Back to Journals » Clinical Epidemiology » Volume 15
Twenty-Three-Year Trends in the Use of Potentially Nephrotoxic Drugs in Denmark
Authors Enevoldsen FC , Christiansen CF , Jensen SK
Received 13 November 2022
Accepted for publication 19 February 2023
Published 7 March 2023 Volume 2023:15 Pages 275—287
DOI https://doi.org/10.2147/CLEP.S397415
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lars Pedersen
Frederik Cosedis Enevoldsen,1 Christian Fynbo Christiansen,1,2 Simon Kok Jensen1,2
1Department of Clinical Medicine, Aarhus University, Aarhus, Denmark; 2Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark
Correspondence: Frederik Cosedis Enevoldsen, Department of Clinical Epidemiology, Aarhus University Hospital, Olof Palmes Allé 43-45, 8200 Aarhus N, Central Denmark Region, Denmark, Tel +45 87 16 72 12, Email [email protected]
Background: The occurrence of acute and chronic kidney diseases has been rising in the last decades. Although drug use is a common risk factor for impaired kidney function, changes in utilization of potential nephrotoxic drugs have received little attention.
Purpose: To describe temporal trends in the utilization of potentially nephrotoxic drugs in Denmark between 1999 and 2021.
Methods: Specific drugs known or suspected to be nephrotoxic were identified in the literature. Data on the sold defined daily doses (DDDs) of potentially nephrotoxic drugs between 1999 and 2021 were retrieved using the Danish Register of Medical Product Statistics. Trends in sales of DDDs per 1000 inhabitants per day were tabulated and illustrated graphically.
Results: From 1999 to 2021, the total sale of all selected drugs increased from 286 to 457 DDDs per 1000 inhabitants per day. The overall sale reached a preliminary peak in 2012 with 449 DDDs per 1000 inhabitants per day and remained relatively stable thereafter until reaching an all-time high in 2021 with 457 DDDs per 1000 inhabitants per day. Contributing with the majority in volume, sales of drugs inhibiting the renin-angiotensin-aldosterone system (RAAS) increased dramatically throughout the period. The same was observed for acetaminophen, methotrexate, tacrolimus, and iodinated contrast dye. In contrast, the sales of diuretics, acetylsalicylic acid, and ciclosporin decreased during the last decade of the study period.
Conclusion: From 1999– 2021 considerable changes in sales of potentially nephrotoxic drugs were observed. In general, the sales increased, in volume predominated by RAAS inhibiting drugs. This increase in sales of potential nephrotoxins could contribute to an increasing occurrence of kidney diseases.
Keywords: adverse events, nephrotoxins, drug utilization, acute kidney injury, kidney disease
Introduction
Drug use is a common risk factor for kidney disease, including acute kidney injury (AKI) and chronic kidney disease (CKD).1,2 These diseases are associated with an increased risk of short- and long-term mortality, prolonged hospital admission, and dependence on dialysis.3 The occurrence of kidney diseases has been rising during the last 20 years.4–7 The pathogenic mechanisms of drugs causing nephrotoxicity are diverse, and several drugs can cause nephrotoxicity by more than one pathogenic mechanism. These include, but are not limited to, altered intraglomerular hemodynamics, crystal nephropathy, inflammation, rhabdomyolysis, direct tubular cell toxicity, crystal nephropathy, chronic interstitial nephropathy, and interference with renal hemodynamics (Figure 1).1,8–19
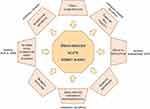 |
Figure 1 Mechanisms of drug-induced acute kidney injury. Inspired by Schetz et al.8 Abbreviations: ACE-I, Angiotensin Converting Enzyme Inhibitor; ARB, Angiotensin Receptor Blocker; MTX, methotrexate; NSAID, non-steroidal anti-inflammatory drug. |
Recently, several Danish studies have examined changes in the utilization of different groups of drugs, generally demonstrating increasing use and sales. These include antithrombotic drugs,20 antihypertensive drugs,21 statins,22 antidiabetic drugs,23 antiarrhythmic drugs,24 and opioids.25 However, only a few population-based studies have examined changes in utilization of potentially nephrotoxic drugs.20,26–28 In the Nordic countries, the total sales of acetaminophen (paracetamol) increased from 2000 to 2015.26 Between 1999 and 2014, the use of low-dose acetylsalicylic acid in Denmark increased before a slight decline after 2010.20 Moreover, recent cohort studies have investigated the use among critically ill and in patients with chronic kidney disease, demonstrating widespread use of potential nephrotoxic drugs.27,28 To our knowledge, no previous studies have specifically examined trends in the use of potentially nephrotoxic drugs in a nationwide, population-based setting. Such information would contribute to our understanding of changes in risk factors for kidney disease in the general population. Therefore, we examined temporal changes in the sales of selected potentially nephrotoxic drugs from 1999 to 2021 in Denmark.
