Back to Journals » OncoTargets and Therapy » Volume 9
Tumor heterogeneity as a rationale for a multi-epitope approach in an autologous renal cell cancer tumor vaccine
Authors Wittke S, Baxmann S, Fahlenkamp D, Kiessig S
Received 10 July 2015
Accepted for publication 9 December 2015
Published 27 January 2016 Volume 2016:9 Pages 523—537
DOI https://doi.org/10.2147/OTT.S92182
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Video abstract presented by Wittke et al.
Views: 259
Stefan Wittke,1 Susann Baxmann,2 Dirk Fahlenkamp,3 Stephan T Kiessig2
1University of Applied Sciences Bremerhaven, Faculty of Biotechnology Bremerhaven, 2Ruhr-Plasma-Centre GmbH, Bochum, 3Department of Urology, Zeisigwald Bethanien Hospital, Chemnitz, Germany
Purpose: An autologous tumor vaccine already used successfully in the immune therapy of renal cell carcinoma was investigated in detail. The evaluation of potential tumor markers should allow for the assessment of potency according to pharmaceutical regulations.
Methods: A panel of 36 tumor-associated antigens and cellular marker proteins was characterized in a total of 133 tumor cell lysates by methods such as ELISA, Western blots, and topological proteomics. The induction of tumor-associated antigen-specific antibodies was demonstrated by immunization in mice.
Results: Tumor heterogeneity was demonstrated: none of the tumor-associated antigens investigated were detectable in each tumor lysate. In parallel, the coincidental presence of potential danger signals was shown for HSP-60 and HSP-70. The presence of both antigen and danger signal allowed a successful induction of an immune response in a murine model.
Conclusion: The verified tumor heterogeneity indicates the need for a multi-epitope approach for the successful immunotherapy in renal cell carcinoma.
Keywords: renal cell carcinoma, kidney cancer, tumor-associated antigens, tumor marker, ELISA, Western Blot, immunotherapy, therapeutic vaccine, potency testing, topological proteomics
Introduction
Renal cell carcinoma is an orphan disease with an incidence of less than 1.6:10.000.1 Only about 3% of all malignant tumors in adults develop in the kidney and 85% of these tumors are identified as renal clear cell carcinoma (RCC). Most cases are discovered incidentally and 25%–30% are already in a metastatic stage. The median age of patients at primary diagnosis is 60 years and the male to female ratio is 3:2.
Standard therapy of organ-confined and locally advanced RCC is partial or radical nephrectomy, whereas patients with distant metastasis are often treated with nonspecific immunotherapy or, more recently, with targeted therapy.2–4 The 5-year relative survival rate for patients diagnosed with RCC between 1996 and 2002 was 65.6% in the US compared to 68.5% in Germany (diagnosis between 2000 and 2002).5
After radical nephrectomy, despite the significant stage-related risk of tumor progression, no effective adjuvant treatment without major side effects is currently approved. Drugs that show some efficacy in patients with metastatic RCC such as interferon-α and interleukin-2 (IL-2) failed to demonstrate a benefit in the adjuvant setting.6–8 Until now, only a 1997 initiated prospective randomized Phase III trial showed a significant effect in overall survival after radical nephrectomy accompanied by treatment with an autologous renal tumor cell vaccine.8 Furthermore, by comparing data from a compassionate use program with a historical group of patients observed for more than 10 years and treated by radical nephrectomy, May et al10 demonstrated the same significant effect on the overall survival (42.3 months) for T3 tumors.
In addition, discussions on common tumor markers or tumor-associated antigens (TAA) as potential targets for immunotherapy are ongoing especially since authorities like the European Medical Agency (EMA) and the US Food and Drug Administration request additional information about the potency and potential risks of these autologous applied antigens.11,12 The potential risk might be the induction of an autoimmune disease. In this context, Zinkernagel (personal communication, 2008) defined any immunological antitumor reaction as autoimmune that has to be differentiated from an autoimmune disease.
Thus, the first step of the current study was the protein–chemical characterization of RCCs, followed by a variety of immune (histo-)chemical investigations which depended on the availability of respective mono- or polyclonal antibodies, summarized by Gouttefangeas et al.13 Only a few antigens like carbonic anhydrase IX (CA-IX) were noticed as being present and occurring with high frequency in RCC. Additionally, tumor markers or TAAs in patients’ serum were investigated during the ’70s and ’80s and again, no ambiguous tumor marker for RCC, irrespective of the histological type, could be defined. Even gene-based assays added to the palette in the ’90s gave no clear hint of a common “RCC-specific” antigen.
