Back to Journals » OncoTargets and Therapy » Volume 13
Tumor Suppressor microRNA-138 Suppresses Low-Grade Glioma Development and Metastasis via Regulating IGF2BP2
Authors Yang Y, Liu X, Cheng L, Li L, Wei Z, Wang Z, Han G, Wan X, Wang Z, Zhang J, Chen C
Received 28 September 2019
Accepted for publication 25 February 2020
Published 13 March 2020 Volume 2020:13 Pages 2247—2260
DOI https://doi.org/10.2147/OTT.S232795
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Yang Yang,1,2,* Xinyu Liu,3,* Lulu Cheng,2 Li Li,2 Zhenyu Wei,4 Zong Wang,2 Gang Han,2 Xuefeng Wan,2 Zaizhong Wang,2 Jianhua Zhang,5 Chuanliang Chen1
1Henan Key Laboratory for Medical Imaging of Neurological Diseases, People’s Hospital of Zhengzhou University, Zhengzhou 450003, People’s Republic of China; 2Department of Neurosurgery, Zhumadian Central Hospital, Zhumadian 463000, People’s Republic of China; 3School of Intelligent Manufacturing, The Huanghuai University, Zhumadian 463000, People’s Republic of China; 4Department of Neurosurgery, Second Affiliated Hospital of Xinxiang Medical College, Xinxiang 453000, People’s Republic of China; 5Medical Engineering Technology and Data Mining Institute of Zhengzhou University, Zhengzhou 450000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chuanliang Chen
Henan Key Laboratory for Medical Imaging of Neurological Diseases, People’s Hospital of Zhengzhou University, Zhengzhou 450003, People’s Republic of China
Email [email protected]
Background: Low-grade gliomas (LGG), approximately constitute one-third of all types of gliomas, are prone to relapse and metastasis. MicroRNA-138 (miR-138) is reported to be dysregulated in diverse human tumors and mainly function as a tumor suppressor. In this study, we analyzed the expression profile and function of miR-138 in LGG.
Methods: Quantitative PCR (qPCR) and public database bioinformatics analysis were performed to determine the miR-138 levels in LGG. MiR-138 overexpression in LGG cells was achieved by miR-138 mimics transfection. Cell proliferation was assessed by CCK8, EdU and colony formation assays. Cell invasion and migration were analyzed by transwell and wound-healing assays. Xenograft model was employed to study the role of miR-138 in LGG growth in vivo. The target of miR-138 was validated by multiple methods, such as luciferase reporter assay, RT-qPCR and Western blot. Bioinformatics analysis was conducted to explore the molecular mechanisms by which miR-138 contributed to LGG progression.
Results: miR-138 was significantly downregulated in LGG tumor tissues and low expression of miR-138 was significantly associated with poor prognosis as well as relapse and metastasis in LGG patients. Functional analysis indicated that ectopic miR-138 expression suppressed LGG cell growth and invasive phenotype in vitro, and inhibited tumor development in vivo. Moreover, miR-138 directly targeted and repressed insulin-like growth factor 2 mRNA binding protein 2 (IGF2BP2) by targeting the 3ʹ-UTR of IGF2BP2, inhibiting epithelial to mesenchymal transition (EMT) to attenuate LGG aggressiveness. In addition, we found that elevated IGF2BP2 expression correlates with poor survival of LGG patients.
Conclusion: miR-138 may function as a tumor inhibitor by directly inhibiting IGF2BP2 and suppressing EMT in the progression of LGG.
Keywords: miR-138, tumor prognosis, LGG, IGF2BP2, EMT
Introduction
Glioma is a common type of brain tumor. Despite the technical advances like surgical resection and chemo-radiotherapy, the prognosis of glioma patients is dismal.1 Low-grade gliomas (LGG) account for one-third of all gliomas.2 Though LGG is typically slow growing, it has much high morbidity and mortality due to the recurrence and malignant progression.3,4 Therefore, it is critical and essential to identify new targets and develop novel strategies for treatment of patients with LGG.