Methods
Setting
We conducted this study in Denmark between 1 January 1999 and 31 December 2021. In 2021 Denmark had a population of approximately 5.8 million inhabitants.29 All Danish inhabitants have equal access to universal tax-supported healthcare including hospitals, general practitioners, and partial reimbursement for prescribed drugs.
Data Source
Information on the sale of potentially nephrotoxic drugs according to the Anatomical Therapeutic Chemical (ATC) classification system is available from the publicly accessible Danish database Medical Statistics (MEDSTAT).30 Based on the Register of Medical Product Statistics,31 the database has provided aggregated statistics on annual drug sales from the Danish primary and hospital sectors since 1996 and 1997, respectively.32 Registration includes both prescription and non-prescription drug sales in defined daily doses (DDD), a statistical measure of drug consumption defined by WHO. DDD represents the assumed average maintenance dose for the main indication in adults, thus facilitating comparison of trends in drug use, independent of prices or pack sizes. Reporting of drug sales to the Register of Medical Product Statistics is mandatory and data from Medical Statistics are considered valid and complete from 1999 onwards.32 However, certain formulations containing ATC groups J01 (antibacterials for systemic use) and L01 (antineoplastic drugs) were incompletely recorded until 2011. Therefore, trends in the sales of these drugs before 2012 should be interpreted with caution.33
Statistical Analysis
Trend in sales (DDDs) of different groups of potentially nephrotoxic drugs were illustrated graphically. The selection and grouping of drugs altering hemodynamics, drugs associated with chronic interstitial nephropathy, crystal nephropathy, tubular cell toxicity, and diuretics, was adapted from Naughton et al1 (Table 1). Glucosamine (ATC: M01AX05) was excluded from the main group of non-steroidal anti-inflammatory drugs (NSAID) (ATC: M01A), because it does not share pharmacodynamic properties with other NSAIDs.34 DDDs were not available for cisplatin (ATC: L01XA01) and radiocontrast. As the sold amount of cisplatin was too small to assess from MEDSTAT, total sales were obtained through correspondence with the Danish Health Data Authority and grams sold per 1000 inhabitants per day were calculated using census statics from Statistics Denmark.29,30 Radiocontrast was limited to contrast dyes containing iodine (ATC: V08A).
 |
Table 1 Selected Potentially Nephrotoxic Drugs with ATC Codes |
Results
During 1999–2021, the aggregated annual sales of all selected drugs increased from 286 to 457 DDDs per 1000 inhabitants per day (Figure 2 and Table 2). The overall use reached a preliminary peak in 2012 with 449 DDDs per 1000 inhabitants per day and remained relatively stable hereafter until reaching an all-time high in 2021.
 |
Table 2 Sold Amount (Units) per 1000 Inhabitants per Day |
 |
Figure 2 Sales of selected drugs between 1999 and 2021 in DDDs per 1000 inhabitants per day. Abbreviation: DDD, Defined Daily Dose. |
Drugs Altering Intraglomerular Hemodynamics
Drugs altering intraglomerular hemodynamics comprised the largest subgroup from 2004 and onwards and included angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin receptor blockers (ARBs), and NSAIDs (Figure 2). Predominated by ACE-Is and ARBs, the sales of drugs with effect on intraglomerular hemodynamics increased from 70.8 to 246.8 DDDs per 1000 inhabitants per day (Figure 3A). From 1999 to 2021 the sale of ARBs rose steadily from 11.6 to 116 DDDs per 1000 inhabitants per day. The sale of ACE-Is increased from 28.7 DDDs per 1000 inhabitants per day in 1999 to 111.6 DDDs per 1000 inhabitants per day in 2012 and remained relatively stable thereafter. NSAIDs did not share the same trend, as a rise from 30.5 DDDs per 1000 inhabitants per day in 1999 to 59.1 DDDs per 1000 inhabitants per day in 2004 was followed by a steady decrease to 27 DDDs per 1000 inhabitants per day in 2021.
