Back to Journals » International Journal of General Medicine » Volume 14
Tumor Endothelial Marker TEM7 is a Prognostic Biomarker and Correlating with Immune Infiltrates in Gastric Cancer
Authors Geng L, Chen S , Gong Y, Zhou Y, Yang H, Tang L
Received 30 October 2021
Accepted for publication 9 December 2021
Published 22 December 2021 Volume 2021:14 Pages 10155—10171
DOI https://doi.org/10.2147/IJGM.S347010
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lixin Geng,1,2 Shuai Chen,2 Yu Gong,2 Yan Zhou,2 Haojun Yang,2 Liming Tang2
1Dalian Medical University, Dalian, Liaoning, 116044, People’s Republic of China; 2Center of Gastrointestinal Disease, The Affiliated Changzhou NO.2 People’s Hospital of Nanjing Medical University, Changzhou, Jiangsu, People’s Republic of China
Correspondence: Liming Tang
Center of Gastrointestinal Disease, The Affiliated Changzhou NO.2 People’s Hospital of Nanjing Medical University, 68 Gehu Road, Wujin District, Changzhou, Jiangsu, People’s Republic of China
Email [email protected]
Introduction: Tumor endothelial marker 7 (TEM7) is included in the endothelial cells of tumors as a greatly expressed protein. In previous studies, it has been confirmed that TEM7 is highly expressed in gastric cancer (GC) cells and related to tumor invasion and migration. However, the relationship between TEM7 gene expressions, prognostic and tumor-infiltrating lymphocyte in GC is still unclear.
Methods: We obtained the expression of TEM7 in GC tissues using Oncomine and TIMER databases and validated it using qRT-PCR. The effect of TEM7 on survival in patients with GC was explored through the Kaplan–Meier Plotter database. Univariate and multifactorial analyses of the TCGA (The Cancer Genome Atlas) database were performed to explore the association of TEM7 expression with clinical traits. TIMER and GEPIA databases were used to analyze the relationship between TEM7 expression and immune infiltration. GSEA pathway enrichment in Kyoto Encyclopedia of Genes and Genomes (KEGG) exposed possible ways.
Results: An expression level of TEM7 was significantly increased in gastric cancer tissues and related with unfavorable survival in GC. Univariate analysis of TCGA (The Cancer Genome Atlas) database indicated that excessive level of TEM7 expression was linked with age, tumor grade and TMN classification, and multivariate analysis indicated that age and level of TEM7 expression were independent prognostic factors for a poor prognosis. The level of expression of TEM7 was positively related to tumor-infiltrating CD4+ and CD8+ T cells, neutrophils, macrophages, and dendritic cells other than B cells in GC. In the end, GSEA pathway enrichment exposed possible ways related to immunity.
Discussion: Our study indicates that TEM7 is a prognostic biomarker that makes the decision that the progression of GC and is connected with tumor immune infiltrates in GC.
Keywords: TEM7, gastric cancer, biomarker, prognosis, immune infiltration
Introduction
In statistics of 2020 of Global Cancer, gastric cancer is one of the main cancers of the digestive tract, and it is considered as the 5th most common cancer diagnosed, and is considered 4rd of the leading reasons for cancer death worldwide.1 The majority of gastric cancer patients cannot be treated early because it begins as asymptomatic. When gastric cancer patients begin showing symptoms of stomach ache, hemoptysis or weight loss, often it is at an advanced stage, with a poor overall survival (OS) rate.2 Although the level of medical treatment has developed rapidly, it remains a huge challenge to cure gastric cancer via surgery, radio therapies and chemotherapies.3 A path that is promising for treatment of gastrointestinal (GI) cancers is said to be immunotherapy techniques.4 Programmed death-1 (PD-1), programmed death ligand-1 (PD-L1) inhibitors, and cytotoxic T lymphocyte-associated antigen 4 (CTLA4), are Immunotherapy that is considered as a benefit to non-small cell lung carcinoma (NSCLC). Most of the gastric cancer patients who received this immunotherapy had great outcomes.5,6 Most patients with gastric cancer who receive this immunotherapy have excellent results. Unfortunately, only a few agents have been found to be successful in GC, but those GC patients treated with these agents still have minimal survival rates.7 Moreover, it has been found by scientists that Tumor-Associated Macrophages (TAMs), Tumor-Infiltrating Lymphocytes (TILs), and Tumor-Infiltrating Neutrophils (TINs) affect prognosis and immunotherapy.8,9 The immunophenotyping of tumor-immune interactions and determination of immune-related therapeutic targets in gastric cancer must therefore be elucidated as soon as possible.