MicroRNAs (miRNAs) are small non-coding RNAs post-transcriptionally regulating target gene expression.5 Accumulating reports have demonstrated that miRNAs participate in various biological processes, such as cell proliferation, migration, inflammation, and apoptosis.6 Moreover, accumulating studies have been focused on the important roles of miRNAs in the development of diverse cancers.7 Though the functional role of miRNAs and their underlying molecular mechanism in LGG has not been well investigated.
MiR-138, which is located in the chromosomal region 3q44, has been discovered to be dysregulated in diverse human tumors and mainly function as a tumor inhibitor. Decreased miR-138 expression was reported to be associated with poor prognosis in colon cancer.8 Gao et al found that miR-138-5p could reverse gefitinib resistance in NSCLC cells.9 It has been documented that MiR-138 inhibited NSCLC cell growth through repression of EZH2.10 Li et al showed that miR-138 posttranscriptionally regulated LIMK1 to suppress the proliferation of breast cancer cells.11 These studies have revealed an important tumor suppress function of miR-138 in various cancers. Moreover, miR-138 has been demonstrated to function as a tumor suppressor in glioblastoma (GBM) via regulating EZH2/CDK4/6-E2F1 signal loop.12 Wei et al reported that miR-138 exerted anti-glioma efficacy via targeting immune checkpoints CTLA4 and PD-1.13 However, the expression profile and function of miR-138 in LGG are not fully addressed.
In this study, we evaluated the expression pattern and clinical relevance of miR-138 in LGG. MiR-138 overexpression suppressed LGG cell growth, invasion and migration in vitro, and inhibited tumor development in vivo. Insulin-like growth factor 2 mRNA binding protein 2 (IGF2BP2) was validated as a direct target of miR-138 while overexpression of IGF2BP2 could partly abolished the inhibition of LGG cell growth and invasion mediated by miR-138. Mechanistically, we revealed that miR-138/IGF2BP2 axis contributed to LGG progression through regulating EMT process. This discovery may lead to the development of therapeutic interventions for LGG.
Materials and Methods
Human Specimens
A total of 89 patients diagnosed pathologically and clinically with LGG were recruited from the brain surgery department of People’s Hospital of Zhengzhou University. LGG pathological diagnosis was confirmed by histological examination, and classified according to WHO classification system.14 Distant healthy normal tissues be defined as normal brain tissues which 3 cm away from tumor tissues. Tissue samples were collected during surgical resection and were immediately frozen in liquid nitrogen for RNA extraction, as well as stored as formalin-fixed embedded tissue. All patients provided written informed consent and the experiment was conducted in accordance with the Declaration of Helsinki and approved by the Zhengzhou University Institutional Review Board.
Identify the Best Cutoff Value for Survival Analysis
The detailed procedures were described in Supplementary Methods.
Immunohistochemical (IHC) Staining
For IHC staining, antigen-retrieved sections were washed with PBS and PBST (0.1% v/v). After slides were blocked with 3% BSA for 1 h at room temperature, slides were added antibodies (IGF2BP2 1:200 dilution, proteintech, China) and incubated for 1 h at room temperature. Detection was carried out using the HRP-DAB system (Millipore, USA). Sections were semi-quantitatively scored for the IGF2BP2 staining patterns as follows: the staining intensity was quantified as negative, weak, intermediate or strong. For statistical purposes, scores of intermediate and strong were defined as high expression, while negative and weak scores were considered as low expression. Categorizing the IGF2BP2 staining was completed by two independent investigators, who were blinded to the clinicopathologic data.
Cell Culture
LGG cells Res186 and Res259 were from ATCC (Manassas, VA, USA) and cultured following the ATCC cell culture guidelines in an incubator at 37°C with 5% CO2.
