Back to Journals » Clinical Ophthalmology » Volume 16
Twelve-Months Follow-Up Postmarket Study of a Hydrophobic Intraocular Lens Using a Preloaded Automated Injector in an Indian Population
Authors Titiyal JS, Basak SK, Shetty N, Mathur U, Padmanabhan P , Ganesh S , Dey A, Ramamurthy D
Received 25 June 2022
Accepted for publication 8 November 2022
Published 16 December 2022 Volume 2022:16 Pages 4215—4225
DOI https://doi.org/10.2147/OPTH.S379054
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jeewan S Titiyal,1 Samar K Basak,2 Naren Shetty,3 Umang Mathur,4 Prema Padmanabhan,5 Sri Ganesh,6 Arindam Dey,7 Dandapani Ramamurthy8
1Department of Cornea and Refractive Surgery, All India Institute of Medical Sciences, New Delhi, India; 2Department of Cornea and Cataract Services, Disha Eye Hospitals, Kolkata, India; 3Department of Cataract and Refractive Services, Narayana Nethralaya, Bengaluru, India; 4Department of Ophthalmology, Dr. Shroff’s Charity Eye Hospital, New Delhi, India; 5Department of Ophthalmology, Sankara Nethralaya, Chennai, India; 6Department of Phaco and Refractive Surgery, Nethradhama Superspeciality Eye Hospital, Bengaluru, India; 7Alcon Laboratories (India) Private Ltd, Bengaluru, India; 8Department of Cornea, Cataract and Refractive Services, The Eye Foundation, Coimbatore, India
Correspondence: Jeewan S Titiyal, Department of Cornea and Refractive Surgery, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, 110029, India, Email [email protected]
Purpose: To assess real-world clinical outcomes and safety of the Clareon® intraocular lens (IOL) and AutonoMe® automated preloaded delivery system in an Indian population.
Patients and methods: This was a prospective, single-arm, multicenter, 12-month clinical study in patients aged ≥ 20 years with unilateral or bilateral cataracts. Surgery was performed by phacoemulsification followed by implantation of the Clareon monofocal IOL (CNA0T0). Monocular best-corrected distance visual acuity (BCDVA) and uncorrected distance visual acuity (UCDVA) were assessed at 1 week and 1, 6, and 12 months after implantation. Posterior capsular opacification (PCO), surface haze, and glistenings were evaluated at all visits. Surgeons’ satisfaction with automated injector system was also evaluated using a questionnaire. Safety was assessed by monitoring adverse events (AEs).
Results: A total of 151 eyes received the CNA0T0 IOL. Mean ± SD monocular BCDVA improved from 0.53± 0.44 logMAR preoperatively to 0.00± 0.08 logMAR at week 1 and − 0.03± 0.08 logMAR at 12 months after implantation. At 12 months, 137/137 (100%) of eyes achieved BCDVA of 0.3 logMAR or better. Mean ± SD monocular UCDVA was 0.78± 0.40 logMAR preoperatively, 0.11± 0.15 logMAR at week 1, and 0.08± 0.13 logMAR at 12 months after implantation. At 12 months, 132/137 (96%) eyes achieved UCDVA of 0.3 logMAR or better. Serious intraoperative AEs were posterior capsule rupture (n=1) and ciliary zonular dehiscence (n=1). Surgeons reported that the automated preloaded device was more intuitive compared with other push- or screw-style preloaded injector systems. None of the eyes in this study presented surface haze; all were graded as 0 glistenings at all visits. No clinically significant PCO or neodymium-doped yttrium aluminum garnet (Nd:YAG) capsulotomies were reported.
Conclusion: The hydrophobic IOL preloaded in an automated injector system provided good visual and refractive outcomes, as well as no surface haze and grade 0 glistenings. None of the patients required Nd:YAG capsulotomy.