In recent years, tumor endothelial cells (TECs), a set of proteins of cell surface strongly described in endothelial cells of different cancers, have received a huge amount of importance.10 Abnormality among normal endothelial cells and tumor cells gives a chance to determine certain markers, ie, Tumor Endothelial Markers (TEMs) along with tumor angiogenesis.11 TEM7, also called Plexin Domain-Containing 1 (PLXDC1), is one of the TEMS families and it has the most numerous gene isoform among the TEMs.12 TEM7 in the vascular endothelium of various malignant tumors was well known for being an exceedingly expressed gene in cell surface protein.10,13 In contrast, TEM7 expressions were indirectly related with micro vascular density and survival of the patients.14 TEM7, which has been reported, can be a biomarker and has an ability to forecast the prognosis of patients with Colorectal Cancer, osteogenic sarcoma and advanced high-grade serous ovarian cancer.11,15 Therefore, we speculated that gastric cancer is similar to other solid tumors in that there is a large amount of neovascularization in gastric cancer tissues, which affects the development of gastric cancer. We decided to further investigate the role of TEM7 in gastric cancer because we knew that the expression of TEM7 in gastric cancer tissues was higher than that in normal gastric tissues and only a few papers had reported the role of TEM7 in gastric cancer through reading related literature and screening relevant databases.
In this study, we used Oncomine, Gene Expression Profiling Interactive Analysis (GEPIA), the Kaplan–Meier plotter and TCGA databases to explore the relationship between TEM7 gene expression and patients’ clinicopathological characteristics with GC, and provided the potential relationship and mechanism between TEM7 and tumor-immune interaction, so that additional proof for its potential function as a prognostic marker of gastric cancer and its relationship with tumor-immune interaction can be given.
Materials and Methods
Data Acquisition
We extracted the Gastric Cancer patient databases from the TCGA databases, including 32 normal and 375 tumor tissues, including gene expression profiles and clinical information. Afterwards, we used the Perl programming language to link the gene expression information with clinical information and remove unknown or incomplete clinical information and the R software’s survival package was used to inspect the status of survival and gene expression.
The Expression Level of TEM7
(https://www.oncomine.org/resource/login.html) The Oncomine database has been used to establish the level of expression in many cancers of the TEM7 gene.16 The threshold parameters were calculated as follows: 0.05 P-value, 1.5 fold shift, and all gene rankings.
Cell Lines and Clinical Specimens
Gastric cancer cell lines MGC-803, BGC-823, NCI-N87, AGS, SGC-7901 and normal gastric epithelial cells (GES-1) were provided by the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Gastric cancer tissue samples from 30 patients with GC admitted to Changzhou No. 2 People’s Hospital (Changzhou, China) from 2018 to 2019 were used in this study. Paracancerous tumors and tissues were collected during the operation and immediately stored at −80°C.
qRT-PCR
Cell lines and tissues were pretreated in order to extract total RNA. Synthesis of cDNA using Prime Script RT Kit (Takara, Dalian, China). Quantitative PCR was carried out with a 7500 real-time PCR system (ABI, Waltham, MA, USA). PCR primers were synthesized and purchased by SangonBiotech Company (Shanghai, China). TEM7: forward: 5ʹ-ACACGCTGCCAGATAACAGG-3ʹ, reverse: 5ʹ-TCGGCCACATCTACCCACA-3ʹ, GAPDH served as an internal control, and fold change had been calculated using the 2−ΔΔCT technique.
Kaplan–Meier Plotter Database Analysis
In 21 common cancer types, including 6234 breasts, 2190 ovaries, 3452 lungs, and 1440 gastric cancers worldwide, the Kaplan Meier plotter includes a change of 54,675 numbers of genes on survival. (http://kmplot.com/analysis/) In order to identify the correlation between a level of TEM7 expression and level of survival in ovarian, breast, gastric, and lung cancers, a Kaplan–Meier plotter was used, and Hazard Ratio (HR) was determined, 95% of CI, and the log-rank P value of the web page.
TIMER Database Analysis
(https://cistrome.shinyapps.io/timer/) The web server, ie, TIMER is a comprehensive platform for systematic study of several cancer forms of immune infiltrates.17 TIMER algorithms (B cells, CD8+ T cells, CD4+ T cells, Macrophages, Neutrophils, and Dendritic cells) assess the concentration of six immune infiltrates.