RT-qPCR
Total RNA was extracted using the TRIzol Reagent (Life Technologies Inc., Carlsbad, CA, USA), which was maintained at −80°C refrigerator for preservation. RNA was used for cDNA synthesis with the Superscript III Reverse Transcription Reagent (Life Technologies). MiRcute miRNA isolation kit (Tiangen, China) was used for miRNA extraction. Qiagen miScript Reverse Transcription kit (Qiagen, Germany) was used for reverse transcribe. Following the formula recommended by FastStart Universal SYBR Premix Ex Taq™ II (Takara, Japan), the formula containing 14 μL 2 × SYBER Green master mix, 1 μL forward primer (10 μM), reverse primer 1 μL (10 μM), 3 μL cDNA template, 6 μL ddH2O was set up. Later, the reaction system was reacted in Bio-Rad IQ5 thermocycler (Bio-Rad, CA, USA) following the procedures: 90 s at 95°C for start, 25 s at 95°C, 20 s at 65°C, 30 s at 72°C for the amplification. Forty cycles were performed. GAPDH and U6 were used as controls.
Cell Viability Assay
The CCK8 kit (Solarbio, China) was used to assess cell growth following the manufacturer’s protocol. DNA synthesis rate of LGG cells was measured by the EdU staining kit (Ribobio, China). All results were repeated for at least three independent experiments.
Cell Migration and Invasion Assay
Wound-healing assay was utilized to assess the LGG cell migration. 1 × 106 LGG cells were seeded into 6-well plates and cultured for 24 hrs. The wound gaps were created by gently scratching with a pipette. After 24 hrs, the wound width was measured under an inverted microscope. Cell invasion was examined by transwell chamber (Corning, NY, USA) covered with or without matrigel (Corning, NY, USA). A total of approximately 1 × 105 cells were seeded into the top chamber of 24-well plates, while 20% FBS was put into the bottom chamber. The cells migrated or invaded were fixed with 4% paraformaldehyde, staining with Crystal violet and counted with an inverted microscope after 24 hrs.
Luciferase Reporter Assay
IGF2BP2 3ʹUTR containing the wild type or mutated binding sites was cloned into psi-CHECKTM-2 luciferase vector (Promega, USA), respectively. HEK293T cells were transfected with psiCHECKTM-2-Wt-IGF2BP2 or psiCHECKTM-2-mut-IGF2BP2, together with miR-138 mimics, inhibitor or the negative control (GenePharma, China) by Lipofectamine 2000 (Invitrogen, USA). Forty-eight hours later, relative luciferase activities were assessed using the Dual-Luciferase Reporter Assay System (Promega, USA).
Plasmid Construction and Transfection
For IGF2BP2 overexpression, the plasmid pcDNA™3.1 (Invitrogen, USA) was designed to construct pcDNA3.1-IGF2BP2. For transient miRNAs transfection, 3×104 cells/well in 12-well plates were cultured for 24 hrs before transfected with 200 nM pcDNA3.1-IGF2BP2, miRNAs using Lipofectamine 2000.
Western Blot
Proteins were separated by SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membrane. After blocking in 5% BSA/PBST for 2 hrs, the PVDF membrane was probed with WTAP (60188-1-Ig, Proteintech, China), E-cadherin (20874-1-AP, Proteintech, China), N-cadherin (22018-1-AP, Proteintech, China), Slug (ab106077, Abcam, USA), Snail (ab53519, Abcam, USA), β-catenin (51067-2-AP, Proteintech, China), GAPDH (60004-AP, Proteintech, China). Protein expression was detected using enhanced chemiluminescence (KeyGen, Nanjing, China).
Xenograft Model
All animal studies were carried out with the approval of the People’s Hospital of Zhengzhou University Animal Care and Use Committee. Twelve male BABL/c nude mice were obtained from the Shanghai SLAC Animal Center (Shanghai, China). Animals were randomly separated into two groups (NC group or miR-138 overexpression group). Res186 cells (5 x 106 cells) were implanted into the back flanks of the nude mice. The tumor size was recorded every week. The volume of the tumors was calculated: volume (mm3) = length × width2/2. After 5 weeks, the mice were euthanized, and tumors were removed and weighted. This animal experiment was approved by the Animal Ethics Committee of Zhengzhou University. In addition, Guide for the Care and Use of Laboratory Animals was strictly followed in the present study.