Keywords: glistenings, posterior capsular opacification, visual acuity
Introduction
Cataract is the leading cause of blindness in India and worldwide.1,2 Globally, cataract contributed to about 35% of blindness in 2015. There was a substantial variation in the causes of blindness by region, with cataract contributing between 20% (Australasia and high-income North America) and 47% (Oceania) in patients aged 50 years or more.1 Standard-of-care treatment for patients with cataracts is phacoemulsification with intraocular lens (IOL) implantation.3
The Clareon® IOL (Alcon Vision LLC, Fort Worth, TX, USA) is a foldable 1-piece IOL with the same optic design and architecture as the AcrySof® IQ IOL (Alcon Vision LLC). The Clareon IOL uses flexible acrylic material (PEA/HEMA copolymer) with a 1.5% water content (Table 1)4 and an advanced manufacturing process that improves lens clarity by minimizing glistenings, surface nanoglistenings, and surface haze, as demonstrated by in vitro and in vivo clinical studies.4–6
 |
Table 1 Clareon Properties |
The precision edge of the Clareon IOL was designed to minimize edge glare and reduce posterior capsular opacification (PCO). Using optical simulation with a schematic model eye, Clareon IOL showed lower edge glare compared with other lenses.7 Furthermore, a meta-analysis of published studies found that Clareon IOLs had a lower incidence of neodymium-doped yttrium aluminum garnet (Nd:YAG) capsulotomy compared with AcrySof IOLs.8
AutonoMe® (Alcon Vision LLC) is an automated, single-use, preloaded delivery system that can be used to implant the Clareon IOL. This device provides single-handed control of IOL delivery speed. Its nozzle tip was designed to protect the corneal incision by minimizing stress and damage to surrounding tissue. AutonoMe provided a safe IOL delivery and induced a smaller incision enlargement compared with other delivery systems, as demonstrated in several in vitro, ex vivo, and in vivo reports.9–13
The purpose of this post-marketing study was to describe clinical outcomes and safety of the CNA0T0 IOL preloaded in the automated injector throughout 1 year after implantation and to assess real-world surgical experience in India.
Methods
Study Design
This was a prospective, descriptive, single-arm, interventional, multicenter, 12-month follow-up clinical study with patients enrolled from 7 sites in India (Supplementary Table 1), conducted between December 2019 and December 2021 (Clinical Trials Registry India: CTRI/2019/11/022071). Inclusion criteria were adult patients (aged ≥20 years) with age-related unilateral or bilateral cataracts; calculated lens power between +15.0 and +30.0 D in both eyes; and expected postoperative corneal astigmatism <1.00 D. Exclusion criteria were corneal abnormalities; previous corneal transplant; ocular trauma; previous refractive surgery; history of retinal conditions or degenerative eye disorders; amblyopia; pregnancy; and breastfeeding.
The study was conducted in accordance with the principles of the Declaration of Helsinki and in compliance with the International Council for Harmonisation E6 Good Clinical Practice Consolidated Guideline and ISO 14155:2011 clinical investigation of medical devices for human subjects. All study sites obtained institutional ethics committee approval from their respective institutions (Disha Eye Hospital Pvt. Ltd. Ethics Committee; Dr. Shroff Charity Eye Hospital Ethics Committee; All India Institute of Medical Sciences, RP Center – Institute Ethics Committee; Narayana Nethralaya Ethics Committee; Nethradhama Superspeciality Eye Hospital Ethics Committee; The Ethics Committee of the Eye Foundation; Sankara Nethralaya Medical Research Foundation – Vision Research Foundation Ethics Committee). All patients included in this study signed informed consent.
Surgical Procedure
Cataract was removed by standard phacoemulsification procedure using a surgeon’s preferred methodology per local standard of practice. The Clareon monofocal IOL (model CNA0T0) in an aspheric hydrophobic acrylic IOL with Stableforce® modified L-haptics, a 6-mm aspheric optic, and precision edge design (Figure 1A). CNA0T0 IOL was implanted in the capsular bag in the posterior chamber of the eye using the preloaded AutonoMe device that has a CO2-powered mechanism, a 3-mm nozzle, and a depth guard (Figure 1B). IOL power was calculated targeting for emmetropia using the A constant of 119.1 and the preferred IOL calculation formula (the same at each site for all cases). In the case of patients implanted bilaterally, an IOL power adjustment based on the refractive outcomes of the first operated eye was allowed for the second eye. Qualified ophthalmic viscosurgical devices allowed during the surgery included Viscoat® (Alcon Vision LLC), DisCoVisc® (Alcon Vision LLC), Appavisc (Appasamy Associates, Chennai, India), and Provisc® (Alcon Vision LLC). Study IOLs were not implanted if there were surgical complications such as loss of zonular integrity, zonular rupture, extension of the capsulorrhexis interfering with the stability of the IOL, posterior capsule rupture, incorrect or unknown placement of the haptics, mechanical or surgical manipulations of the pupil, or inability to place the IOL in the capsular bag.