The overall image is still inconsistent and requires an immunochemical analysis of RCCs as the basis for comparing the later analysis of the immune response against RCC after therapy with a cell lysate-based tumor vaccine.14 The “best” marker so far is CA-IX15,16 found mostly on tumors of the clear cell type, which represent ~75% of all RCC.17 Nonetheless, this marker is neither present in all tumor cells nor throughout the whole tumor.18 In addition, even if a tumor entity is known to express specific markers, such as MAGE-antigens in melanoma or Her2/neu in a subpopulation of mamma carcinomas, not all tumor cells in a given tumor express these markers at all times.19,20 Consequently, if only a single antigen/epitope can be presented to the immune system, it could be assumed that only the respective tumor cell population will be eliminated by the immune response. In the case of a passive immunization (administration of a monoclonal antibody), this phenomenon is well known.21 In summary, this reduces the tumor mass and therefore the progression-free survival; however, this did not cure the disease.
It is also known that TAAs are not present on all cells of a tumor at all times,19,20 potentially being caused by an oligoclonality of the tumor.22 Consequently, in a therapeutic setting, the presence of the target antigen has to first be demonstrated on an individual level.
Moreover, for an immune response, a second requirement has to be fulfilled: the presence of a danger signal such as heat shock proteins (HSPs).23 Apparently, HSPs are an important part of the antitumor immune response. HSPs afford antigen presentation in antigen-presenting cells. Initially, HSPs bind tumor antigens and subsequently enable their uptake by antigen-presenting cells. Latest investigations revealed that HSP–TAA complexes bind to cell surface proteins that among others elicit the initiation of an innate immune response.24 Calderwood et al25 concluded that HSP-60 and HSP-70 can be processed by antigen-presenting cells and that HSP-derived epitopes subsequently activate regulatory T-cells and suppress inflammatory diseases. In summary, both the antigen and the danger signal have to be present in a tumor vaccine.
The primary objective of the current study was to demonstrate that both a pattern of antigens and danger signals are present in a tumor vaccine manufactured according to the Reniale® scheme. For this purpose, immunochemical methods such as ELISA and Western blotting were used.
Antigens were selected by screening ten clear cell RCCs, either to demonstrate antigens which are frequently present in RCC or to confirm the high heterogeneity as determined from the histology. The panel of antigens was selected based on already published data by Gouttefangeas et al13 and the availability of the assays.
Materials and methods
Patients
Tumor material was received from 133 patients who underwent radical nephrectomy, donating the material on a voluntary basis. All patients gave their written informed consent for the investigations at which the tumor material was removed during the therapeutic nephrectomy. The tumor material (about 1 g) was placed in a sterile RMPI 1640 transport medium (Invitrogen; Karlsruhe, Germany) and shipped at 2°C–8°C within 24 hours to the central laboratory. TNM classification was performed by the local pathologist in the respective hospital.26 Patient characteristics are summarized in Table 1.
  | Table 1 Patient characteristics (N=133) |
Characteristics of patients (Table 1) included in this study highly correlate to typical clinical observations. The median age of patients at primary diagnosis is 60 years and the male to female ratio is 2:1.
Preparation of the TCL
First, macroscopically visible nontumor tissue is removed according to standard procedures.8 In order to obtain a single cell suspension, the remaining tumor tissue is cut into small pieces (~1 mm3) and then passed through a sieve with a defined grid size (50 mesh). Cells are purified by Percoll density gradient centrifugation. The cells are then incubated with interferon-γ and α-tocopherol acetate. The tumor cell suspension is aliquoted in single doses of 1 mL. Threefold rapid freezing at −82°C±5°C and thawing is used to devitalize the cells. For further details see Jocham et al.9
Detection of antigens and cellular marker proteins
Well described and commercially available TAA and cellular marker protein antibody assays were selected to investigate the heterogeneity of tumor cell lysates (TCLs).