Statistical Analysis
All results were presented as mean ± SD (standard deviation). Statistical analysis was performed in GraphPad Prism, version 5.0 (GraphPad Prism Software, GraphPad, San Diego, CA), using the Mann–Whitney test for comparison of two groups and one-way ANOVA followed by Tukey post hoc test for two or more groups. The analysis of correlation between factors was performed by Pearson’s correlation coefficient rank test. The clinicopathological factors differences between miR-138 high- or low-expression groups were analyzed via Chi-square test. Kaplan–Meier and Log-rank test method were performed to determine the survival rate. Prognostic factors for overall survival and recurrence-free survival were identified by univariate and multivariate analyses using the Cox proportional hazards regression model. P-value < 0.05 was considered statistically significant.
Results
miR-138 Is Low-Expressed in LGG Tissues and Associated with Poor Prognosis
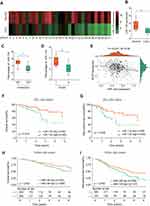
We first examined miR-138 expression levels in a paired LGG cohort (n = 31) by RT-qPCR. The results demonstrated that the expression of miR-138 in LGG was markedly (p < 0.05) lower compared to that in distant healthy normal tissues (3 cm away from tumor tissues) (Figure 1A and B). LGG patients with low miR-138 expression had a higher rate of metastasis (Figure 1C, Supplementary Table 1). Moreover, lower level of miR-138 expression was associated with higher LGG tumor grade (Figure 1D). In addition, miR-138 expression was negatively associated with the cancer proliferation marker Ki67 expression (Figure 1E, p≤0.001, R=−0.26). Taken together, these results indicate that miR-138 expression is down-regulated in human LGG tissues.
To investigate the association between miR-138 expression and LGG progression, we evaluated the clinicopathological features of 89 patients with LGG. Kaplan–Meier survival analysis and Cox proportional hazards regression showed that low-expression levels of miR-138 were significantly associated with shorter overall survival (OS) and recurrence-free survival (RFS) (Figure 1F and G, Supplementary Table 2). The results were further validated by analyzing the TCGA database containing 503 LGG patients (Figure 1H and I). Thus, we hypothesize that low level of miR-138 may promote LGG progression.
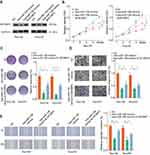
miR-138 Suppresses Proliferation and Invasion of LGG Cells in vitro
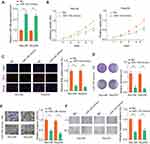
To explore the function of miR-138 in LGG tumorigenesis, Res186 and Res259 cells were overexpressed miR-138 (Lenti-miR-138) or negative control (NC), as evaluated by RT-qPCR (Figure 2A). CCK-8 and colony formation assays demonstrated that miR-138 overexpression remarkably inhibited the growth of LGG cells (Figure 2B and C). EdU staining assays revealed a decreased DNA synthesis rate after miR-138 overexpression (Figure 2D). Additionally, transwell invasion and wound-healing assays were performed to evaluate the role of miR-138 on the invasion of LGG cells. As shown in Figure 2E and F, ectopic miR-139 expression markedly attenuated the metastasis ability of LGG cells. Taken together, our results indicate that miR-138 suppresses LGG cell aggressiveness.
miR-138 Suppresses LGG Tumor Development in vivo
To further confirm the tumor inhibition function of miR-138 in vivo, we set up a xenograft tumor model using Res259 cells. The results of tumor growth through in vivo image analysis indicated that the tumor volumes of Lenti-miR-138 group were markedly smaller than that in control group (Figure 3A). Tumors from Lenti-miR-138 group had smaller tumor volume (Figure 3B), less photon flux (Figure 3C) and decreased tumor weight (Figure 3D) than those from NC group. Consistently, Ki-67 IHC staining showed that the tumor tissues from Lenti-miR-138 group exhibited significantly reduced expression of Ki-67 compared with that in tumors from NC group (Figure 3E and F). These findings suggest that miR-138 suppresses LGG tumor development in vivo.