We extracted an expression of TEM7 in different forms of cancer via gene marker and the relation between the expression of TEM7 and an abundance of immune infiltration, including B cells, CD4+ T cells, macrophages CD8+ T cells, dendritic cells and neutrophils. On the basis of previous research reports, B cells, TAMs, T cells, Natural Killer (NK) cells, monocytes, M1 macrophages, M2 macrophages, neutrophils, Dendritic Cells (DCs), T-Helper (TH) cells, T-Helper 17 (Th17) cells, T (Tfh) Follicular Helper cells, exhausted T cells and Tregs were the gene markers that are useful for assessing of association between TEM7 and immune infiltration.18,19
GEPIA Analysis
(http://gepia.cancer-pku.cn/) Mainly concentrating on 9736 tumors and 8587 standard TCGA and GTEx database samples, GEPIA is an interactive web application for gene expression analysis using the performance of a typical RNA sequencing a data processing pipeline. The findings of the study include more than 20,000 coding and 25,000 noncoding genes, as well as over 14,000 segments of pseudo genes and 400 T cell receptors.20
The GEPIA database is used for generating the correlation between survival rate and gene expression level in different tumors in order to verify the results formed by TIMER.
Gene Set Enrichment Analysis
Gene Set Enrichment Analysis is a technique that computes the statistical importance of the prior defined set of genes and the existence of differences between two states of biology.21 GSEA initially illustrates the list of classification of these genes according to the association between these genes and the expression level of TEM7. One of the major differences noticed by us in the high and low survival of TEM7 groups is discussed in this method of measurement. TEM7 level of speech was the phenotype mark we put forward. Moreover, in order to develop the enriched pathways in each phenotype, we used nominal p-value and Normalized Enrichment Score (NES).22 It was considered that sets of a gene with a discovery rate (FDR) <0.05 were particularly enriched. The R (plyr, ggplot2, grid, grid Extra package) program visualized the outcomes.
Statistical Analysis
From the results provided by Oncomine, p-values, ranks and fold modifications were analyzed. Plots by Kaplan Meier formed curves of survival. In databases like TIMER and GEPIA, an expression of gene correlation is evaluated by using Spearman’s analysis of correlation and the statistical importance threshold is P < 0.05. Datasets collected from TCGA have been combined and carried out by R-4.0.2. The associations between clinical features and TEM7 level of expression were evaluated using logistic regression. Multivariate analysis is used to measure the effect of TEM7 expression level and clinical characteristics of survival and the threshold of statistical significance was P < 0.05.
Results
In gastric cancer, there is a substantially higher level of expression of TEM7 as compared to normal tissues. High level of TEM7 expression in gastric cancer is associated with Overall Survival (OS), Post-Progression Survival (PPS), and Progression-Free Survival (PFS). Moreover, univariate logistic regression analysis indicates that age, tumor grade and TMN classification were significantly interlinked with the increased level of TEM7 expression. Multivariate research has also indicated that an unconventional factor for a bad prognosis is the degree of TEM7 expression. Tumor-infiltrating CD4+ and CD8+ T cells, macrophages, neutrophils, and dendritic cells other than B cells have been positively linked with TEM7 expression levels in GC. In addition, TEM7 showed more associations with CD8+ T cell, T cell (general), Monocytes, TAM, M2 Macrophage, Dendritic cell, Treg, T cell fatigue immune markers. In addition, in the Kyoto Encyclopedia of Genes and Genomes (KEGG), GSEA recognized cell adhesion molecules (CAMs), cytokine receptor interaction, ECM receptor interaction, MAPK signaling pathway, leukocyte transendothelial migration, toll-like receptor signaling pathway as differentially enhanced immunity-based pathway.
The mRNA Expression Levels of TEM7 in Different Types of Tumors
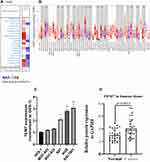
We also studied the mRNA levels of expression of TEM7 in numerous types of tumors and also the corresponding normal tissues of numerous types of cancer via the Oncomine database to examine the variations in TEM7 expression in different tumor tissues. This study found that level of TEM7 expression was particularly higher in tumors of the stomach, head, neck, kidney and pancreas and was particularly lower in tumors of the breast, leukemia, ovary and other cancers compared to the tissues that are normal (Figure 1A and Supplementary Table 1).
In order to obtain further outcomes, a database used to evaluate the expression of TEM7 in different tumors was a TIMER database, based on the TCGA RNA-seq data, and the outcome indicated that TEM7 mRNA expression was particularly higher in Cholangiocarcinoma (CHOL), Colon Adenocarcinoma (COAD), Head and Neck Squamous cell Carcinoma (HNSC), Esophageal Carcinoma (ESCA), Liver Hepatocellular Carcinoma (LIHC), Kidney Renal clear cell Carcinoma (KIRC), Lung Adenocarcinoma (LUAD), Stomach Adenocarcinoma (STAD), Thyroid Carcinoma (THCA) and significantly lower in Breast Invasive Carcinoma (BRCA), Kidney Renal Papillary cell Carcinoma (KIRP) and Uterine Corpus Endometrial Carcinoma (UCEC) as compared to corresponding normal tissues (Figure 1B).
