Back to Journals » Infection and Drug Resistance » Volume 11
Trough concentration of itraconazole and its relationship with efficacy and safety: a systematic review and meta-analysis
Authors Zhang J, Liu Y, Nie X, Yu Y, Gu J , Zhao L
Received 10 April 2018
Accepted for publication 2 June 2018
Published 22 August 2018 Volume 2018:11 Pages 1283—1297
DOI https://doi.org/10.2147/IDR.S170706
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Jingru Zhang,1,2 Yiwei Liu,1 Xiaolu Nie,1 Yuncui Yu,1 Jian Gu,3 Libo Zhao1
1Clinical Research Center, Beijing Children’s Hospital, Capital Medical University, Beijing, China; 2Department of Pharmacy Administration and Clinical Pharmacy, Peking University School of Pharmaceutical Sciences, Beijing, China; 3Department of Pharmacy, Peking University People’s Hospital, Beijing, China
Objectives: The optimum trough concentration of itraconazole for clinical response and safty is controversial. The objective of this systematic review and meta-analysis was to determine the optimum trough concentration of itraconazole and evaluate its relationship with efficacy and safety.
Methods: We searched PubMed, EMBASE, Web of Science, the Cochrane Library, ClinicalTrials.gov, and three Chinese literature databases (CNKI, WanFang, and CBM). We included observational studies that compared clinical outcomes below or above the trough concentration cut-off value which we set as 0.25, 0.5, and 1.0 mg/L. The efficacy outcomes were rate of successful treatment, rate of prophylaxis failure and invasive fungal infection (IFI)-related mortality. The safety outcomes included incidents of hepatotoxicity and other adverse events.
Results: The study included a total of 29 studies involving 2,346 patients. Our meta-analysis showed that compared with itraconazole trough concentrations (Ctrough) of ≥0.25 mg/L, levels of <0.25 mg/L significantly increased the incidence of IFI for prophylaxis (RR =3.279, 95% confidence interval [CI] 1.73–6.206). Moreover, the success rate of treatment decreased significantly at a cut-off level of 0.5 mg/L (RR =0.396, 95% CI 0.176–0.889). An itraconazole trough level of 1.0 mg/L was associated with hepatotoxicity and other adverse events in a review of many studies.
Conclusion: An itraconazole trough concentration of 0.25 mg/L should be considered as the lower threshold for prophylaxis, and a target concentration of 0.5 mg/L should be the lower limit for effective treatment. A trough level of 1.0 mg/L is associated with increased hepatotoxicity and other adverse events (using High Performance Liquid Chromatography [HPLC]).
Keywords: itraconazole, trough concentration, efficacy, safety, meta-analysis
Introduction
Fungal infections exact a significant toll on human health and often compromise the clinical outcomes of patients. Prevalently, invasive fungal infection (IFI) is a leading cause of morbidity and mortality among neutropenic patients after intensive chemotherapy or hematopoietic stem cell transplantation, as well as in other immunocompromised populations.1–3 Itraconazole is a first-generation triazole antifungal agent with broad-spectrum antifungal activity. In clinical practice, it is commonly used for fungal pathogen infections, such as Candida spp., Cryptococcus neoformans, and Aspergillus spp.4 Itraconazole is often recommended as primary therapy for IFI5–9 and as antifungal prophylaxis in immunocompromised patients.10–12 Due to variable and unpredictable oral bioavailability and drug–drug interaction, a satisfactory pharmacokinetics profile cannot be developed with itraconazole in some conditions, making it difficult to determine the optimal dosing regimen.13 Hence, therapeutic drug monitoring (TDM), a technique that timely and appropriately guides drug dosage modifications, is suggested to optimize the treatment.14 Itraconazole trough concentration (Ctrough) is a strong biomarker for drug exposure,15 but most guidelines do not explicitly recommend an optimum trough concentration.