Antigen ELISA
The ELISA test kits were used as listed in Table 2. Sample dilutions were adjusted according to pre-experiments. Free hemoglobin was measured to ensure that no contamination from residual red blood cells of residual plasma influenced the results (Table 2).
  | Table 2 List of assays used and the respective sample dilutions |
Detection of danger signals
The concentrations of HSP-60 and HSP-70 were detected by ELISA test kits (Table 2).
Western blotting
SDS-PAGE and Western blotting were carried out according to standard procedures27 in 4%–20% gradient mini-gels (SERVA, Heidelberg, Germany) utilizing a molecular weight marker (Bio-Rad Laboratories Inc., Hercules, CA, USA). Protein bands were insolubilized onto a PVDF membrane. For immune detection, the antibodies listed in Table 3 were used. Incubations with primary or secondary antibodies/protein-A-HRP were done in TBS 0.1% Tween 20, 1% w/v BSA. Primary and secondary antibodies are described in Table 3. The substrate reaction (insoluble TMB + H2O2) (Seramun, Wolzig, Germany) was carried out for 20 minutes. The reaction was stopped by substrate removal (washing).
  | Table 3 List of used primary and secondary antibodies, their manufacturers, and incubation conditions |
Control tissues
Since it is ethically impossible to receive kidney material from healthy volunteers, kidney tissue material that was not part of the tumor was used as a control if it was available after total nephrectomies. These experiments were performed for five different samples. Division of tumor and nontumor material was performed according to instructions by nephrologists and pathologists.
Statistical analysis
Statistical analysis was carried out by descriptive methods using Microsoft Excel. Since the purpose of the study was to investigate tumor heterogeneity and not to differentiate between tumor and nontumor tissue, data of ELISA and Western blots were categorized into “0” for negative to “1” for positive (higher than the detection limit of the respective assay).
Topological proteomics
Topological proteomics was used to demonstrate the heterogeneity of tumor tissue and cell suspensions. Investigations were performed according to Schubert on a total of three tissue and cell suspension samples.28 The antibody panel used includes commercial antibodies against CD3, CD4, CD8, CD10, CD11b, CD20, CD21, CD22, CD34, CD40, CD45RA, CD45RO, CD79, CD90, CD56, cadherin, CA-IX, Cytokeratin (pan), Cytokeratin 19, HIF-1a, hTert, Ki67, Keratin 8, Keratin 18, p53, and neuron-specific enolase (NSE). As controls, stains using 7AAD, propidium iodine and WGA and ConA were used. The assays were carried out according to standard procedures by Meltec GmbH (Magdeburg, Germany).
Induction of an immune response in an animal model
Immunization of mice
Five selected batches of TCLs were used in an animal model (28 female Balb/c Ola mice, 6–8 weeks old) by Newlab (Gronau, Germany). Mice were immunized twice (200 μL intradermally) according to the approval by the University Münster ethics committee without any adjuvant in 14-day intervals, corresponding to 21 days in-life phase. Immune sera were tested for specific IgG antibodies against CA-IX, NSE, and cytokeratins by ELISA. Sera of nontreated mice were used as controls.
Detection of the induced humoral immune response by ELISA (antibodies against CA-IX, NSE, and cytokeratins)
Microtiter plates (Nunc, Maxisorb, Langenselbold, Germany) were coated with 50 μL/well overnight at 4°C in 0.1 mol/L carbonate buffer pH 9.6 containing 1 μg/mL antigen ([CA-IX: recombinant human carbonic anhydrase IX, RD Systems, Wiesbaden-Nordenstadt, Germany]; [NSE: native human neuron specific enolase, purified, AbD serotec, Düsseldorf, Germany]; cytokeratin: Peptide sequence: QRGELAIKDANAKLSELEAALQRAKQ, Dept Biochem, Charité Humboldt Univ, Berlin).29 Washing steps were carried out using PBS, 0.1% v/v Tween 20. Incubation steps using PBS, 0.1% Tween 20, 3% Gelafusal (Serumwerk, Bernburg AG, Germany) were adjusted at 90 minutes at room temperature for the murine serum (dilution 1:10) and the protein-A-HRP (Seramun). An amount of 100 μL TMB (Seramun) was used as chromogenic substrate (30 minutes). Reaction was stopped using 1 mol/L H2SO4. ELISA results after reading at 450 nm were calculated against a pooled high titered standard serum each with 100 AU/mL as anti-CA-IX, anti-NSE, and anti-cytokeratin. All common test criteria (intra- and interserial imprecision, dilution linearity, repeatability) were analyzed in a validation procedure and were in acceptable known ranges.
