trend = 0.422) (Table 2). Both lower (≤30 mg/dL) and higher (>70 mg/dL) HDL-C levels were associated with higher all-cause mortality risk. We found a similarly increased risk trend for cardiovascular mortality, but the association was not significant (P > 0.05), perhaps as a result of the relatively limited sample size and cardiovascular deaths (Table 2).
 |
Table 2 Multivariate Cox Regression Analysis of HDL-C Levels with All-Cause and Cardiovascular Mortality |
 |
Figure 2 Adjusted cubic spline model of the association between hazard ratio of all-cause mortality (A) and cardiovascular mortality (B) and HDL-C in hypertensive patients. |
Subgroup Analyses
We performed subgroups analyses stratified by age gender, race, BMI, diabetes, cardiovascular disease, and taking lipid-lowering drugs, as provided in Table 3. Age, gender, race and BMI interacted significantly with the association between HDL-C levels and all-cause mortality. There were also interactions among age, race, HDL-C levels, and cardiovascular mortality (P for interaction <0.05). A clear U-shaped association was found in hypertensive subjects without diabetes and not taking lipid-lowering drugs (Table 3). In addition, we conducted threshold effect analysis by subgroups including age, gender, and race. HRs with 95% CI for per 1 mmol/L increase in HDL-C on the left and right of the cut-off value were summarized in Table S1. Moreover, the nature of associations of HDL-C levels with all-cause and cardiovascular mortality by subgroups including age (<65 and ≥65 years), gender (male and female), race (White and Non-white), were shown using restricted cubic splines (Figure S2-S7).
 |
Table 3 Subgroups Analyses for All-Cause and Cardiovascular Mortality |
Discussion
In this population-based study, using data from the NHANES 1999–2014, we found a U-shaped relationship of HDL-C with all-cause mortality and a non-linear association between HDL-C and cardiovascular mortality. Both lower and higher levels of HDL-C appeared to be associated with increased with mortality risk in patients with hypertension. The lowest all-cause mortality and cardiovascular mortality risk were observed at HDL-C 66 mg/dL and 46 mg/dL (cut-off values), respectively, while the optimal HDL-C range was 51–60 mg/dL for all-cause mortality. Besides, there were significant interactions between age, gender, race, HDL-C and risk of all-cause or cardiovascular mortality.
Our results were consistent with previous studies that indicated a non-linear relationship between HDL-C concentrations and mortality. A recent meta-analysis of 37 prospective cohort studies involving 3,524,505 participants demonstrated a J-shaped dose–response relationship between HDL-C level and death from all causes and cardiovascular disease in the general population. The study indicated that both the lowest and highest HDL-C levels were related to increased all-cause and cardiovascular mortality risk.15 Similarly, Sun et al found that, in the general Chinese adults, the relation of HDL-C and all-cause mortality was U-shaped and that HDL-C≥80 mg/dl was significantly correlated with greater risk of all-cause death.20 Another study using data from the NHANES 1999–2010 showed that extremely high (≥100 mg/dL) or low (<30 mg/dL) levels increased risk of all-cause deaths and deaths from coronary heart disease and stroke in American adults, but the results were not stratified by hypertension status.30 Among the elderly, Mao, et al31 found that HDL-C <61 mg/dL was related to a 18% higher all-cause mortality risk and HDL-C >87 mg/dL increased the risk by 56% compared with the group with HDL-C concentrations ranging from 61 to 87 mg/dL. They also observed a non-linear association of HDL-C with cardiovascular mortality.31 In our study, the cut-off points of HDL-C were higher for all-cause mortality than cardiovascular mortality (66 vs 46 mg/dL), which were similar to Maoet al31 Similar nature of non-linear associations (U-shaped or J-shaped) were also confirmed in some other studies.18,19,32,33 These findings were inconsistent with studies that illustrated an inverse linear association between HDL-C and mortality.4,6,34 Notably, due to the relatively small sample size and the different criteria used to divide HDL-C levels, these studies might fail to detect the potential U-shaped or non-linear association in the study subjects.35 Moreover, other studies claimed that there was no association of higher concentrations of HDL-C with mortality risk.14,22,23 The reason for our study was different from these findings may be mainly due to various populations and different adjusted confounding factors.