To our knowledge, there have been no randomized controlled trials on the target trough level, so there is no conclusive evidence on the relationship between the optimum trough concentration of itraconazole and its efficacy or safety. However, numerous observational studies have concluded minimum itraconazole level cut-off values, including 0.25,16,17 0.5,18,19 and 1.0 mg/L.20A 2014 guideline from the British society for medical mycology recommended an itraconazole trough concentration of 0.5–1.0 mg/L to prevent and treat IFI,21 which was based on some observational studies22–30 and a previous meta-analysis in 2003.31 This guideline also proposed an increased incidence of toxicity at higher itraconazole concentrations citing two studies which quantified itraconazole concentrations.32,33
Evidence for itraconazole target and critical trough concentrations described in these studies however, remains controversial and has significant limitations. For example, most of the observational studies which contributed to the aforementioned guideline were published more than 20 years ago. These studies often had no clear inclusion/exclusion criteria. Furthermore, the meta-analysis in the guideline31 has drawbacks such as lack of standardized outcome definitions among included studies, no detailed itraconazole concentration data, no well-established methodology to perform quality assessment and subgroup analysis to explore the heterogeneity. More importantly, the previous and other meta-analyses34,35 were all focused on the evidence of anti-mycoses efficacy or prophylaxis IFIs, rather than on the relationship between itraconazole concentration and efficacy or safety. Therefore, further evaluation of available literature is indicated to provide consistent recommendations for optimizing trough concentration. The objective of this systematic review and meta-analysis was to evaluate the relationship between the reported itraconazole trough concentration and the efficacy/safety of itraconazole.
Methods
Data sources
We performed this meta-analysis according to the Cochrane Handbook for Systematic Reviews and the Meta-analysis of Observational Studies in Epidemiology guidelines.36 Two reviewers independently searched PubMed, EMBASE, Web of Science, the Cochrane Library, ClinicalTrials.gov, and three Chinese literature databases (CNKI, WanFang, and CBM) from inception until October 2017. We also examined reference lists of retrieved articles and related reviews. We used the search terms “itraconazole” and “concentration”. We set no restrictions on language or study design.
Study selection
Two reviewers (JZ and YL) independently conducted initial screening and assessed titles, abstracts, and citations in greater detail. We included studies if: i) it was an observational study; ii) itraconazole was used for treatment or prophylaxis; iii) TDM was performed; iv) trough concentrations at steady state were reported for included patients; v) sufficient data about rate of treatment success, rate of prophylaxis failure, mortality or incidence of itraconazole-related adverse events (eg, hepatotoxicity) were reported; vi) sample size was ≥10 patients; and (vii) full text of the publication was available. The same reviewers retrieved and assessed the full text of potentially relevant articles using the same criteria. Disagreements were resolved through discussion.
Our exclusion criteria included: i) data came from simulated patients or pharmacokinetic models rather than from real patients; ii) concentrations were not troughs; iii) concentrations were not measured at steady state; iv) concentrations were measured by bioassay.
Cut-off value establishment
Previous studies37–39 showed the MIC90 (MIC at which 90% of isolates were inhibited) of itraconazole for most yeasts and molds was between 0.25 and 1.0 mg/L. Some studies had shown a target itraconazole trough concentration of 0.25 mg/L.16,17 A guideline21 and other observational studies18,19 suggested 0.5–1.0 mg/L as itraconazole trough concentration. Patients with Ctrough of 1.0 mg/L were associated with a high level of clinical response according to Kim et al’s 2014 study20 and others.40 Therefore, we established the stepwise cut-off values for itraconazole efficacy and safety as 0.25, 0.5, and 1.0 mg/L.
Data extraction and outcomes
The efficacy outcomes included were: IFI-related mortality, treatment success, and prophylaxis failure. Prophylaxis failure was evaluated by the incidence of IFIs, wherein, a high-risk ratio (RR) meant a high treatment success rate or prophylaxis failure rate. The major safety outcomes were hepatotoxicity and occurrence of adverse events. The pooled analysis for treatment success included only treatment studies, while the analysis for prophylaxis failure included only prophylaxis studies, and the analysis of side effects included all studies.