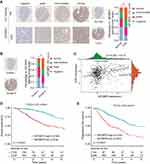
miR-138 Negatively Regulates IGF2BP2 in LGG Cells
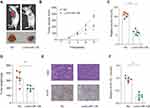
Given the findings that miR-138 inhibited the growth of LGG cells, we next investigated the potential underlying mechanism. Publicly available algorithm TargetScan was used to search for the target of miR-138. Among hundreds of predicted targets, insulin-like growth factor 2 mRNA binding protein 2 (IGF2BP2) was chosen not only for the reason that it was identified as an oncogene but also because of its relatively satisfactory scores of predicted binding sites (Figure 4A). The predicted complementary binding sequences between miR-138 and IGF2BP2 were shown in Figure 4A. Luciferase reporter vectors containing wild-type 3ʹ-UTR of IGF2BP2 or mutated 3ʹ-UTR of IGF2BP2 were constructed and luciferase reporter assay confirmed that miR-138 inhibited luciferase activity in HEK293 cells transfected with reporter plasmid containing WT IGF2BP2 3ʹ-UTR, but not in HEK293 cells transfected with reporter plasmid containing mutant IGF2BP2 3ʹ-UTR (Figure 4B). RT-qPCR (Figure 4C) and Western blot (Figure 4D) results demonstrated that the IGF2BP2 expression was suppressed in LGG cells with miR-138 overexpression, and IGF2BP2 expression was enhanced upon miR-138 inhibitor treatment. Moreover, Pearson correlation analysis found that miR-138 expression was negatively correlated with the expression of IGF2BP2 in TCGA datasets (Figure 4E, p≤0.001, R=−0.34). IHC staining of IGF2BP2 also confirmed that miR-138 expression was negatively associated with IGF2BP2 expression in our independent ZZU LGG cohort (Figure 4F). Together, these data validate that IGF2BP2 is a direct target of miR-138 and miR-138 negatively regulates IGF2BP2 in LGG.
Overexpression of IGF2BP2 Partially Reverses the Suppressive Function of miR-138 in LGG Cells
To further validate the interaction between miR-138 and IGF2BP2, Res186 and Res259 cells were transfected with miR-138 mimics, negative control, with or without IGF2BP2 overexpression vector (Figure 5A). Cell proliferation assessed by CCK-8 showed that ectopic IGF2BP2 partially reversed the suppressive effect of miR-138 in LGG cells (Figure 5B). Similarly, overexpression of IGF2BP2 restored DNA colony formation ability inhibited by miR-138 overexpression (Figure 5C). Additionally, overexpression of IGF2BP2 partially relieved the inhibition of invasion and migration ability caused by miR-138 overexpression (Figure 5D and E). The data confirm that miR-138 suppresses LGG cell growth and invasion by negatively regulating IGF2BP2 expression.
Clinical Relevance of IGF2BP2 Expression in LGG
IGF2BP2 has been documented to be frequently upregulated in various human cancers, including glioma.15–18 However, its expression pattern and prognostic value in LGG remain unclear. We performed IHC staining of IGF2BP2 using the LGG or paired normal control samples obtained from ZZU LGG TMA cohorts and the results revealed the significantly elevated expression of IGF2BP2 in LGG tissues (Figure 6A). In addition, IGF2BP2 expression was remarkably higher in LGG patients with advanced TNM stage (Stage III–IV) than that in patients with TNM stage I–II (Figure 6B). Moreover, Pearson correlation analysis found that expression of IGF2BP2 was positively correlated with the expression of Ki67 (Figure 6C, p≤0.001, r=0.31). TCGA-LGG cohort analysis indicated that LGG patients with higher IGF2BP2 expression had significantly poor OS and RFS than those with lower IGF2BP2 expression (Figure 6D and E). Thus, we further confirm the prognostic value of IGF2BP2 in an independent ZZU LGG TMA cohort.