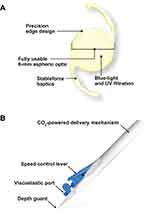 |
Figure 1 Clareon IOL (A) and preloaded AutonoMe delivery system (B). Abbreviations: IOL, intraocular lens; UV, ultraviolet. |
Assessments
Monocular best-corrected distance visual acuity (BCDVA) and uncorrected distance visual acuity (UCDVA) were assessed at 1 week, 1 month, 6 months, and 12 months after implantation. Both BCDVA and UCDVA were evaluated at 4 m using the Early Treatment Diabetic Retinopathy Study chart under photopic conditions (~85 cd/m2) and summarized using descriptive statistics. The difference between predicted refraction and manifest refraction was evaluated at all visits.
Presence of PCO and its clinical relevance (absent, clinically non-significant, clinically significant, or clinically significant requiring Nd:YAG capsulotomy) was recorded at all follow-up visits.
Intraocular lens clarity assessments included evaluations of surface haze and glistenings at all follow-up visits. Using a specific slit lamp configuration, investigators reported the presence or absence of surface haze and quantified the glistenings according to a grading scale adapted from Miyata et al.14
An IOL Delivery System Performance Questionnaire (a 17-item instrument asking surgeons’ preferences) was used to assess the ease of IOL insertion, hand posture of the surgeon, the control over IOL delivery, and surgeon satisfaction. Surgeons with a minimum of 14 implantations were included in this assessment (one eligible surgeon from each site).
Safety
Safety endpoints included adverse events (AEs), device deficiencies, and surgical complications. AEs were reported from the time informed consent was signed to study exit and summarized using descriptive statistics. Secondary surgical interventions were also included in the safety assessment.
Results
Subjects
A total of 151 eyes (111 patients) were implanted with the study IOLs (Supplementary Figure 1). At 1 year, 14 eyes were lost to follow-up, and 137 eyes (102 patients) completed the study. Mean age was 59.7±9.3 years, and 58/111 (52%) patients were female. Mean baseline corneal astigmatism was 0.59±0.28 and 0.45±0.26 D in the first and second eyes, respectively. Detailed demographics and baseline characteristics of the population are shown in Table 2.
 |
Table 2 Patient Demographics and Baseline Characteristics (All-Implanted Analysis Set) |
Visual Acuity Outcomes
At 1 month after implantation, 135/138 eyes (98%; 95% CI, 92–100%) achieved BCDVA of 0.2 logMAR or better. Mean ± SD monocular BCDVA improved from 0.53±0.44 logMAR preoperatively to 0.00±0.08 logMAR at week 1 after implantation and remained stable at −0.03±0.08 logMAR 12 months after implantation (Figure 2A; Supplementary Table 2). The cumulative distribution of monocular BCDVA in patients was stable between 1 and 12 months. Monocular BCDVA of 0.00 logMAR or better was achieved by 96/138 (70%) and 111/137 (81%) eyes at month 1 and 12, respectively. Monocular BCDVA of 0.3 logMAR or better was achieved by 137/138 (99%) and 137/137 (100%) eyes at months 1 and 12, respectively (Figure 2B).
Mean ± SD monocular UCDVA was 0.78±0.40 logMAR preoperatively, 0.11±0.15 logMAR at week 1, and 0.08±0.13 logMAR at 12 months after implantation (Figure 3A; Supplementary Table 2). The cumulative distribution of monocular UCDVA is summarized in Figure 3B. Monocular UCDVA of 0.00 logMAR or better was achieved by 49/138 (36%) and 51/137 (37%) eyes at months 1 and 12, respectively. Monocular UCDVA of 0.3 logMAR or better was achieved by 121/138 (88%) and 132/137 (96%) eyes at months 1 and 12, respectively.
Absolute prediction error remained stable throughout the study. Mean ± SD absolute prediction error was 0.24±0.17 D at week 1 and 0.25±0.17 D at month 12 (Figure 4A; Supplementary Table 2). At 12 months, 124/137 (91%) of all eyes were within 0.5 D, and 137/137 (100%) were within 1.0 D (Figure 4B).
 |
Figure 4 Mean absolute prediction error (A) and cumulative distribution of absolute prediction error (B). Error bars represent 95% CI. |
Intraocular Lens Clarity Outcomes and Posterior Capsular Opacification Observations
None of the eyes implanted with the CNA0T0 IOL presented any surface haze, and all were graded as 0 glistenings at all visits throughout the 1-year postoperative follow-up. There were no observations for grade 1 or above glistenings. There was no clinically significant PCO reported, and no Nd:YAG capsulotomy was performed.