Correlation analysis
Correlation analysis was carried out to show dependencies between the initial antigen levels in the TCL and the respective antibody titer as well as for the levels of danger signals using Microsoft™ Excel statistic tools.
Results
Detection of antigens, cellular marker proteins, and danger signals in TCLs
The detection of antigens and danger signals in TCLs was carried out stepwise according to the limitations given by the amount of tumor material. In a first step, an unbiased screening using a large panel of 36 antigens (Table 4) was used to investigate tumor heterogeneity. The number of antigens tested per TCL ranges from a minimum of one up to 20 different antigens, respectively.
  | Table 4 List of antigens and cellular proteins in tumor cell lysates |
If antigens were detected by ELISA, Western blotting, or activity detection (hTert), the results were summarized in a single column (Tables 4 and 5).
Overall, the antigen concentrations did not show any correlation to the concentration of free hemoglobin in the TCL (TPA R2=0.0532, CYFRA R2=0.0709, NSE R2=0.0003). Therefore, it can be concluded that they are not derived from the concomitant blood or blood cells. Also, no dependency on the tumor stage (calculated for TPA, NSE, TPS, and CYFRA; data not shown) was observed.
The proteins with highest overall frequencies of occurrence (FOCs) and highest number of tumors investigated (FOC >70%; n>10) were CD10, ALAT, CA-IX, LDH, TPA, NSE, TPS, MMP2, ASAT, CYFRA, NMP9, NMP22, CXCR4, and CA50 (Table 4). Additionally, danger signals such as HSP-60 and/or HSP-70 and urea were determined and found in nearly all of the TCLs (FOC >90%; n>10) investigated.
In a second step, the study focused on antigens with the highest overall FOCs (FOC >85%) and highest number of tumors investigated (n>10). The most frequent antigens were CA-IX, NSE, TPS, TPA, CYFRA, and as danger signal HSP-60 and HSP-70. Table 5 summarizes these experiments whereas it has to be emphasized that the number of TCLs investigated by a certain assay increased significantly (n>70).
As expected, even for the most frequent entity of RCC, clear cell carcinoma, no distinct change in the TAA pattern could be observed (Table 5). Only for Ca-IX an increase of the FOC from 84% to 91% and surprisingly for NSE from 80% to 87% could be noticed. In summary, Tables 4 and 5 demonstrate the high individuality of TCLs for their antigen pattern (Figure 1). None of the TAAs/danger signals in more than 10 TCLs investigated was found as a constant antigen in each TCL, even when grouped according to histological entities (Table 5). In addition, no significant sex-specific changes in the TAA expression were observed for the RCC or the clear cell carcinoma subgroup, respectively (Figure 1). No correlation was observed for the TNM stages vs the different antigen concentrations after adjusting all total protein concentration to 1 mg/mL (data not shown).
Since kidney tissue from healthy volunteers was not applicable, the removed nontumor tissue was investigated as a control. Five different nontumor tissue samples (Table 6) were investigated according to CYFRA, TPA, TPS, and NSE. Furthermore, an additional three tissue samples were investigated according to CA-IX (Table 7). The results show a clear differentiation between normal and tumor tissue whereas the values were highly variable.
  | Table 7 Absolute values of carbonic anhydrase IX (CA-IX) in nontumor tissue and the respective tumor tissue |
Topological proteomics
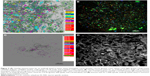
TAA heterogeneity in tumor tissue and single cell suspension was demonstrated by topological proteomics. As shown in Figure 2A and B, none of the antigens investigated were present in all tumor regions as well as on each single cell. This was found for “typical” antigens as CA-IX, NSE, or other (epithelial) markers (Figure 2C, Control tissue: Figure 2D).