miR-138/IGF2BP2 Contributes to Progression of LGG Through Epithelial–Mesenchymal Transition/β-Catenin Pathway
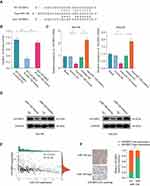
To analyze the molecular mechanisms by which miR-138/IGF2BP2 promotes growth, migration and invasion in LGG cells, we conducted comprehensive bioinformatics analysis based on TCGA database. Gene Set Variation Analysis (GSVA) results showed that high level of IGF2BP2 expression was associated with the activation of EMT pathway (Figure 7A). Consistently, pathway enrichment analysis showed that focal adhesion and ECM receptor interaction pathway were markedly enriched in IGF2BP2-high expression samples, indicating the promoting-metastasis role of IGF2BP2 in LGG (Figure 7B and C). Moreover, the GSEA further confirmed the significant positive correlation between high IGF2BP2 expression and EMT-related gene signatures (Figure 7D and E). To further confirm whether miR-138/IGF2BP2 contributes to progression of LGG through promoting EMT process, rescue experiments were performed. As shown in Figure 8A, miR-138 overexpression led to markedly decrease in EMT-related proteins (N-cadherin, Slug, β-Catenin) and increase of E-cadherin, which were reversed by ectopic IGF2BP2 expression. Furthermore, IHC staining results confirmed the effect of miR-138 overexpression on expression of EMT-related proteins in nude mice tumor tissues (Figure 8B). In summary, these findings suggest that miR-138/IGF2BP2 may contribute to progression of LGG through regulating EMT process.
Discussion
Previous studies have indicated that up- or downregulation of miRNAs is frequently observed in glioma tissues, and is involved in the glioma progression. For example, miR-128 could inhibit cell self-renewal by antagonizing Bmi-1.19 MiR-146 inhibited tumor metastasis by negatively regulating MMPs in glioma.20 Peng et al demonstrated that low miR-200b expression suppressed LGG cell growth and metastasis through targeting CREB1.21
MiR-138 is highly enriched in the brain and regulates dendritic spine morphogenesis via targeting acyl-protein thioesterase 1 (APT1).22 It is clear that miR-138 has a multifaceted role in glioma and its regulatory function remains complicated. Low expression of miR-138 was found in GBM and associated with poor survival of GBM patients.12 MiR-138 promoted acquired alkylator resistance in GBM via targeting the Bcl2-interacting mediator.23 However, other studies indicated that miR-138 might be an oncogenic miRNA as miR-138 was highly expressed in tumor-initiating glioma stem cell.24,25 In this study, we found that miR-138 was significantly low-expressed in human LGG tissues in comparison with that in control tissues. Low expression of miR-138 was positively associated with metastasis, higher grade and poor prognosis of LGG patients. Interestingly, integrated mRNAseq and microRNAseq analysis suggested that miR-138 was closely associated with LGG patient outcome and could be prognostic miRNA signature.26 These results indicate that miR-138 is a tumor suppressor in the development of LGG.
In general, miRNAs exert their functions through regulating their specific target genes.6 In the present study, IGF2BP2 was discovered as a target of miR-138 by bioinformatics analysis. Further, we demonstrated that the IGF2BP2 was significantly upregulated in LGG patient specimens and high expression of IGF2BP2 was negatively associated with poor prognosis in LGG patients. Moreover, we found that IGF2BP2 overexpression attenuated the inhibitory function of ectopic miR-138 expression on cell proliferation and invasion. Theses finding indicate IGF2BP2 may act as oncogene in LGG. Consistent with our study, increasing evidence has confirmed that IGF2BP2 plays a crucial oncogenetic role in diverse human cancers, including rhabdomyosarcoma,27 breast cancer,28 colorectal cancer,16 non-small cell lung cancer, gastric cancer,18 and glioblastoma.29 Taken together, these results strongly sustain that miR-138/IGF2BP2 axis plays a critical role in the pathogenesis and worse prognosis of LGG.
To further explore the molecular mechanisms by which miR-138 contributes to LGG progression, bioinformatics analysis was conducted and the result indicated that high IGF2BP2 expression was markedly correlated with gene signatures of EMT. Aberrant expression of E-cadherin, Vimentin and Snail is correlated with tumor metastasis. In accordance with our findings, a variety of miRNAs has been reported to contribute to cancer progression through regulating EMT process.30 In the present study, we found that N-cadherin and Slug were downregulated, E-cadherin was upregulated in LGG cells where miR-138 was overexpressed. However, knockdown of IGF2BP2 could abolish these changes. Taken together, these results suggest that the EMT process contributes to the miR-138/IGF2BP2-mediated development of LGG. It is worth mentioning that multiple signaling pathways are involved in the IGF2BP2 function in LGG, as analyzed by GSVA and KEGG. Our preliminary data indicated that IGF2BP2 overexpression also enhanced NF-κB signaling (Supplementary Figure 1). Studies reported that HMGA2 enhanced IGF2BP2 expression by binding to the AT-rich region of the first intron of the IGF2BP2 gene in cooperation with NF-κB.31 Together with our results, there might be a positive feedback mechanism of IGF2BP2/NF-κB signaling loop. Future studies are needed to further address the signaling pathways regulated by IGF2BP2.