To further confirm that the expression level of TEM7 in gastric cancer tissues was higher than that in paracancerous tissues, we detected this in five gastric cancer cell lines and gastric epithelial cells (GSE-1) (Figure 1C). The expression levels of TEM7 in SGC-7901 (P < 0.01), AGS (P < 0.01) and MGC-803 (P < 0.05) were higher than that in gastric epithelial cells. In addition, using qRT-PCR technology to identify the expression of TEM7 at the transcriptional level, it was found that the interpretation level of TEM7 mRNA expression in GC tissues was fundamentally higher than that in neighboring non-tumor tissues. (p = 0.0053) (Figure 1D).
The Potential of TEM7 to Be a Prognostic Biomarker in Human Cancers
We used the Kaplan–Meier plotter database to analyze the correlation between the expression level of TEM7 and survival rate in different human tumors. The outcome indicates a high expression level of TEM7 corresponds to a poor prognosis in gastric cancer (OS: HR = 1.71, 95% CI = 1.44–2.03, P-value = 7.9e-10; FP: HR =1.83, 95% CI = 1.49–2.24, P-value = 6.5e-09; PPS: HR = 2.32, 95% CI = 1.84–2.92, P-value = 1.9e-13; Figure 2A–C), ovarian cancer (OS: HR = 1.26, 95% CI = 1.11–1.43, P-value = 0.00041; PFS: HR = 1.27, 95% CI = 1.12–1.44, P-value = 0.00021; PS: HR = 1.53, 95% CI = 1.29–1.81, P-value = 7e-07; Figure 2D–F), lung cancer (OS: HR = 0.77, 95% CI = 0.68–0.87, P-value = 4e-05; FPS: HR = 0.75, 95% CI = 0.58–0.97, P-value = 0.027; FP: HR = 0.94, 95% CI = 0.78–1.14, P-value = 0.55; Figure 2G–I). However, in breast cancer, OS: HR = 0.89, 95% CI = 0.72–1.1, P-value = 0.28; PPS: HR = 1.16, 95% CI = 0.91–1.48, P-value = 0.22; DMFS, HR = 1.09, 95% CI = 0.9–1.32, P-value = 0.39: RFS: HR = 0.77, 95% CI = 0.69–0.86, P-value = 3.1e-06; Figure 2J–M. Univariate correlation analysis of the use of Cox regression found, as shown in Table 1, that age, stage and TMN classifications are particularly linked with overall survival. In analysis of multivariate, age and the degree of TEM7 expression (Figure 3) are independent prognostic factors.
 |
Table 1 A. Association with Overall Survival and Clinicopathologic Characteristic in TCGA Patients Using Cox Regression. B. Multivariate Survival Using Cox Regression |
 |
Figure 3 Multivariate Cox analysis of TEM7 expression and other clinical pathological factors. (As age and TEM7 expression level are independent prognostic factors). Notes: *P < 0.05, ***P < 0.001. |
The Correlation Between Clinical Features and TEM7 Expression Level in Gastric Cancer
To identify an association between clinical features and expression level of TEM7 in gastric cancer, a Kaplan–Meier Plotter database is used in order to analyze and the results are as follows (Table 2), high expression level of TEM7 corresponding to Overall Survival (OS) and Progression-Free Survival (PFS) in Female (OS: HR = 1.9, P-value = 0.0003; PFS: HR = 1.92, P-value = 0.0008), male (OS: HR = 1.9, P-value= 6.90E-09; PFS: HR = 1.78, P-value = 2.70E-06), intestinal of Lauren classification (OS: HR = 2.2, P-value = 1.50E-06; PFS: HR = 1.46, P-value = 0.036), Diffuse of Lauren classification (OS: HR = 1.8, P-value = 0.0008; PFS: HR = 1.75, P-value = 0.0016), surgery alone (OS: HR = 1.82, P-value = 6.00E-05; PFS: HR = 1.65, P-value= 0.0005), other adjuvant (OS: HR = 3.45, P-value = 0.01; PFS: HR = 2.94, P-value= 0.0114), HER2 negative (OS: HR = 1.73, P-value = 2.20E-06; PFS: HR = 1.73, P-value= 4.40E-05) and HER2 positive (OS: HR = 1.79, P-value = 1.1E-05; FPS: HR = 2.05, P-value= 1.20E-05). Above all, high expression level corresponding to poor OS and PFS belong to stage 3 (OS: HR = 1.54, P-value= 0.0032; PFS: HR = 1.8, P-value= 0.0022), stage 4 (OS: HR =1.56, P-value = 0.0237; PFS: HR = 1.49, P-value = 0.0431). N category that refers to lymph node involvement include N1, N2, N3 were linked with TEM7 level of expression may affect a prognosis in patients of gastric cancer. In conclusion, the level of expression of TEM7 can change the prognosis in gastric cancer patients with different clinical characteristics, especially in advanced cancer patients.