Two authors extracted data independently (JZ and YL) and resolved any disagreements by discussion or by a third investigator (XN). We extracted study characteristics, participants’ baseline characteristics, methods for measuring itraconazole concentration, type of trough concentration (initial, mean or maximum), cut-off value of itraconazole trough concentration, and pre-specified study outcomes of efficacy and safety from each study under review. If the study already contained a cut-off value, we considered patient groups below the pre-defined cut-off value as the intervention group, and those above the pre-defined cut-off value as the control. If studies contained no control group, we collected them into a single arm comparison. When individual patient data were available, we used all our pre-defined cut-off values to divide patients into two groups in the same way and extracted the number of events. When the trough concentration was measured multiple times for each patient, we used the mean value of multiple measurements, and we only used the median value when the mean was not available. If neither mean nor median was available, we used the reported trough concentration for that patient in the article. If there were multiple data for the same outcome in an article, we chose the outcome data measured at Day 14 considering itraconazole accumulates slowly and generally reaches concentrations steadily after 7–15 days of dosing.41,42
Quality assessment
Two independent reviewers (JZ and YL) completed the assessment. We applied the Newcastle–Ottawa Quality Assessment Scale to assess the quality of the included studies with control,43 and used a star system (maximum of nine stars) to evaluate the methodological quality of each study. For observational studies without control, we applied a modified version of the Scale that does not evaluate the comparability part, and possible scores ranged from 0 to 6.44 Higher scores indicated better quality. We resolved any disagreements between the reviewers through discussion. A third reviewer (XN) was available to settle any disputes.
Data analysis
We performed all analyses using the Open Meta-Analyst software (Tufts Medical Center, Boston, MA, USA). The I2 statistic was used to assess heterogeneity among studies. I2 values over 25%, 50%, and 75% represented low, moderate, and considerable heterogeneity, respectively.45 To assess variations between studies in addition to sampling error within studies, we selected the fixed-effects model if I2<50%, and the random-effects model when I2≥50%. The DerSimonian–Laird or the Mantel–Haenszel method was used to calculate the PR or RR and 95% confidence interval (CI) for each study. The 95% CI of outcome among distinct groups did not overlap, showing that outcomes were statistically significant. P<0.05 was considered statistically significant.
Subgroup analyses
To explore the heterogeneity among different studies, we performed a subgroup analysis when more than two studies were included in the analysis of each cut-off level. For the treatment outcome, studies were stratified by: i) studies reporting single drug therapy compared with studies including patients on combination therapy (at least some patients on combination therapy); ii) studies located in Asian countries compared with in non-Asian countries. For the prophylaxis outcome, studies were stratified by location in Asian countries or European countries, or in America and Australia.
Sensitivity analysis
We performed sensitivity analysis to examine whether a single study substantially influenced the core results. We excluded each study and evaluated its effect on the summary estimates and heterogeneity of the main analysis, then reported the results for sensitivity analysis if the conclusions differed.
Results
Literature searches and study inclusion
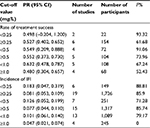
The literature selection process is summarized in Figure 1. A total of 7,007 articles were initially identified. After initial screening, 68 full-text, potentially relevant articles were selected, 39 studies were excluded owing to inadequate clinical outcomes data, concentration was not a trough or at steady-state, or itraconazole alone, or measured by bioassay, among other reasons. Ultimately, 29 articles involving 2,346 patients were included for meta-analysis.16–19,22–24,30,46–65
  | Figure 1 Flow chart of study selection. Abbreviations: TDM, therapeutic drug monitoring; PK, pharmacokinetic. |
Study characteristics
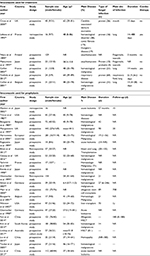
A summary of descriptions of included studies is reported in Table 1. Of these 29 studies, seven studies used itraconazole for treatment30,46–51 and 22 studies used itraconazole for prophylaxis.16–19,22–24,52–65 Fourteen were conducted in European countries, six were in Japan,16,48,50,53,55,63 three were in China,61,64,65 three were in America,23,57,59 two in Australia,18,62 and one in South Korea.20 Among these, two studies were conducted in children who used itraconazole for prophylaxis.54,56 Ten studies used serum samples,16,20,24,30,52,57,60–62,64 while the other 15 used plasma samples.17,18,20,22,23,46–49,51,55,56,58,59,63 The remainder did not report whether serum or plasma sample was used. All the included studies measured itraconazole concentrations by HPLC.