Detection of immunogenicity in mice
All five test items demonstrated the typical property “immunogenicity” using the induction of specific IgG antibody titers detectable in the immunoassays (Table 8). This leads to the conclusion that both components for an immune response were present in all TCL in a biological active form. Here, CA-IX, NSE, and/or a cytokeratin epitope plus at least one of the danger signals were present (Table 5). The results of the immunization (anti-CA-IX, anti-NSE, and anti-cytokeratin) correlate poorly to the antigen levels in the TCL and to the level of danger signals (data not shown). This was expected, since the mode of action in vivo30 does not show a dose–response relationship as known for other classical medicinal products.
Discussion
Tumor vaccines are currently being discussed as a highly potential opportunity for the treatment of minimal residual disease after cancer resection in RCC and other carcinomas. Currently, a tumor vaccine for prostate cancer is approved by the FDA.31 May et al10 and Jocham et al9 published independent data for a successful treatment of RCC using an autologous TCL. Maslak et al32 used a peptide vaccine for the treatment of acute myeloid leukemia. Baek et al33 presented data from Phase I/II clinical trials for the combination therapy of renal cell carcinoma or breast cancer patients with dendritic cell vaccine and IL-2. According to the authors, the data indicated that DC vaccine combined with IL-2 is well tolerated by the patients without major side effects. In addition, the DC vaccine induced the specific immunity against introduced antigens.
In the current study, initially a subset of 36 different TAAs – all of them related to different tumor diseases13,34 – was investigated. Based on these initial data, a subset of five TAAs (CA-IX, NSE, TPA, TPS, and CYFRA) and an additional two typical stress/danger proteins, the HSPs HSP-60/-70, were selected for further experiments. HSPs – molecular chaperones which control protein folding and prevent protein aggregation – were selected due to their potential of activating the immune response. Both HSP-60 and HSP-70 were detected in nearly every tumor (92%–98%) and at least one of the HSPs investigated was present in every TCL investigated. Recent studies demonstrated that tumor-derived HSPs, like HSP-70 and HSP-90,35 initiate tumor-specific CTL responses and protective immunity.36 In addition, Wan et al23 reported that HSP-70-like protein 1 (HSP-70L1), a novel HSP derived from human dendritic cells, has potent adjuvant effects that polarize responses toward Th1.
Since CA-IX typically shows increased levels, it is described as a major indicator for renal cell carcinoma.37,38 Recently, the combination of CA-IX and HSP-110 was described as a tumor vaccine which showed its potential in a tumor prevention model, inhibiting the growth of RENCA tumors in BALB/c mice.39 Consequently, CA-IX was selected as one of the most interesting TAAs. Our results support the findings by Vissers et al37 observing an overall FOC of 84% for CA-IX and a FOC of 91% in clear cell carcinoma.
Nonetheless, a positive test for CA-IX does not correlate with the observation that CA-IX can be observed in each tumor cell. Using FACS analytics (data not shown) or topological proteomics, we were able to demonstrate on the contrary that a number of tumor cells showed no CA-IX but did show other typical TAAs. These findings correlate to those of Al-Ahmadie et al40 who observed that all clear cell RCCs had diffuse expression of CA-IX, while papillary and collecting duct subtypes had focal expression of CA-IX.
NSE has been detected in patients with certain tumors, such as neuroblastoma, small cell lung cancer, medullary thyroid cancer, carcinoid tumors, endocrine tumors of the pancreas, and melanoma. Measurement of NSE levels in patients with carcinoid and pancreas tumors provides information about the extent of the disease and the patient’s prognosis (outlook), as well as about the patient’s response to treatment.41,42 However, only a few studies correlate NSE with renal cell carcinoma. Ronkainen et al43 evaluated neuroendocrine markers such as serotonin, CD56, NSE, chromogranin A, and synaptophysin in renal cell carcinoma by immunohistochemistry with special emphasis on patient outcome. In all, 48% of the tumors were positive for NSE and tumors with an immune-positivity for NSE had a shorter (but insignificant) RCC-specific survival. In addition, no relationship between stage and immunoreactivity for NSE was observed. Our findings partly contradict these results since we were able to detect NSE in about 87% of the TCLs. Nonetheless, our data emphasize that NSE is a highly relevant TAA in renal cell carcinoma regardless whether it is a clear cell carcinoma or not. Finally, we investigated typical cytokeratins and, as expected, in more than 80% of the TCLs investigated, these cytokeratins were detectable. It has to be emphasized that these findings were not limited to clear cell carcinoma.44