 |
Table 2 Correlation of TEM7 mRNA Expression and Prognosis in Gastric Cancer with Different Clinicopathological Factors by Kaplan–Meier Plotter |
The TEM7 Expression Level Correlates with the Infiltration Levels of Immune Cells in Gastric Cancer
Tumor-infiltrating lymphocyte situations associated with the time of survival in several human tumors.23,24 The database of TIMER was used to analyze the correlation in order to explain the relationship between the TEM7 level of expression and the infiltrating level of immune cells in 39 types of cancer. Following are the findings: the level of expression of TEM7 is particularly linked with purity of tumor in 29 types of cancer, TEM7 expression was closely related with the level of infiltration of B cells in 17 types of cancer, CD8+T cells in 20 types of cancer, CD4+T cells in 28 types of cancer, macrophages in 29 types of cancer, neutrophils in 25 types of cancer, and dendritic cells in 28 types of cancer (Figure 4 and Supplementary Figure 1).
 |
Figure 5 Continue. |
Higher expression of TEM7 in gastric cancer was linked with poor outcomes and positively correlated with infiltration levels of CD8+ T cells (cor = 0.241, P-value= 2.66e-06), CD4+ T cells (cor = 0.363, P-value= 7.19e-13), macrophages (cor = 0.591, P-value= 3.17e-36), neutrophils (cor = 0.326, P-value= 1.32e-10) and dendritic cells (cor = 0.432, P-value= 2.87e-18).
Analysis of a Correlation Between TEM7 and Immune Marker Sets
For further analysis, TIMER database and GEPIA database were used for investigating the association among TEM7 expression and tumor-infiltrating immune cell status depending on immune marker sets of different immune cells for gastric cancer. The tumor-infiltrating immune cells include T cells (general), CD8+ T cells, monocytes, TAMs, neutrophils, NK cells, M1 and M2 macrophages and DCs. In addition, various subsets of T cells in gastric cancer tissues are also studied by us, including T helper 1 (Th1), Th2, and Th17, regulatory T (Tregs), depleted T cells and follicular helper T (Tfh). Cholangiocarcinoma was used as control. The correlation was adjusted by tumor purity. An essential factor that affects the immune infiltration analysis in samples of tumor is known as tumor purity (Table 3).
 |
Table 3 Correlation Analysis Between TEM7 and Relate Genes and Markers of Immune Cells in TIMER |
The analysis indicates that the degree of expression of TEM7 was closely linked to the expression of most immune marker systems in different immune cells and T cell sets, whereas the connection was not relevant in Cholangiocarcinoma (Figure 5 and Table 4).
 |
Table 4 Correlation Analysis Between TEM7 and Marker Genes of Immune Cells in GEPIA |
We found that levels of expression of maximum CD8+ T cell, T cell (general), Monocytes, TAM, Dendritic cell, M2 Macrophage, Treg, T cell fatigue marker sets have good associations in gastric cancer with TEM7 expression. Specifically, we showed CD2 of T cell (general), CD86 and CD115 of Monocytes, CCL2, CD68 and IL10 of TAM, CD163, VSIG4 and MS4A4A of M2 Macrophage, HLA-DPB1, HLA-DRA, HLA-DPA1, BDCA-1, BDCA-4 and CD11c of Dendritic cell, FOXP3, CCR8, STAT5B and TGFβ of Treg, TIM-3 of T cell exhaustion represented a relation with TEM7 expression in gastric cancer (P < 0.001; correlation value >0.30).
Gene Sets Enriched in TEM7 Expression Phenotype
In order to differentiate the ways involved in gastric cancer, between high and low LncRNA data sets of TEM7 expression, GSEA-based TEM7-related signaling pathways were being used and indicated considerable differences in KEGG Collection enrichment (FDR <0.05, NOM P-value <0.05). Due to the limited space, only six high routes and immunity-related expressions are described here (Table 5).
 |
Table 5 Gene Sets Enriched in Phenotype |
Six Immunity-related KEGG items including cytokine receptor interaction, cell adhesion molecule cams, ECM receptor interaction, MAPK indicating pathway, transendothelial leukocyte migration and toll-like receptor signaling pathway were shown to significantly differential enrichment in the high-expression TEM7 phenotype and there are no low-expression immunity-related signal routes in the TEM7 phenotype (Figure 6).
All of these indicate the potential role of TEM7 in GC growth.