Drugs Associated with Chronic Interstitial Nephropathy
The group of drugs associated with chronic interstitial nephropathy consisted of lithium, acetaminophen and acetylsalicylic acid (NSAIDs were included in 3A). In total, the sales peaked in 2010 at 139.5 DDDs per 1000 inhabitants per day, preceded by a rise from 108.4 DDDs per 1000 inhabitants per day in 1999 (Figure 3B). From 2010 to 2014, the sales decreased to 122.7 DDDs per 1000 inhabitants per day and remained stable thereafter.
The sales of acetaminophen steadily increased from 48.4 to 77.3 DDDs per 1000 inhabitants per day during the 23-year period. Following an increase from 58.9 to 74.1 DDDs per 1000 inhabitants per day from 1999 to 2009, acetylsalicylic acid sales decreased to 43.5 DDDs per 1000 inhabitants per day between 2009 and 2021. Sales of lithium remained stable between 1.1 and 1.2 DDDs per 1000 inhabitants per day during the study period.
Drugs Associated with Crystal Nephropathy
Drugs associated with crystal nephropathy included methotrexate (MTX), sulfonamide antibiotics, and aciclovir. Being overshadowed by MTX, sales of sulfonamides decreased from 0.5 to 0.2 DDDs per 1000 inhabitants per day, while sales of aciclovir increased from 0.1 to 0.5 DDD per 1000 inhabitants per day. Sales of MTX increased fivefold during the study period, from 1.3 to 6.88 DDDs per 1000 inhabitants per day (Figure 3C).
Drugs Associated with Tubular Cell Toxicity
Trends in sales of drugs associated with tubular cell toxicity varied between the selected subgroups of drugs (Figure 3D). The sales of tacrolimus had the most pronounced annual rise increasing from 0.0 to 0.6 DDD per 1000 inhabitants per day during the study period. The opposite was seen for ciclosporin, decreasing from 0.4 to 0.1 per 1000 inhabitants per day. The sales of aminoglycosides and amphotericin B were stable during the period ranging from 0.0 to 0.1 DDD per 1000 inhabitants per day. Overall, a clear trend in the sales of these drugs was not present. In total, the sales remained between 0.5 and 0.8 DDD per 1000 inhabitants per day. During the study period a steady increase in the sales iodinated contrast dye was observed, growing from 2.0 grams per 1000 inhabitants per day in 1999 to 9.8 grams per 1000 inhabitants per day in 2021 (Figure 4A). Between 2012 and 2021 the sales of cisplatin remained relatively stable around micrograms per 1000 inhabitants per day (Figure 4B).
Diuretics
The group of diuretics included loop diuretics, potassium sparing diuretics, thiazides, and sulfonamide diuretics. Overall, the number of sold DDDs decreased during the study period. After an increase from 104.1 DDDs per 1000 inhabitants per day in 1999 to 112.9 DDDs per 1000 inhabitants per day in 2007, the sales decreased to 80.2 DDDs per 1000 inhabitants per day in 2021 (Figure 3E). The decreasing trend was observed for most subgroups.
Discussion
This is the first nationwide, population-based study on long-term trends in utilization of potential nephrotoxic drugs. We found considerable changes in the sales of potentially nephrotoxic drugs during the 23-year period from 1999 to 2021. Sales of drugs inhibiting the renin-angiotensin-aldosterone system (RAAS) contributed with the majority in volume and increased dramatically throughout the period. Increases in sales were also observed for acetaminophen, methotrexate, tacrolimus, and contrast dye. In contrast, the use of diuretics, acetylsalicylic acid, and ciclosporin decreased during the last decade of the study period.