In summary, a heterogenic mixture of different antigens was found in a total of 133 tumors. Even when only clear cell carcinomas are evaluated, a highly individual antigen/protein pattern was found in ELISA, topological proteomics, and also in FACS analysis on the cellular level. Furthermore, a sex-specific difference in TAA expression as described by Sun et al45 for brain cancer could not be verified. Most tumor gene and antigen patterns in RCC are not consistent.46–48
In conclusion, a combination of different TAAs (multi-epitope approach) seems reasonable for the adjuvant vaccination of patients suffering from renal cancer. In addition, concluding from this highly individual antigen pattern, the artificial composition of an individualized tumor vaccine seems to be impossible. Therefore, the use of the autologous composition could be an appropriate opportunity for the manufacturing of a tumor vaccine in RCC patients. When different regions of the tumor tissue are used for the manufacturing process, a probability is given to include as many epitopes as possible in a “multi-epitope vaccine.” A multi-epitope approach could therefore be appropriate in the fight against (micro) metastasis as can be concluded from antigenic heterogeneities found in several tumors.2,10,30,49
The study design addresses current discussions initiated by the EMA and the FDA according to the requirements on potency testing in medicinal products by comparing the TAA distribution in tumor lysates and initiated immune response in mice, respectively. Two immunologic properties of a vaccine need to be proven by appropriate assays; First, the antigenicity as the property of an antigen to be recognized by the specific immune system, and second, the immunogenicity which requires the combination of the antigenicity and the presence of a danger signal, resulting in the induction of a specific immune response. Therefore, one of the most important considerations is to address regulatory issues like mode of action and potency of a tumor vaccine.11 Mode of action was not the focus of this study but was demonstrated for murine TCLs in an earlier study using a mouse model.30 The potency of a tumor vaccine based on its immunogenicity. Thus, the proven prevalence of both antigens and danger signals could be the key for a successful immunization. The presence of danger signals was shown by inducing a cytokine response (MCP1) after stimulating PBMCs in vitro with a TCL (data not shown) and by the detection of the respective proteins (HSP-60 and HSP-70) in TCLs.
Of course, the general antigenicity of a human TCL (as used in this study) in mice could be expected in the chosen experimental setup due to the species barrier. But, if tumor vaccines made from autologous tumor cells contain antigens and danger signals, this mixture should be able to induce a specific, TAA-related immune response in mice without adding any adjuvants. The immune response surrogate should be detectable in sera of mice by classical serological techniques like ELISA.
To demonstrate the coexistence of both antigen and danger signals, an animal model was selected. The humoral immune response in mice was investigated after two intradermal shots with a TCL. All five test TCLs were immunogenic and induced IgG antibody titers detectable in the immunoassays despite the low concentrations (ng/mL) of each individual antigen in the multi-epitope vaccines. In summary, the immunogenic potency of the TCLs was proven and the mouse model used could be capable of addressing the requirements by the FDA and EMA for potency testing.50
Conclusion
This leads to the conclusion that all components needed for a (broad) immune response were present in all TCLs in an immunological active form. Since the presence of both (a mixture of TAA and danger signal) was proven in the animal model used, one may conclude that at least one antigen and one danger signal is required for the induction of an immune response. This could define a minimal requirement for a tumor vaccine, autologous as well as artificially composed.
Acknowledgment
The authors acknowledge financial support from LipoNova AG (Hannover, Germany).
Disclosure
The authors report no conflicts of interest in this work.