Conclusion
For the first time, we demonstrate that miR-138 is downregulated in LGG and low expression of miR-138 is associated with poor clinical prognosis of LGG patients. MiR-138 impedes LGG growth, metastasis and EMT process of LGG cells via negatively regulating IGF2BP2. Collectively, miR-138/IGF2BP2 axis might serve as a promising therapeutic target for LGG treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bush NAO, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev. 2017;40(1):1–14. doi:10.1007/s10143-016-0709-8
2. Blionas A, Giakoumettis D, Klonou A, Neromyliotis E, Karydakis P, Themistocleous MS. Paediatric gliomas: diagnosis, molecular biology and management. Ann Transl Med. 2018;6(12):251. doi:10.21037/atm
3. Tateishi K, Nakamura T, Yamamoto T. Molecular genetics and therapeutic targets of pediatric low-grade gliomas. Brain Tumor Pathol. 2019;36(2):74–83. doi:10.1007/s10014-019-00340-3
4. Carabenciov ID, Buckner JC. Controversies in the therapy of low-grade gliomas. Curr Treat Options Oncol. 2019;20(4):25. doi:10.1007/s11864-019-0625-6
5. Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2019;20(1):5–20. doi:10.1038/s41580-018-0059-1
6. Gregory RI, Shiekhattar R. MicroRNA biogenesis and cancer. Cancer Res. 2011;65(9):3509–3512. doi:10.1158/0008-5472.CAN-05-0298
7. Garzon R, Fabbri M, Cimmino A, Calin GA, Croce CM. MicroRNA expression and function in cancer. Trends Mol Med. 2006;12(12):580–587. doi:10.1016/j.molmed.2006.10.006
8. Long L, Huang G, Zhu H, Guo Y, Liu Y, Huo J. Down-regulation of miR-138 promotes colorectal cancer metastasis via directly targeting TWIST2. J Transl Med. 2013;11(1):1–10. doi:10.1186/1479-5876-11-275
9. Gao Y, Fan X, Li W, Ping W, Deng Y, Fu X. miR-138-5p reverses gefitinib resistance in non-small cell lung cancer cells via negatively regulating G protein-coupled receptor 124. Biochem Biophys Res Commun. 2014;446(1):179–186. doi:10.1016/j.bbrc.2014.02.073
10. Zhang H, Zhang H, Zhao M, et al. MiR-138 inhibits tumor growth through repression of EZH2 in non-small cell lung cancer. Cell Physiol Biochem. 2013;31(1):56–65. doi:10.1159/000343349
11. Li D, Song H, Wu T, et al. MiR-138-5p targeting LIMK1 suppresses breast cancer cell proliferation and motility. RSC Adv. 2017;7(82):52030–52038. doi:10.1039/C7RA09042K
12. Qiu S, Huang D, Yin D, et al. Suppression of tumorigenicity by microRNA-138 through inhibition of EZH2-CDK4/6-pRb-E2F1 signal loop in glioblastoma multiforme. Biochim Biophys Acta. 2013;1832(10):1697–1707. doi:10.1016/j.bbadis.2013.05.015
13. Wei J, Nduom EK, Kong LY, et al. MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints. Neuro Oncol. 2016;18(5):639–648. doi:10.1093/neuonc/nov292
14. Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi:10.1007/s00401-016-1545-1
15. Chen Z, Wu C, Li X, Zhang X, Huang L. miR-1193 inhibits glioma cells proliferation, migration, and invasion via targeting IGF2BP2. J Biomater Tissue Eng. 2018;8:1558–1565. doi:10.1166/jbt.2018.1904
16. Ye S, Song W, Xu X, Zhao X, Yang L. IGF2BP2 promotes colorectal cancer cell proliferation and survival through interfering with RAF‐1 degradation by miR‐195. FEBS Lett. 2016;590(11):1641–1650. doi:10.1002/1873-3468.12205