Limitations
Our study has a number of strengths, including a long study period, utilization of prospectively collected nationwide data, and accurate population figures. Despite these strengths, several limitations must be considered when interpreting the results: First, it should be noted that the data used for the study are on drug sales and not drug use, why drug adherence merit mentioning here. The prevalence of non-adherence has been reported between 20 and 54%, but small changes over time cannot be ruled out.35–38 While sales may vary from the actual use, it is unlikely that the overall observed trends in sales are not accompanied by similar changes in use. Second, a potential confounder to the observed trends in drug sales is the overall increase in patients’ comorbidity burden.39,40 We did not stratify by demographic variables such as age or sex as this was not possible for the aggregated statistics on total sales in MEDSTAT. Therefore, we are not able to provide data on the potential impact of the rising proportion of older individuals with more comorbidities as well as exposure to more diagnostic and therapeutic procedures. Third, while the use of DDDs allows comparison of sales of a drug over time, caution must be taken when evaluating the overall change in nephrotoxic burden as adverse event profiles and the degree of nephrotoxicity vary widely between drug groups. Although rating systems for the nephrotoxic potential of drugs exist, comparisons of direct nephrotoxicity of different drugs are rarely available. For instance, if a drug is five times more toxic and prescribed twice as much as another, the former would have an overall nephrotoxic impact ten times larger than the latter of the two. A recent study aimed to assign a nephrotoxic potential for 167 drugs, rating these on a scale from 0 to 3, with 3 corresponding to a definite nephrotoxic potential.41 However, the overall changes in nephrotoxic potential arising from changes in the sales of numerous drugs are difficult to assess. In addition, the aggregated data used in this study does not disclose patients receiving multiple potentially nephrotoxic drugs, which could in theory have synergistic (or antagonistic) effects on kidney function. Keeping this in mind, monitoring of kidney function in high-risk patients through blood or urine samples is pivotal. Lastly, even though changes to the DDD are generally avoided, they sometimes undergo revision.42 Such alterations could potentially complicate the interpretation.
Interpretation
ACE-is and ARBs
Between 1999 and 2012 an almost fourfold rise in the use of ACE-Is was observed, whereafter the use remained stable. Likewise, the use of ARBs increased dramatically during the study period, being ten times higher in 2021 than in 1999. Due to effectiveness and a favorable safety profile ACE-Is and ARBs are currently first-line drugs in the treatment of high blood pressure.43–45 The rising trend in use correlates with a gradual development of a more aggressive approach to blood pressure control, especially among a growing population of elderly. Though the renal benefits of RAAS inhibitors have been well established, the treatment is also known to inhibit regional hemodynamic autoregulation, which may lower the threshold for developing or worsening AKI in certain circumstances.46 Importantly, it is not unusual that ACE-Is and ARBs are taken together with diuretics and NSAIDs, a combination known as “the triple whammy”, which is associated with an increased risk of AKI.47 The changes in use of RAAS inhibitors are substantial, and it cannot be ruled out that the drugs could contribute to the development of AKI in some cases, although the widespread use might conversely lower the overall incidence of AKI and CKD due to their nephroprotective properties.
NSAIDs
Since 2004 the sales of NSAIDs have been declining. This can be explained by multiple factors, including withdrawal of selective COX-2 inhibitors due to the risk of cardiovascular events48 and a bigger awareness of other serious side effects, such as gastrointestinal bleeding.49 In 2008 and 2009 respectively, the Danish Medicines Agency and Danish Society for Cardiology recommended that diclofenac should be used with caution due to the risk of cardiovascular toxicity.50 However, NSAIDs known to increase the risk of AKI through alterations of intraglomerular hemodynamics are still widely used and easily accessible.51
Acetaminophen
During the study period the use of acetaminophen increased 1.5-fold. A minor, abrupt fall in 2013 can be attributed to Danish legislators restricting sales of large packs of painkillers to prescriptions only, which was done to prevent suicidal poisonings.26 Similarly, an 18-year-old minimum age for purchase was implemented in 2011.52 Both legislative initiatives were followed by a significant decrease in the number of non-opioid analgesic poisonings.52
Acetylsalicylic Acid
Acetylsalicylic acid was commonly used between 1999 and 2021, but the sales decreased from 2009 and onward. This could be due to Danish and European guidelines no longer recommended low-dose acetylsalicylic acid as routine thromboprophylaxis in patients >65 years without cardiovascular disease.53
Contrast Dye
The use of iodinated radiocontrast increased almost fivefold between 1999 and 2021. As diagnostic procedures using iodinated radiocontrast are becoming more routine, the use of the agents is growing. Additionally, parts of the dramatic increase might be due to an increasing number of invasive procedures in a growing population of elderly.39,40 Importantly, the group of iodinated contrast agents (V08A) comprises several different kinds of solutions, which are not necessarily comparable regarding level of toxicity. However, generally the use of all subgroups increased during the study period.