References
National Cancer Institute [homepage on the Internet]. Bethesda. Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Kidney and Renal Pelvis Cancer. Available from: http://seer.cancer.gov/statfacts/html/kidrp.html. | ||
Doehn C, Kausch I, Melz S, Behm A, Jocham D. Cytokine and vaccine therapy of kidney cancer. Expert Rev Anticancer Ther. 2004;4(6):1097–1111. | ||
Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24(35):5601–5608. | ||
Parton M, Gore M, Eisen T. Role of cytokine therapy in 2006 and beyond for metastatic renal cell cancer. J Clin Oncol. 2006;24(35):5584–5592. | ||
SEER Stat Fact Sheets: Kidney and Renal Pelvis Cancer. Available from: www.seer.cancer.gov. Accessed November 8, 2015. | ||
Pizzocaro G, Piva L, Colavita M, et al. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: a multicentric randomized study. J Clin Oncol. 2001;19(2):425–431. | ||
Clark JI, Atkins MB, Urba WJ, et al. Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma: a cytokine working group randomized trial. J Clin Oncol. 2003;21(16): 3133–3140. | ||
Atzpodien J, Reitz M. Metastatic renal carcinoma long-term survivors treated with s.c. interferon-alpha and s.c. interleukin-2. Cancer Biother Radiopharm. 2005;20(4):410–416. | ||
Jocham D, Richter A, Hoffmann L, et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet. 2004;363(9409):594–599. | ||
May M, Brookman-May S, Hoschke B, et al. Ten-year survival analysis for renal carcinoma patients treated with an autologous tumour lysate vaccine in an adjuvant setting. Cancer Immunol Immunother. 2010;59(5):687–695. | ||
Guideline on potency testing of cell based immunotherapy medicinal products for the treatment of cancer. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003814.pdf. Accessed November 8, 2015. | ||
Guideline on the evaluation of anticancer medicinal products in man: 13th Dec. 2012, EMA/CHMP/205/95/Rev.4 Oncology Working Party. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/01/WC500137128.pdf. Accessed November 8, 2015. | ||
Gouttefangeas C, Stenzl A, Stevanović S, Rammensee HG. Immunotherapy of renal cell carcinoma. Cancer Immunol Immunother. 2007;56(1):117–128. | ||
Doehn C, Jocham D. Vaccination immunotherapy - an update. Scand J Surg. 2004;93(2):163–169. | ||
Lam JS, Pantuck AJ, Belldegrun AS, Figlin RA. G250: a carbonic anhydrase IX monoclonal antibody. Curr Oncol Rep. 2005;7:109–115. | ||
Said J. Biomarker discovery in urogenital cancer. Biomarkers. 2005;10 Suppl 1:S83–S86. | ||
Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–811. | ||
Iakovlev VV, Pintilie M, Morrison A, Fyles AW, Hill RP, Hedley DW. Effect of distributional heterogeneity on the analysis of tumor hypoxia based on carbonic anhydrase IX. Lab Invest. 2007;87: 1206–1217. | ||
Jones TD, Eble JN, Wang M, Maclennan GT, Jain S, Cheng L. Clonal divergence and genetic heterogeneity in clear cell renal cell carcinomas with sarcomatoid transformation. Cancer. 2005;104:1195–1203. | ||
Li G, Passebosc-Faure K, Lambert C, et al. Flow cytometric analysis of antigen expression in malignant and normal renal cells. Anticancer Res. 2000;20:2773–2778. | ||
Krishnamurthy S, Bischoff F, Ann Mayer J, et al. Discordance in HER2 gene amplification in circulating and disseminated tumor cells in patients with operable breast cancer. Cancer Med. 2013;2(2):226–233. | ||
Lee JT, Herlyn M. Old disease, new culprit: tumor stem cells in cancer. J Cell Physiol. 2007;213(3):603–609. | ||
Wan T, Zhou X, Chen G, et al. Novel heat shock protein Hsp70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood. 2004;103(5):1747–1754. | ||
Murshid A, Gong J, Calderwood SK. Heat-shock proteins in cancer vaccines: agents of antigen cross-presentation. Expert Rev Vaccines. 2008;7(7):1019–1030. | ||
Calderwood SK. Tumor heterogeneity, clonal evolution, and therapy resistance: an opportunity for multitargeting therapy. Discov Med. 2013;15(82):188–194. | ||
Fritz A, Percy C, Jack A, et al, editors. International Classification of Diseases for Oncology. 3rd ed. WHO, Geneva; 2000, ISBN 92-4-154534-8. | ||
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. | ||
Schubert W. Topological proteomics, toponomics, MELK-technology. Adv Biochem Eng Biotechnol. 2003;83:189–209. | ||