17. Lochhead P, Imamura Y, Morikawa T, et al. Insulin-like growth factor 2 messenger RNA binding protein 3 (IGF2BP3) is a marker of unfavourable prognosis in colorectal cancer. Eur J Cancer. 2012;48(18):3405–3413. doi:10.1016/j.ejca.2012.06.021
18. Liu X, Chen Z, Zhao X, et al. Effects of IGF2BP2, KCNQ1 and GCKR polymorphisms on clinical outcome in metastatic gastric cancer treated with EOF regimen. Pharmacogenomics. 2015;16(9):959–970. doi:10.2217/pgs.15.49
19. Godlewski J, Nowicki MA, Williams S, et al. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68(22):9125. doi:10.1158/0008-5472.CAN-08-2629
20. Xia H, Qi Y, Ng SS, et al. microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res. 2009;1269:158–165. doi:10.1016/j.brainres.2009.02.037
21. Peng B, Hu S, Jun Q, et al. MicroRNA-200b targets CREB1 and suppresses cell growth in human malignant glioma. Mol Cell Biochem. 2013;379(1–2):51–58. doi:10.1007/s11010-013-1626-6
22. Siegel G, Obernosterer G, Fiore R, et al. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol. 2009;11(6):705–716. doi:10.1038/ncb1876
23. Stojcheva N, Schechtmann G, Sass S, et al. MicroRNA-138 promotes acquired alkylator resistance in glioblastoma by targeting the Bcl-2-interacting mediator BIM. Oncotarget. 2016;7(11):12937–12950. doi:10.18632/oncotarget.v7i11
24. Chan XH, Nama S, Gopal F, et al. Targeting glioma stem cells by functional inhibition of a prosurvival oncomiR-138 in malignant gliomas. Cell Rep. 2012;2(3):591–602. doi:10.1016/j.celrep.2012.07.012
25. Di Pascale F, Nama S, Muhuri M, et al. C/EBPbeta mediates RNA polymerase III-driven transcription of oncomiR-138 in malignant gliomas. Nucleic Acids Res. 2018;46(1):336–349. doi:10.1093/nar/gkx1105
26. Dai J, Bing Z, Zhang Y, et al. Integrated mRNAseq and microRNAseq data analysis for grade III gliomas. Mol Med Rep. 2017;16(5):7468–7478. doi:10.3892/mmr.2017.7545
27. Li Z, Zhang Y, Ramanujan K, Ma Y, Kirsch DG, Glass DJ. Oncogenic NRAS, required for pathogenesis of embryonic rhabdomyosarcoma, relies upon the HMGA2–IGF2BP2 pathway. Cancer Res. 2013;73(10):3041–3050. doi:10.1158/0008-5472.CAN-12-3947
28. Li X, Li Y, Lu H. MiR-1193 suppresses proliferation and invasion of human breast cancer cells through directly targeting IGF2BP2. Oncol Res. 2017;25(4):579–585. doi:10.3727/97818823455816X14760504645779
29. Janiszewska M, Suva ML, Houtkooper RH, Clementschatlo V, Stamenkovic I. The RNA-binding protein Imp2 regulates oxidative phosphorylation that is key to glioblastoma cancer stem cell maintenance. FASEB J. 2012;26.
30. Zare M, Bastami M, Solali S, Alivand M. Aberrantly miRNA promoter methylation and EMT-involving miRNAs in breast cancer metastasis: diagnosis and therapeutic implications. J Cell Physiol. 2017;233(5):3729–3744. doi:10.1002/jcp.26116
31. Cleynen I, Brants JR, Peeters K, et al. HMGA2 regulates transcription of the Imp2 gene via an intronic regulatory element in cooperation with nuclear factor-kappaB. Mol Cancer Res. 2007;5(4):363–372. doi:10.1158/1541-7786.MCR-06-0331
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.