As revealed in subgroup analyses, the association of HDL-C concentrations and mortality risk in our study was significantly interacted by age, gender and race. A cohort from northern rural China draw a U-shaped relationship between HDL-C concentrations and all-cause mortality in younger participants (<65 years old), but not in those >65 years, which was not in accordance with the results of the elderly.17,31 Banach et al4 found an HDL-C paradox in Mexican-American ethnicity participants, among which higher HDL-C levels had greater all-cause mortality risk compared with a lower level. Moreover, in patients with chronic renal insufficiency, lower risk of all-cause and cardiovascular mortality for HDL-C >60 mg/dL was only found in women.32 Our study might have some public and clinical implications in individuals with extremely low or high HDL-C concentrations. The measurement of HDL-C concentration was recommended by current guidelines for cardiovascular risk assessment in patients with hypertension.36,37 Based on our results, clinicians were able to be aware of that the association between HDL-C and mortality risk was non-linear and the common thoughts of blindly increasing HDL-C concentration might be not suitable in hypertensives. These findings might also be useful in determining which population will benefit from appropriate management of HDL-C levels.
The underlying mechanisms of higher HDL-C level and elevated risk of mortality, so-called HDL-C paradox, remain unclear. One possible reason was genetic variance. Studies have found that some variants of certain genes such as CETP, SCARB1, ABCA1, and LIPC could increase the HDL-C concentration as well as the risk of adverse health outcomes.19,38–40 An alternative reason was the complicated composition of HDL-C. Differences in particle size, number, shape, electrophoresis speed, or lipid and protein composition of HDL-C might contribute variously to the predictive ability of cardiovascular events. Additionally, extremely large HDL particle sizes at high HDL-C concentrations might cause particles like low-density lipoproteins trapping in the arterial intima, which might accelerate the development of atherosclerosis.41 Another plausible explanation was the concentration-dependent biphasic effects of high-density lipoprotein on tube formation and angiogenesis of endothelial progenitor cells.42 Most importantly, in participants with very high level of HDL-C, the function of HDL-C might already be compromised. Dysfunctional HDL-C might in turn promote cardiovascular risk rather than benefit due to the great amount of HDL-C. In fact, cumulative evidence has proved that HDL-C efflux capacity was a better predictor of cardiovascular risk than HDL-C concentration. Therefore, cholesterol efflux dysfunction might be a critical reason for the association of higher HDL-C with increased incidence of cardiovascular events and death.6,43 These findings confirm that further mechanism investigations need to focus more on the HDL phenotype and function so as to better understand the role of HDL-C in cardiovascular disease risk.
The strength of the current research was to include a representative sampling design, rigorous and standard protocol for data collection, and linkage to national mortality data, which made our results of prospective relationship more reliable. However, some limitations should be considered for cautious interpretation. First, despite we have fully adjusted for many risk factors, residual confounding might exist due to unrecognized confounders such as alcohol consumption and steroid usage, as they might result in higher HDL-C levels. Second, self-reported data such as medical history and medication usage might cause recall bias. Third, the number of deaths from cardiovascular disease was relatively small and the statistical power might be weak. Longer duration of follow-up is needed to further confirm a significant association. Fourth, the current study outcome of interest was mortality rather than cardiovascular events. Fifth, the baseline HDL-C levels of study participants might change during follow up, which might result bias in estimated HR. Finally, because of the observational property of the NAHNES, our findings cannot conclude a causal relationship between HDL-C and mortality risk.
Conclusion
In patients with hypertension, we found a U-shaped association of HDL-C concentration with all-cause and a non-linear association with cardiovascular mortality. Both lower and higher HDL-C levels were related to higher probability of mortality risk. Different to the traditional belief, our findings suggested that “the higher HDL-C concentration the better” did not hold in hypertensive individuals. Based on results of the current study, the lowest all-cause mortality risk was observed at HDL-C 66 mg/dL (concentration) and 51–60 mg/dL (range). The functional damage of HDL-C might be one important reason for higher HDL-C increasing mortality risk. More prospective studies in the future are needed to confirm our findings.
Ethical Statement
Our experimental study was approved by the institutional medical ethical committee the Guangdong General Hospital, Guangzhou, China. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consents were obtained from all participants.
Acknowledgment
We are grateful to all the participants.
Funding
This work was supported by the Science and Technology Program of Guangzhou (No.201604020143, No.201604020018, No.201604020186, and No.201803040012), the National Key Research and Development Program of China (No.2017YFC1307603, No.2016YFC1301305), and the Key Area R&D Program of Guangdong Province (No.2019B020227005).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–e595.
2. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381.