Discussion
Plexin domain-containing 1 (PLXDC1), initially recognized as a strongly expressed protein in the vascular endothelium of human tumors, is classified as Tumor Endothelial Marker 7 (TEM7).10 It is a molecular target candidate for antiangiogenic therapy. In previous studies, elevated expression of TEM7 in tumor endothelial cells and its function in tumor angiogenesis have been well demonstrated.13,25 A number of cancer tissues, including colorectal cancer, osteosarcoma, and glioblastoma epithelium, also express TEM7. According to our knowledge, limited literature is available on the possible prognostic and immune-related effect of TEM7 on gastric cancer. It has been reported that TEM7 is highly expressed in gastric cancer. According to previous reports of TEM7, the high expression of TEM7 in gastric cancer can promote the migration and invasion of gastric cancer cells,26 and TEM7 is related to angiogenesis under normal and pathological conditions.12,27 In our study, we verified the above findings through TCGA database and made further statistical analysis of the data. Using univariate and multivariate COX analysis, we obtained more important results. TEM7 can be used as an independent prognostic factor to judge the prognosis of patients with gastric cancer. Combined with the results of our current study, the high expression of TEM7 is related to the late stage of gastric cancer. We speculate that the reason for the high expression of TEM7 in patients with gastric cancer, especially in patients with advanced gastric cancer, is that gastric cancer cells in advanced patients have stronger ability of migration and invasion, which leads to distant metastasis of gastric cancer cells, and the vascular network of advanced gastric cancer is more abundant. It provides more nutrition for the progression of gastric cancer, but the specific regulation mechanism of high expression of TEM7 in patients with gastric cancer is still unclear. Here, the results show that expression levels of TEM7 link with several human cancer prognosis, high expression levels relate to a poor prognosis in several cancers, such as gastric, ovarian, and lung cancer. Interestingly, TEM7 expression level correlated with relatively late stage (stage 3+4) and lymph node metastasis. Therefore, TEM7 can be a predictor of tumor stage and metastasis. Besides, in analysis of multivariate, TEM7 expression level is an unconventional prognostic factor. Therefore, TEM7 can be a tumor stage and metastasis indicator. Moreover, our outcomes showed that TEM7 expression level strongly correlated with immune infiltration and sets of immune markers of different immune cells. In conclusion, it is revealed in our study that it is the potential of TEM7 to be a prognostic biomarker and strong correlation with tumor immunology.
In our analysis, Oncomine, GEPIA and Kaplan-Meier Plotter databases are used to investigate the level of TEM7 expression and the relationship between TEM7 rate of expression and the rate of survival in different human tumors. TEM7 expression was up-regulated in cancer of the brain, CNS, breast, esophageal, gastric, kidney, liver, pancreatic and sarcoma. Although some data sets showed that in leukemia, lymphoma, ovarian cancer and prostate cancer, TEM7 expression was down-regulated. However, the results from a GEPIA database show that TEM7 expression is up-regulated in Cholangiocarcinoma, Colon adenocarcinoma, Esophageal carcinoma, Kidney renal clear cell carcinoma, Head and Neck squamous cell carcinoma, Liver Hepatocellular carcinoma, Lung adenocarcinoma, Stomach adenocarcinoma and Thyroid carcinoma, whereas some data sets showed that TEM7 expression was down regulated in Kidney renal papillary cell, Breast invasive carcinoma, and Uterine corpus endometrial carcinoma. The reason for the inconsistent results may be due to the methods of data collection and fundamental causative mechanisms. The expression of TEM7 in gastric cancer, however, was consistent in both databases. In addition, Kaplan-Meier Plotter findings show that high TEM7 expression levels are associated with low survival rates for gastric, ovarian, and lung cancer. In addition, a high level of TEM7 expression associated with worse patient prognosis at a relatively late step (stage 3+4). The above findings recommend that TEM7 is a possible biomarker indicator for gastric cancer.
Outcomes from the databases of TIMER and GEPIA indicate that degree of expression of TEM7 is linked with immune cell infiltration status in gastric cancer. The TEM7 expression is positively connected with infiltration of immune cells including CD8+ and CD4+ T cells, neutrophils, macrophages, and DCs. It suggests the level of expression of TEM7 is related to tumor immune; therefore, it can affect the survival rates of patients of gastric cancer. In addition, the degree of expression of TEM7 shows a clear correlation with immune cell markers like T cell CD2 (general), Monocytes CD86 and CD115, TAM CCL2, CD68 and IL10, M2 Macrophage CD163, VSIG4 and MS4A4A, HLA-DPB1, HLA-DRA, HLA-DPA1, BDCA-1, BDCA-4 and Dendritic cell CD11c, FOXP3, CCR8, STAT5B and TGFβ of Treg, T cell exhaustion TIM-3. Upregulated and ectopic tumor FOXP3, partly through the TGF-βpathway, can promote gastric cancer proliferation, migration, and invasion.28 The expression of TIM3 has been observed in dysfunctional CTLs in both solid tumors and hematological cancers.29,30 In conclusion, TEM7 plays a vital role in modulating tumor immunity in gastric cancer.