Surgeon-Reported Preferences
Seven surgeons from 7 study sites met the criteria to respond to the surgeon’s preferences questionnaire. Delivery systems currently used by the surgeons included Monarch (Alcon Vision LLC), Tecnis iTec (Johnson & Johnson Vision Care, Inc., Santa Ana, CA, USA), and Vivinex iSert (Hoya Surgical Optics, Inc., Chino Hills, CA, USA). Surgeons observed the automated injector system used in this study to be more intuitive for delivering the IOL compared with push- or screw-style preloaded injector systems. In all cases, preparation of the CNA0T0 preloaded in the automated injector before implantation was considered “very easy” or “easy.” IOL insertion was “very easy” or “easy”, and IOL delivery was “very controllable” or “controllable” (Supplementary Table 3).
Safety Outcomes
There were 2 serious intraoperative AEs reported: posterior capsule rupture on the day of the surgery (n=1) and ciliary zonular dehiscence (n=1; Table 3). The most common nonserious ocular treatment-emergent AEs were meibomianitis (first eye, n=2; second eye, n=2) and corneal edema (first eye, n=3; Table 3). There were 6 cases reported as device deficiencies (difficult to fold or unfold, n=5; delivery system issue, n=1).
 |
Table 3 Ocular Treatment-Emergent Adverse Events (Safety Analysis Set) |
Discussion
This study was designed to gather real-world experience in an Indian population on the clinical and safety outcomes of the hydrophobic IOL preloaded in an automated injector system. The use of this device resulted in good visual and refractive outcomes in this multicenter 1-year study. At 12 months after implantation, mean BCDVA was −0.03 logMAR, and mean UCDVA was 0.08 logMAR. Most eyes achieved BCDVA and UCDVA of 0.2 logMAR or better (99% and 83%, respectively). At 12 months, 100% of eyes had absolute prediction error within 1.0 D. None of the eyes presented clinically relevant PCO (no Nd:YAG capsulotomy was performed). The CNA0T0 IOL demonstrated high clarity characteristics with no surface haze reported, and all eyes presented with grade 0 glistenings. The automated injector received high marks for usability and performance from surgeons.
The visual outcomes of this study are consistent with previous reports for Clareon IOLs. In a recent single-arm multicenter clinical trial in 350 patients in the United States, Clareon IOLs implanted using a manual delivery system (Monarch III D cartridge and handpiece, Alcon Vision LLC) demonstrated mean monocular BCDVA of −0.05 logMAR at 12 months after implantation; 99% of patients achieved monocular BCDVA of 0.24 logMAR or better at 12 months.4 Mean monocular UCDVA was 0.04 logMAR at month 12, and 94% of patients achieved UCDVA of 0.24 or better. Absolute prediction error was within 1.0 D in 98% of patients at month 12.4 Similarly, in a 1-year study in 384 patients in Japan, the Clareon IOL demonstrated good visual acuity and low postoperative refractive error. At 12 months, 95% of eyes achieved BCDVA of 20/25 (~0.2 logMAR) or better, and refractive error in 90% of eyes was within 1.0 D.6 A 4- to 6-week follow-up study that evaluated the Clareon AutonoMe system in 144 eyes in Germany also reported good visual acuity and low postoperative refractive error; in patients without ocular comorbidities, BCDVA improved from 0.27 to 0.03 logMAR.15
The material and design of the Clareon IOL were aimed to reduce the risk of PCO and to achieve low levels of surface haze and glistenings. The current study reported no clinically significant PCO throughout the 12 months. A meta-analysis with Clareon and AcrySof IOLs estimated that the probability of Nd:YAG capsulotomy within the first 3 years was numerically lower with Clareon IOLs (2%) compared with AcrySof IOLs (4%).8 Overall incidence of Nd:YAG was expected to be 0.62 cases per 100 surgeries per year for Clareon compared with 1.46 per 100 surgeries per year for AcrySof IOLs. However, PCO rates can vary among studies; Japanese patients reported that 0.9% of eyes (2/230) required Nd:YAG capsulotomy during 12 months of follow-up.6 In another study, Nd:YAG capsulotomy was performed in 1.5% of eyes during the 12-month follow-up.16