3. Castelli WP, Garrison RJ, Wilson PW, et al. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256(20):2835–2838. doi:10.1001/jama.1986.03380200073024
4. Penson P, Long DL, Howard G, et al. Associations between cardiovascular disease, cancer, and very low high-density lipoprotein cholesterol in the REasons for Geographical and Racial Differences in Stroke (REGARDS) study. Cardiovasc Res. 2019;115(1):204–212. doi:10.1093/cvr/cvy198
5. Okamura T, Hayakawa T, Kadowaki T, et al. The inverse relationship between serum high-density lipoprotein cholesterol level and all-cause mortality in a 9.6-year follow-up study in the Japanese general population. Atherosclerosis. 2006;184(1):143–150. doi:10.1016/j.atherosclerosis.2005.03.042
6. Saleheen D, Scott R, Javad S, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3(7):507–513. doi:10.1016/S2213-8587(15)00126-6
7. Keene D, Price C, Shun-Shin MJ, et al. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349(jul18 2):g4379. doi:10.1136/bmj.g4379
8. Bowman L, Hopewell JC, Chen F, et al. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N Engl J Med. 2017;377:1217–1227.
9. Di Bartolo BA, Duong M, Nicholls SJ. Clinical trials with cholesteryl ester transfer protein inhibitors. Curr Opin Lipidol. 2016;27(6):545–549. doi:10.1097/MOL.0000000000000352
10. Kingwell BA, Chapman MJ, Kontush A, et al. HDL-targeted therapies: progress, failures and future. Nat Rev Drug Discov. 2014;13:445–464.
11. Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384(9943):618–625. doi:10.1016/S0140-6736(14)61217-4
12. Angeloni E, Paneni F, Landmesser U, et al. Lack of protective role of HDL-C in patients with coronary artery disease undergoing elective coronary artery bypass grafting. Eur Heart J. 2013;34(46):3557–3562. doi:10.1093/eurheartj/eht163
13. Silbernagel G, Schöttker B, Appelbaum S, et al. High-density lipoprotein cholesterol, coronary artery disease, and cardiovascular mortality. Eur Heart J. 2013;34(46):3563–3571. doi:10.1093/eurheartj/eht343
14. Hirata A, Okamura T, Sugiyama D, et al. The Relationship between Very High Levels of Serum High-Density Lipoprotein Cholesterol and Cause-Specific Mortality in a 20-Year Follow-Up Study of Japanese General Population. J Atheroscler Thromb. 2016;23(7):800–809. doi:10.5551/jat.33449
15. Zhong GC, Huang SQ, Peng Y, et al. HDL-C is associated with mortality from all causes, cardiovascular disease and cancer in a J-shaped dose-response fashion: a pooled analysis of 37 prospective cohort studies. Eur J Prev Cardiol. 2020;2047487320914756.
16. Oh IH, Hur JK, Ryoo JH, et al. Very high high-density lipoprotein cholesterol is associated with increased all-cause mortality in South Koreans. Atherosclerosis. 2019;283:43–51. doi:10.1016/j.atherosclerosis.2019.01.035
17. Li X, Guan B, Wang Y, et al. Association between high-density lipoprotein cholesterol and all-cause mortality in the general population of northern China. Sci Rep. 2019;9(1):14426. doi:10.1038/s41598-019-50924-4
18. Hamer M, O’Donovan G, Stamatakis E. High-Density Lipoprotein Cholesterol and Mortality: too Much of a Good Thing? Arterioscler Thromb Vasc Biol. 2018;38(3):669–672. doi:10.1161/ATVBAHA.117.310587
19. Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38(32):2478–2486. doi:10.1093/eurheartj/ehx163
20. Yu S, Guo X, Li GX, et al. Lower or higher HDL-C levels are associated with cardiovascular events in the general population in rural China. Lipids Health Dis. 2020;19(1):152. doi:10.1186/s12944-020-01331-6
21. Sharif S, van der Graaf Y, Nathoe HM, et al. HDL Cholesterol as a Residual Risk Factor for Vascular Events and All-Cause Mortality in Patients With Type 2 Diabetes. Diabetes Care. 2016;39(8):1424–1430. doi:10.2337/dc16-0155
22. Harari G, Green MS, Magid A, et al. Usefulness of Non-High-Density Lipoprotein Cholesterol as a Predictor of Cardiovascular Disease Mortality in Men in 22-Year Follow-Up. Am J Cardiol. 2017;119(8):1193–1198. doi:10.1016/j.amjcard.2017.01.008
23. Sung KC, Ryu S, Wild SH, et al. An increased high-density lipoprotein cholesterol/apolipoprotein A-I ratio is associated with increased cardiovascular and all-cause mortality. Heart. 2015;101(7):553–558. doi:10.1136/heartjnl-2014-306784
24. Doran B, Guo Y, Xu J, et al. Prognostic value of fasting versus nonfasting low-density lipoprotein cholesterol levels on long-term mortality: insight from the National Health and Nutrition Examination Survey III (NHANES-III). Circulation. 2014;130(7):546–553. doi:10.1161/CIRCULATIONAHA.114.010001
25. Bucholz EM, Rodday AM, Kolor K, et al. Prevalence and Predictors of Cholesterol Screening, Awareness, and Statin Treatment Among US Adults With Familial Hypercholesterolemia or Other Forms of Severe Dyslipidemia (1999–2014). Circulation. 2018;137(21):2218–2230. doi:10.1161/CIRCULATIONAHA.117.032321
26. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. doi:10.1093/clinchem/18.6.499
27. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266.
28. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. doi:10.1001/jama.2013.284427
29. American Diabetes Association. 2. 2. Classification and Diagnosis of Diabetes: standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(Supplement 1):S14–S31. doi:10.2337/dc20-S002
30. Mazidi M, Mikhailidis DP, Banach M. Associations between risk of overall mortality, cause-specific mortality and level of inflammatory factors with extremely low and high high-density lipoprotein cholesterol levels among American adults. Int J Cardiol. 2019;276:242–247. doi:10.1016/j.ijcard.2018.11.095
31. Li ZH, Lv YB, Zhong WF, et al. High-Density Lipoprotein Cholesterol and All-Cause and Cause-Specific Mortality Among the Elderly. J Clin Endocrinol Metab. 2019;104(8):3370–3378. doi:10.1210/jc.2018-02511
32. Navaneethan SD, Schold JD, Walther CP, et al. High-density lipoprotein cholesterol and causes of death in chronic kidney disease. J Clin Lipidol. 2018;12(4):1061–1071.e1067. doi:10.1016/j.jacl.2018.03.085
33. Bowe B, Xie Y, Xian H, et al. High Density Lipoprotein Cholesterol and the Risk of All-Cause Mortality among U.S. Veterans. Clin J Am Soc Nephrol. 2016;11(10):1784–1793. doi:10.2215/CJN.00730116
34. Kaysen GA, Ye X, Raimann JG, et al. Lipid levels are inversely associated with infectious and all-cause mortality: international MONDO study results. J Lipid Res. 2018;59:1519–1528.
35. Ko DT, Alter DA, Guo H, et al. High-Density Lipoprotein Cholesterol and Cause-Specific Mortality in Individuals Without Previous Cardiovascular Conditions: the CANHEART Study. J Am Coll Cardiol. 2016;68(19):2073–2083. doi:10.1016/j.jacc.2016.08.038
36. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104.
37. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248.
38. Frikke-Schmidt R. Genetic variation in the ABCA1 gene, HDL cholesterol, and risk of ischemic heart disease in the general population. Atherosclerosis. 2010;208(2):305–316. doi:10.1016/j.atherosclerosis.2009.06.005
39. Genga KR, Trinder M, Kong HJ, et al. CETP genetic variant rs1800777 (allele A) is associated with abnormally low HDL-C levels and increased risk of AKI during sepsis. Sci Rep. 2018;8(1):16764. doi:10.1038/s41598-018-35261-2
40. Zanoni P, Khetarpal SA, Larach DB, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351(6278):1166–1171. doi:10.1126/science.aad3517
41. Zh L, Yb L, Wf Z, et al. High-density lipoprotein cholesterol and all-cause and cause-specific mortality among the elderly. Sci Rep. 2019;104:3370–3378.
42. Huang CY, Lin FY, Shih CM, et al. Moderate to high concentrations of high-density lipoprotein from healthy subjects paradoxically impair human endothelial progenitor cells and related angiogenesis by activating Rho-associated kinase pathways. Arterioscler Thromb Vasc Biol. 2012;32(10):2405–2417. doi:10.1161/ATVBAHA.112.248617
43. Otocka-Kmiecik A, Mikhailidis DP, Nicholls SJ, et al. Dysfunctional HDL: a novel important diagnostic and therapeutic target in cardiovascular disease? Prog Lipid Res. 2012;51(4):314–324. doi:10.1016/j.plipres.2012.03.003
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