Conclusion
This study is the first to provide nationwide, population-based data on long-term trends in the use of potentially nephrotoxic drugs. During the past 23 years, considerable changes in the use of potentially nephrotoxic drugs have occurred. Notably, sales of drugs inhibiting RAAS, methotrexate, tacrolimus, and acetaminophen increased steadily. Additionally, the use of iodinated radiocontrast increased almost fivefold. In contrast, sales of other drugs of interest, including diuretics, acetylsalicylic acid, and ciclosporin, decreased. The reported increases in total use of potential nephrotoxins aligns with reported increases in occurrence of kidney diseases globally. However, whether the temporal changes in use of potential nephrotoxins is causally related to a possible increase in the incidence of AKI and CKD requires further examination.
Abbreviations
ACE-I, Angiotensin converting enzyme inhibitor; AKI, Acute kidney injury; ARB, Angiotensin receptor blocker; ATC, Anatomical therapeutical chemical classification; CKD, Chronic kidney disease; COX-2, Cyclooxygenase-2; DDD, Defined daily dose; MTX, Methotrexate; NSAID, Non-steroid anti-inflammatory drug; RAAS, Renin-angiotensin-aldosterone system.
Data Sharing Statement
The data reported in this article are publicly available through the online database Medical Statistics.30
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was funded by the Independent Research Fund Denmark (grant number: 0134-00407B).
Disclosure
The authors have no conflicts of interest to disclose in this work.
References
1. Naughton CA. Drug-induced nephrotoxicity. Am Fam Physician. 2008;78(6):743–750.
2. Rolland AL, Garnier AS, Meunier K, Drablier G, Briet M. Drug-Induced Acute Kidney Injury: a Study from the French Medical Administrative and the French National Pharmacovigilance Databases Using Capture-Recapture Method. J Clin Med. 2021;10(2):168. doi:10.3390/jcm10020168
3. Negi S, Koreeda D, Kobayashi S, et al. Acute kidney injury: epidemiology, outcomes, complications, and therapeutic strategies. Semin Dial. 2018;31(5):519–527. doi:10.1111/sdi.12705
4. Sawhney S, Fraser SD. Epidemiology of AKI: utilizing Large Databases to Determine the Burden of AKI. Adv Chronic Kidney Dis. 2017;24(4):194–204. doi:10.1053/j.ackd.2017.05.001
5. Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007;72(2):208–212. doi:10.1038/sj.ki.5002297
6. Stack AG, Li X, Kaballo MA, et al. Temporal trends in acute kidney injury across health care settings in the Irish health system: a cohort study. Nephrol Dial Transplant. 2020;35(3):447–457. doi:10.1093/ndt/gfy226