The surface roughness of the IOL, measured using atomic force microscopy, may affect the incidence of PCO.17 Surface roughness has also been shown to affect surface haze; the Clareon IOL had a smoother surface and was associated with a lower surface haze compared with other commercially available IOLs (Tecnis ZCB00, Tecnis Obtiblue ZCB00V, enVista MX60 [Bausch & Lomb, Rochester, NY, USA], and Eternity W-60 [Santent, Inc., Osaka, Japan]).5,18 In the current clinical study, none of the eyes presented with surface haze, supporting the findings from these in vitro reports. Our results were consistent with previous in vitro and in vivo clinical studies that reported grade 0 glistenings at 12 months in patients implanted with Clareon IOLs.4–6,16
The edge architecture of the Clareon IOL contributed to the low PCO formation and reduced the edge-reflected and edge-transmitted glare. A study using ray tracing demonstrated that the Clareon IOL produced less edge glare compared with other commercially available IOLs (Tecnis ZCB00, enVista MX60, Eternity W-60, and Vivinex XY1 [Hoya Surgical Optics, Inc.]).7
In the current study, AutonoMe received high marks for usability and performance from surgeons in India, which was consistent with responses collected from surgeons in Japan.6 None of the patients in this study of the single-use preloaded device had endophthalmitis. Previous assessments of AutonoMe found few technical issues associated with IOL delivery and reported no serious or severe events.10 A previous study (n=40) that evaluated corneal tissue trauma and corneal incision structure found that the corneal thickness increase at the incision was smaller for AutonoMe compared with the Monarch III device at 1 hour and 1 day after implantation.11 In another clinical study (n=48) that analyzed effects of different injector systems on the architecture and morphology of corneal incisions, AutonoMe produced less trauma to the wound and reduced wound retraction compared with other delivery systems.19 Furthermore, AutonoMe devices were associated with a more precise and regular architecture of the corneal incision that appeared less stressed compared with Monarch III devices.19
Limitations of this study include a lack of a comparator group, descriptive analyses of the data, a small number of surgeons responding to the questionnaire, and potential for bias. No toric Clareon IOLs were available during this study; hence, the refractive outcomes may be affected by eyes with astigmatism near 1 D. Future studies will need to provide a more in-depth description of device malfunctions and an analysis of the surgeons’ learning curve. Strengths of the study include its real-world setting, 1-year follow-up, and multicenter design.
In conclusion, this real-world study evaluated clinical outcomes and safety of the hydrophobic CNA0T0 IOL implanted using an automated injector delivery system in an Indian population. Most eyes achieved good visual and refractive outcomes. Only grade 0 glistenings were observed, and there were no reports of surface haze. No eyes required Nd:YAG capsulotomy. The automated, single-use, preloaded injector system was reported to be intuitive and easy to use by surgeons.
Data Sharing Statement
The data used to support the primary findings of this study are available upon reasonable request from the study sponsor, Alcon Research LLC.
Acknowledgments
Medical writing assistance was provided by Natalia Zhukovskaya, PhD, of ICON plc (Blue Bell, PA), and was funded by Alcon.
Funding
This study was funded by Alcon Research LLC. Alcon assisted with the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Disclosure
JS Titiyal, SK Basak, S Ganesh, P Padmanabhan, N Shetty, and U Mathur have no conflict of interest to disclose. A Dey is an employee of Alcon. D Ramamurthy is a consultant to Alcon.
References
1. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi:10.1016/S2214-109X(17)30393-5
2. Vashist P, Senjam SS, Gupta V, et al. Blindness and visual impairment and their causes in India: Results of a nationally representative survey. PLoS One. 2022;17(7):e0271736 doi:10.1371/journal.pone.0271736.