According to the relevant literature we reviewed, there are few articles about TEM7 and immunity, so the mechanism between TEM7 and immune infiltration is not clear. We used GSEA to identify signaling pathways involved in TEM7 expression. The possible key pathways related to immunity in gastric cancer that is regulated by TEM7 include cell adhesion molecules (CAMs), MAPK signaling pathway, ECM receptor interaction, leukocyte transendothelial migration, cytokine receptor interaction, and toll like receptor signaling pathway. Syndecan-1, E-cadherin and integrin β3 together make cell adhesion molecules (CAMs) and also takes part in the bond between cell and extracellular matrix.31 The development of syndecan-1 has been up-regulated in stomach and other tissues during epithelial regeneration and rearrangement.32,33 E-cadherin is presented in normal cells and highly related to annexation of cancer cells.32 Survival, movement, proliferation and inflammatory reaction of cells are regulated by Integrin.34 By controlling the interaction of cytokines, cytokine-cytokine receptor interactions can control the occurrence and progression of cancer.35 The malignant behavior and development of cancer can be affected by cell–cell interactions, soluble factor signaling and the highly complex nature of ECM.36–39 An integral part of the inflammatory response is recruitment of leukocytes from circulation to the sites of inflammation, and transendothelial migration of leukocytes is a crucial step in such recruitment.36–39 We hypothesized that TEM7 may affect immune infiltration in gastric cancer through these pathways.
There are multiple drawbacks to our analysis. Firstly, we used several databases to investigate the function of TEM7; the results may be distorted by various methods in data collection and analysis. Secondly, in the database, the sample size of individual tumors is small; the findings may not be representative. Thirdly, to validate our conclusion, we did not perform in vitro or animal studies.
In conclusion, our findings indicate that TEM7 is a possible biomarker indicator of gastric cancer and can be used to determine immune cell infiltration levels in tumor tissues.
Conclusion
The conclusion of our study is that it is a prognostic biomarker. Determination of the progression of cancer is done through TEM7 and it is correlated with the Tumor Immune Infiltrates in gastric cancer as well.
Highlight
- Advanced patients with GC can be guided through the expression level of TEM7.
- TEM7 is an independent risk factor for predicting the prognosis of patients with GC.
- Tumor-infiltrating cells have been positively linked with TEM7 expression level in GC.
Data Sharing Statement
Data was analyzed from TCGA (https://portal.gdc.cancer.gov), Oncomine database (https://www.oncomine.org/resource/login.html), GEPIA (http://gepia.cancer-pku.cn/), TIMER web server (https://cistrome.shinyapps.io/timer/) and Kaplan-Meier plots (https://kmplot.com/analysis/). We declare that the data and materials in this study will be provided free of charge to scientists for noncommercial purposes.
Ethics Approval and Consent to Participate
Human gastric cancer tissues and adjacent non-tumor tissues were collected from 30 patients admitted to the Gastrointestinal Surgery Department of the Second People’s Hospital of Changzhou, Nanjing Medical University, China, from 2018 to 2019. The project was approved by the hospital ethics committee with the written informed consent of each patient included in the study. Clinical samples were conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province, China (BK20181155); the Young Scientists Foundation of Changzhou No.2 People’s Hospital (2018K010); Changzhou High-Level Medical Talents Training Project (RC201602).
Disclosure
All authors declare that there is no conflict of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977–1010. doi:10.1016/S0140-6736(14)62038-9
3. Wu W, Zhen T, Yu J, et al. Circular RNAs as new regulators in gastric cancer: diagnosis and cancer therapy. Front Oncol. 2020;10:1526. doi:10.3389/fonc.2020.01526
4. Procaccio L, Schirripa M, Fassan M, et al. Immunotherapy in gastrointestinal cancers. Biomed Res Int. 2017;2017:1–17. doi:10.1155/2017/4346576
5. Barbee MS, Ogunniyi A, Horvat TZ, et al. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 2015;49(8):907–937. doi:10.1177/1060028015586218
6. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi:10.1056/NEJMoa1501824
7. Song Z, Wu Y, Yang J, et al. Progress in the treatment of advanced gastric cancer. Tumor Biol. 2017;39(7):101042831771462. doi:10.1177/1010428317714626
8. Lu J, Xu Y, Wu Y, et al. Tumor-infiltrating CD8+ T cells combined with tumor-associated CD68+ macrophages predict postoperative prognosis and adjuvant chemotherapy benefit in resected gastric cancer. BMC Cancer. 2019;19(1):920. doi:10.1186/s12885-019-6089-z
9. Zhang H, Liu H, Shen Z, et al. Tumor-infiltrating neutrophils is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer. Ann Surg. 2018;267(2):311–318. doi:10.1097/SLA.0000000000002058
10. St CB, Rago C, Velculescu V, et al. Genes expressed in human tumor endothelium. Science. 2000;289(5482):1197–1202. doi:10.1126/science.289.5482.1197
11. Pietrzyk L. Biomarkers discovery for colorectal cancer: a review on tumor endothelial markers as perspective candidates. Dis Markers. 2016;2016:4912405. doi:10.1155/2016/4912405
12. Wang XQ, Sheibani N, Watson JC. Modulation of tumor endothelial cell marker 7 expression during endothelial cell capillary morphogenesis. Microvasc Res. 2005;70(3):189–197.