7. Cockwell P, Fisher LA. The global burden of chronic kidney disease. Lancet. 2020;395(10225):662–664.
8. Schetz M, Dasta J, Goldstein S, Golper T. Drug-induced acute kidney injury. Curr Opin Crit Care. 2005;11(6):555–565. doi:10.1097/01.ccx.0000184300.68383.95
9. Oliveira JF, Silva CA, Barbieri CD, Oliveira GM, Zanetta DM, Burdmann EA. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009;53(7):2887–2891. doi:10.1128/AAC.01430-08
10. Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014;2014:967826. doi:10.1155/2014/967826
11. Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508. doi:10.2215/CJN.04800908
12. Rocha PN, Kobayashi CD. Incidence, Predictors, and Impact on Hospital Mortality of Amphotericin B Nephrotoxicity Defined Using Newer Acute Kidney Injury Diagnostic Criteria. Antimicrob Agents Chemother. 2015;59(8):4759–4769. doi:10.1128/AAC.00525-15
13. McCullough PA. Radiocontrast-induced acute kidney injury. Nephron Physiol. 2008;109(4):p61–72. doi:10.1159/000142938
14. Vlachopanos G, Schizas D, Hasemaki N, Georgalis A. Pathophysiology of Contrast-Induced Acute Kidney Injury (CIAKI). Curr Pharm Des. 2019;25(44):4642–4647. doi:10.2174/1381612825666191210152944
15. Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11(6):694–703. doi:10.1634/theoncologist.11-6-694
16. McCrae JC, Morrison EE, MacIntyre IM, Dear JW, Webb DJ. Long-term adverse effects of paracetamol - a review. Br J Clin Pharmacol. 2018;84(10):2218–2230. doi:10.1111/bcp.13656
17. Fored CM, Ejerblad E, Lindblad P, et al. Acetaminophen, aspirin, and chronic renal failure. N Engl J Med. 2001;345(25):1801–1808. doi:10.1056/NEJMoa010323
18. Perneger TV, Whelton PK, Klag MJ. Risk of kidney failure associated with the use of Acetaminophen, aspirin, and nonsteroidal antiinflammatory drugs. N Engl J Med. 1994;331(25):1675–1679. doi:10.1056/NEJM199412223312502
19. Markowitz GS, Perazella MA. Drug-induced renal failure: a focus on tubulointerstitial disease. Clin Chim Acta. 2005;351(1–2):31–47. doi:10.1016/j.cccn.2004.09.005
20. Adelborg K, Grove EL, Sundbøll J, Laursen M, Schmidt M. Sixteen-year nationwide trends in antithrombotic drug use in Denmark and its correlation with landmark studies. Heart. 2016;102(23):1883–1889. doi:10.1136/heartjnl-2016-309402
21. Sundbøll J, Adelborg K, Mansfield KE, Tomlinson LA, Seventeen-Year Nationwide SM. Trends in Antihypertensive Drug Use in Denmark. Am J Cardiol. 2017;120(12):2193–2200. doi:10.1016/j.amjcard.2017.08.042
22. Mortensen MB, Falk E, Schmidt M. Twenty-Year Nationwide Trends in Statin Utilization and Expenditure in Denmark. Circ Cardiovasc Qual Outcomes. 2017;10(7). doi:10.1161/CIRCOUTCOMES.117.003811
23. Bang C, Mortensen MB, Lauridsen KG, Bruun JM. Trends in antidiabetic drug utilization and expenditure in Denmark: a 22-year nationwide study. Diabetes Obes Metab. 2020;22(2):167–172. doi:10.1111/dom.13877
24. Poulsen CB, Damkjær M, Løfgren B, Schmidt M. Trends in Antiarrhythmic Drug Use in Denmark Over 19 Years. Am J Cardiol. 2020;125(4):562–569. doi:10.1016/j.amjcard.2019.11.009
25. Nissen SK, Pottegård A, Ryg J. Trends of Opioid Utilisation in Denmark: a Nationwide Study. Drugs Real World Outcomes. 2019;6(4):155–164. doi:10.1007/s40801-019-00163-w
26. Wastesson JW, Martikainen JE, Zoëga H, Schmidt M, Karlstad Ø, Pottegård A. Trends in Use of Paracetamol in the Nordic Countries. Basic Clin Pharmacol Toxicol. 2018;123(3):301–307. doi:10.1111/bcpt.13003
27. Bosi A, Xu Y, Gasparini A, et al. Use of nephrotoxic medications in adults with chronic kidney disease in Swedish and US routine care. Clin Kidney J. 2022;15(3):442–451. doi:10.1093/ckj/sfab210
28. Ehrmann S, Helms J, Joret A, et al. Nephrotoxic drug burden among 1001 critically ill patients: impact on acute kidney injury. Ann Intensive Care. 2019;9(1):106. doi:10.1186/s13613-019-0580-1