3. Lundstrom M, Barry P, Henry Y, Rosen P, Stenevi U. Evidence-based guidelines for cataract surgery: guidelines based on data in the European Registry of Quality Outcomes for Cataract and Refractive Surgery database. J Cataract Refract Surg. 2012;38(6):1086–1093. doi:10.1016/j.jcrs.2012.03.006
4. Lehmann R, Maxwell A, Lubeck DM, et al. Effectiveness and safety of the Clareon monofocal intraocular lens: outcomes from a 12-month single-arm clinical study in a large sample. Clin Ophthalmol. 2021;15:1647–1657. doi:10.2147/OPTH.S295008
5. Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J Cataract Refract Surg. 2019;45(10):1490–1497. doi:10.1016/j.jcrs.2019.05.017
6. Oshika T, Sasaki N, Clinical Study Group on New Intraocular Lens and Delivery System. One-year multicenter evaluation of a new hydrophobic acrylic intraocular lens with hydroxyethyl methacrylate in an automated preloaded delivery system. J Cataract Refract Surg. 2022;48(3):275–279. doi:10.1097/j.jcrs.0000000000000746
7. Das KK, Werner L, Collins S, Hong X. In vitro and schematic model eye assessment of glare or positive dysphotopsia-type photic phenomena: comparison of a new material IOL to other monofocal IOLs. J Cataract Refract Surg. 2019;45(2):219–227. doi:10.1016/j.jcrs.2018.09.017
8. Von Tress M, Marotta JS, Lane SS, Sarangapani R. A meta-analysis of Nd:YAG capsulotomy rates for two hydrophobic intraocular lens materials. Clin Ophthalmol. 2018;12:1125–1136. doi:10.2147/OPTH.S161380
9. Liu J, Wolfe P, Hernandez V, Kohnen T. Comparative assessment of the corneal incision enlargement of 4 preloaded IOL delivery systems. J Cataract Refract Surg. 2020;46(7):1041–1046. doi:10.1097/j.jcrs.0000000000000214
10. Oshika T, Sasaki N. Experimental study on delivery performance of an automated preloaded intraocular lens injector system for corneal and sclerocorneal incisions. J Ophthalmol. 2021;2021:5548493. doi:10.1155/2021/5548493
11. Mastropasqua L, Toto L, D’Ugo E, et al. In vivo and in vitro results of an automated preloaded delivery system for IOL implantation in cataract surgery. Int Ophthalmol. 2020;40(1):125–134. doi:10.1007/s10792-019-01154-0
12. Negishi K, Masui S, Torii H, Nishi Y, Tsubota K. Refractive stability of a new single-piece hydrophobic acrylic intraocular lens and corneal wound repair after implantation using a new automated intraocular lens delivery system. PLoS One. 2020;15(9):e0238366. doi:10.1371/journal.pone.0238366
13. Yildirim TM, Labuz G, Baur ID, et al. Corneal incision enlargement in two preloaded intraocular lens injectors: an intraindividual in vivo study. J Refract Surg. 2021;37(5):331–336. doi:10.3928/1081597X-20210204-01
14. Miyata A, Uchida N, Nakajima K, Yaguchi S. Clinical and experimental observation of glistening in acrylic intraocular lenses. Jpn J Ophthalmol. 2001;45(6):564–569. doi:10.1016/S0021-5155(01)00429-4
15. Bedar MS, Kellner U. Klinische Erfahrung mit der Clareon®-IOL und dem AutonoMe®-Implantation system [Clinical experience with the ClareonⓇ IOL and the AutonoMeⓇ implantation system]. Ophthalmologe. 2020;117(11):1100–1104. German. doi:10.1007/s00347-020-01075-9
16. Stanojcic N, O’Brart D, Hull C, et al. Visual and refractive outcomes and glistenings occurrence after implantation of 2 monofocal, aspheric, hydrophobic acrylic IOLs. J Cataract Refract Surg. 2020;46(7):986–994. doi:10.1097/j.jcrs.0000000000000201
17. Lombardo M, De Santo MP, Lombardo G, Barberi R, Serrao S. Analysis of intraocular lens surface properties with atomic force microscopy. J Cataract Refract Surg. 2006;32(8):1378–1384. doi:10.1016/j.jcrs.2006.02.068
18. De Giacinto C, Porrelli D, Turco G, et al. Surface properties of commercially available hydrophobic acrylic intraocular lenses: comparative study. J Cataract Refract Surg. 2019;45(9):1330–1334. doi:10.1016/j.jcrs.2019.04.011
19. Cennamo M, Favuzza E, Salvatici MC, et al. Effect of manual, preloaded, and automated preloaded injectors on corneal incision architecture after IOL implantation. J Cataract Refract Surg. 2020;46(10):1374–1380. doi:10.1097/j.jcrs.0000000000000295
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