Johansson A, Sandström P, Ullén A, et al. Epitope specificity of the monoclonal anticytokeratin antibody TS1. Cancer Res. 1999;59(1):48–51. | ||
Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17(11):3520–3526. | ||
Doehn C, Esser N, Pauels HG, et al. Mode-of-action, efficacy, and safety of a homologous multi-epitope vaccine in a murine model for adjuvant treatment of renal cell carcinoma. Eur Urol. 2009;56:123–133. | ||
Maslak P, Scheinberg D. Targeted therapies for the myeloid leukaemias. Expert Opin Investig Drugs. 2000;9(6):1197–1205. | ||
Baek S, Kim CS, Kim SB, et al. Combination therapy of renal cell carcinoma or breast cancer patients with dendritic cell vaccine and IL-2: results from a phase I/II trial. J Transl Med. 2011;9:178–188. | ||
Weinschenk T, Gouttefangeas C, Schirle M, et al. Integrated functional genomics approach for the design of patient-individual antitumor vaccines. Cancer Res. 2002;62(20):5818–5827. | ||
Kurotaki T, Tamura Y, Ueda G, et al. Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J Immunol. 2007;179(3):1803–1813. | ||
Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P, Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001;167(9):4844–4852. | ||
Vissers JL, De Vries IJ, Schreurs MW, et al. The renal cell carcinoma-associated antigen G250 encodes a human leukocyte antigen (HLA)-A2.1-restricted epitope recognized by cytotoxic T lymphocytes. Cancer Res. 1999;59(21):5554–5559. | ||
Zisman A, Pantuck AJ, Bui MH, et al. LABAZ1: A metastatic tumor model for renal cell carcinoma expressing the carbonic anhydrase type 9 tumor antigen. Cancer Res. 2003;63(16):4952–4959. | ||
Kim HL, Sun X, Subjeck JR, Wang XY. Evaluation of renal cell carcinoma vaccines targeting carbonic anhydrase IX using heat shock protein 110. Cancer Immunol Immunother. 2007;56(7):1097–1105. | ||
Al-Ahmadie HA, Alden D, Qin LX, et al. Carbonic anhydrase IX expression in clear cell renal cell carcinoma: an immunohistochemical study comparing 2 antibodies. Am J Surg Pathol. 2008;32(3):377–382. | ||
Manfé AZ, Norberto L, Marchesini M, Lumachi F. Usefulness of chromogranin A, neuron-specific enolase and 5-hydroxyindolacetic acid measurements in patients with malignant carcinoids. In Vivo. 2011;25(6):1027–1029. | ||
Neuss H, Koplin G, Raue W, Reetz C, Mall JW. Analysing the serum levels of tumour markers and primary tumour data in stage III melanoma patients in correlation to the extent of lymph node metastases – a prospective study in 231 patients. Acta Chir Belg. 2011;111(4):214–218. | ||
Ronkainen H, Soini Y, Vaarala MH, Kauppila S, Hirvikoski P. Evaluation of neuroendocrine markers in renal cell carcinoma. Diagn Pathol. 2010;5:28. | ||
Holthöfer H, Miettinen A, Paasivuo R, et al. Cellular origin and differentiation of renal carcinomas. A fluorescence microscopic study with kidney-specific antibodies, antiintermediate filament antibodies, and lectins. Lab Invest. 1983;49(3):317–326. | ||
Sun T, Plutynski A, Ward S, Rubin JB. An integrative view on sex differences in brain tumors. Cell Mol Life Sci. 2015;72(17):3323–3342. | ||
Höglund M, Gisselsson D, Soller M, Hansen GB, Elfving P, Mitelman F. Dissecting karyotyping patterns in renal cell carcinoma: an analysis of the accumulated cytogenetic data. Cancer Genet Cytogenet. 2004;153:1–9. | ||
Massari F, Ciccarese C, Porcaro AB, et al. Quantitative score modulation of HSP90 and HSP27 in clear cell renal cell carcinoma. Pathology. 2014;46(6):523–526. | ||
Syring I, Müller SC, Ellinger J. Novel tumor markers in the serum of testicular germ cell cancer patients: a review. Current Biomarker Findings. 2014;4:133–137. | ||
Yang Y, Li J, Mao S, Zhu H. Comparison of immunohistology using pan-CK and EMA in the diagnosis of lymph node metastasis of gastric cancer, particularly micrometastasis and isolated tumor cells. Oncol Lett. 2013;5(3):768–772. | ||
U.S. Food and Drug Administration [homepage on the Internet]. Silver Spring, MD. Guidance for Industry. Potency Tests for Cellular and Gene Therapy Products. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM243392.pdf. Accessed November 08, 2015. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.