29. Statistics Denmark. Available from: https://www.dst.dk/en.
30. Medical Statistics (MedStat). The Danish Health Data Authority. Available from: https://www.medstat.dk/en.
31. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41. doi:10.1177/1403494810394717
32. Schmidt M, Hallas J, Laursen M, Friis S. Data Resource Profile: Danish online drug use statistics (MEDSTAT). Int J Epidemiol. 2016;45(5):1401–1402g. doi:10.1093/ije/dyw116
33. ”Data basis and description”, Medical Statistics (MedStat), The Danish Health Data Authority. Available from: https://medstat.dk/en/view/datagrundlag_og_beskrivelse.
34. Anderson JW, Nicolosi RJ, Borzelleca JF. Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem Toxicol. 2005;43(2):187–201. doi:10.1016/j.fct.2004.11.006
35. Omori DM, Potyk RP, Kroenke K. The adverse effects of hospitalization on drug regimens. Arch Intern Med. 1991;151(8):1562–1564. doi:10.1001/archinte.1991.00400080064011
36. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi:10.1097/01.mlr.0000114908.90348.f9
37. Molloy GJ, Messerli-Bürgy N, Hutton G, Wikman A, Perkins-Porras L, Steptoe A. Intentional and unintentional non-adherence to medications following an acute coronary syndrome: a longitudinal study. J Psychosom Res. 2014;76(5):430–432. doi:10.1016/j.jpsychores.2014.02.007
38. Voils CI, King HA, Neelon B, et al. Characterizing weekly self-reported antihypertensive medication nonadherence across repeated occasions. Patient Prefer Adherence. 2014;8:643–650. doi:10.2147/PPA.S60715
39. Oksuzyan A, Höhn A, Krabbe Pedersen J, Rau R, Lindahl-Jacobsen R, Christensen K. Preparing for the future: the changing demographic composition of hospital patients in Denmark between 2013 and 2050. PLoS One. 2020;15(9):e0238912. doi:10.1371/journal.pone.0238912
40. Beard JR, Bloom DE. Towards a comprehensive public health response to population ageing. Lancet. 2015;385(9968):658–661. doi:10.1016/S0140-6736(14)61461-6
41. Gray MP, Barreto EF, Schreier DJ, et al. Consensus Obtained for the Nephrotoxic Potential of 167 Drugs in Adult Critically Ill Patients Using a Modified Delphi Method. Drug Saf. 2022;45(4):389–398. doi:10.1007/s40264-022-01173-4
42. ”Application for DDD alterations”, WHO Collaborating Centre for Drug Statistics Methodology. Available from: https://www.whocc.no/ddd/application_for_ddd_alterations/.
43. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913.
44. Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet. 2000;356(9246):1955–1964.
45. Taylor AA, Siragy H, Nesbitt S. Angiotensin receptor blockers: pharmacology, efficacy, and safety. J Clin Hypertens. 2011;13(9):677–686. doi:10.1111/j.1751-7176.2011.00518.x
46. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46–61. doi:10.1038/ki.2014.293
47. Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ. 2013;346(jan08 12):e8525. doi:10.1136/bmj.e8525
48. Jüni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet. 2004;364(9450):2021–2029. doi:10.1016/S0140-6736(04)17514-4
49. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006;55(12):1731–1738. doi:10.1136/gut.2005.080754
50. Schmidt M, Hallas J, Friis S. Potential of prescription registries to capture individual-level use of aspirin and other nonsteroidal anti-inflammatory drugs in Denmark: trends in utilization 1999-2012. Clin Epidemiol. 2014;6:155–168. doi:10.2147/CLEP.S59156
51. Ungprasert P, Cheungpasitporn W, Crowson CS, Matteson EL. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015;26(4):285–291. doi:10.1016/j.ejim.2015.03.008
52. Morthorst BR, Erlangsen A, Chaine M, et al. Restriction of non-opioid analgesics sold over-The-counter in Denmark: a national study of impact on poisonings. J Affect Disord. 2020;268:61–68. doi:10.1016/j.jad.2020.02.043
53. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol. 2016;23(11):Np1–np96. doi:10.1177/2047487316653709
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