13. Nanda A, Buckhaults P, Seaman S, et al. Identification of a binding partner for the endothelial cell surface proteins TEM7 and TEM7R. Cancer Res. 2004;64(23):8507–8511. doi:10.1158/0008-5472.CAN-04-2716
14. Carpenter RL, Paw I, Zhu H, et al. The gain-of-function GLI1 transcription factor TGLI1 enhances expression of VEGF-C and TEM7 to promote glioblastoma angiogenesis. Oncotarget. 2015;6(26):22653–22665. doi:10.18632/oncotarget.4248
15. Fuchs B, Mahlum E, Halder C, et al. High expression of tumor endothelial marker 7 is associated with metastasis and poor survival of patients with osteogenic sarcoma. Gene. 2007;399(2):137–143. doi:10.1016/j.gene.2007.05.003
16. Rhodes DR, Kalyana-Sundaram S, Mahavisno V, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9(2):166–180. doi:10.1593/neo.07112
17. Li T, Fan J, Wang B, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi:10.1158/0008-5472.CAN-17-0307
18. Danaher P, Warren S, Dennis L, et al. Gene expression markers of tumor infiltrating leukocytes. J Immunother Cancer. 2017;5(1):18. doi:10.1186/s40425-017-0215-8
19. Sousa S, Maatta J. The role of tumour-associated macrophages in bone metastasis. J Bone Oncol. 2016;5(3):135–138. doi:10.1016/j.jbo.2016.03.004
20. Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi:10.1093/nar/gkx247
21. Subramanian A, Kuehn H, Gould J, et al. GSEA-P: a desktop application for gene set enrichment analysis. Bioinformatics. 2007;23(23):3251–3253.
22. Tu Z, Xiong J, Xiao R, et al. Loss of miR-146b-5p promotes T cell acute lymphoblastic leukemia migration and invasion via the IL-17A pathway. J Cell Biochem. 2019;120(4):5936–5948.
23. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi:10.1073/pnas.0506580102
24. Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4.
25. Carson-Walter EB, Watkins DN, Nanda A, et al. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61(18):6649–6655.
26. Zhang ZZ, Hua R, Zhang J-F, et al. TEM7 (PLXDC1), a key prognostic predictor for resectable gastric cancer, promotes cancer cell migration and invasion. Am J Cancer Res. 2015;5(2):772–781.
27. Yamaji Y, Yoshida S, Ishikawa K, et al. TEM7 (PLXDC1) in neovascular endothelial cells of fibrovascular membranes from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49(7):3151. doi:10.1167/iovs.07-1249
28. Zhang L, Xu J, Zhang X, et al. The role of tumoral FOXP3 on cell proliferation, migration, and invasion in gastric cancer. Cell Physiol Biochem. 2017;42(5):1739–1754. doi:10.1159/000479442
29. Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi:10.1084/jem.20100643
30. Zhou Q, Munger ME, Veenstra RG, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117(17):4501–4510. doi:10.1182/blood-2010-10-310425
31. Day RM, Hao X, Ilyas M, et al. Changes in the expression of syndecan-1 in the colorectal adenoma-carcinoma sequence. Virchows Arch. 1999;434(2):121–125. doi:10.1007/s004280050315
32. Siitonen SM, Kononen JT, Helin HJ, et al. Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. Am J Clin Pathol. 1996;105(4):394–402. doi:10.1093/ajcp/105.4.394
33. Tanabe H, Yokota K, Kohgo Y. Localization of syndecan-1 in human gastric mucosa associated with ulceration. J Pathol. 1999;187(3):338–344.
34. Yao M, Zhou X-D, Zha X-L, et al. Expression of the integrin alpha5 subunit and its mediated cell adhesion in hepatocellular carcinoma. J Cancer Res Clin Oncol. 1997;123(8):435–440. doi:10.1007/BF01372547
35. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572. doi:10.1038/nri.2017.49
36. Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1(1):46–54. doi:10.1038/35094059
37. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–5912. doi:10.1038/onc.2008.271
38. Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–1219. doi:10.1126/science.1176009
39. Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196(4):395–406. doi:10.1083/jcb.201102147
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.