Evaluation of efficacy
Table 2 displays a summary of outcomes for each study, and Tables 3–7 exhibit summaries of meta-analysis and subgroup analysis and sensitivity analysis for efficacy. Figures 2 and 3 and Figures S1–S10 show forest plots.
  | Table 3 Summary of meta-analysis for efficacy (without control arm) Abbreviations: CI, confidence interval; IFI, invasive fungal infection |
  | Table 5 Summary of subgroup analysis for treatment success (without control arm) Abbreviations: CI, confidence interval; NA, . |
  | Table 7 Summary of subgroup analysis for prophylaxis failure (with control arm) Abbreviations: CI, confidence interval; NA, not applicable. |
Our meta-analysis demonstrated there were no significant differences at all cut-off values for efficacy (without control arm) (Table 3). In comparing efficacy, including studies with control arm, we found a significant difference at the cut-off level of 0.25 mg/L for incidence of IFI (RR =3.279, 95% CI 1.73–6.206) (Table 4 and Figure 2). For treatment success, our meta-analysis based on two studies, showed that the success rate decreased at a cut-off level of 0.5 mg/L (RR =0.396, 95% CI 0.176–0.889) (Table 4, Figure 3). Only one study contributed data for IFI-related mortality so we were unable to pool the data.60
Subgroup analysis showed that the rate of prophylaxis failure significantly increased at a cut-off level of <0.25 mg/L in USA + Australia subgroup patients (PR =0.524, 95% CI 0.310–0.737) compared with other concentration regimens. There were no significant differences at other cut-off levels (Tables 5–7).
Sensitivity analysis
We identified moderate to considerable inter-study heterogeneity during some of the meta-analyses, so we used a random-effects model when I2≥50% and the source of heterogeneity assessed in subgroup analysis according to combination therapy and study location (Tables 5–7). The heterogeneity we observed may also be due to diseases, itraconazole dose, sample size, age, and/or the criteria used for assessment – these factors led us to also conduct sensitivity analysis. The trial by Caillot et al in 2001 with relatively small sample size or including complete and partial responders for outcome was omitted and analyzed again to test the stability of the final pooled results.51 The results showed that, in ≥0.25 mg/L and ≥1.0 mg/L, I2 was significantly decreased to less than 50%. When we removed another study with relatively small sample size or proven and possible IFI, there was a decline to less than 50% of I2 in <0.5 mg/L range group.63 Though I2 decreased, the 95% CI did not change significantly, demonstrating that the meta-analysis results were robust. Sensitivity analysis results were summarized in Tables S1 and S2.
Evaluation of safety
Due to different reported safety outcomes and variable definitions of hepatotoxicity, we could not pool the data to perform a meta-analysis. In general, itraconazole was well tolerated in most patients.
Regarding treatment, Miller66 reported that two of 13 patients with itraconazole Ctrough <0.25 mg/L had adverse events grade 3 or 4 related to study treatment. Matsumoto et al48 found patients with Ctrough <0.25 mg/L experienced mild diarrhea (one case), mild drug eruption (one case), and abnormal GOT and GPT (one case). Lebeau et al46 reported that two patients presented with liver function disorders with concentration 0.25–0.5 mg/L and 0.5–1.0 mg/L, respectively. Nine patients (43%) with Ctrough 0.5–1.0 mg/L experienced severe adverse events in Caillot’s study.49 Among these nine cases, there was only one severe reaction definitely related to itraconazole treatment. In a study including a concentration more than 1.0 mg/L, researchers observed adverse events such as liver dysfunction in four patients and heart failure in five of a total of 24 patients in Yoshida et al’s study.50 In another trial,51 only two events were considered definitely related to the treatment: rash in one patient and rigors during drug administration in another. Thirteen patients (42%) experienced adverse events possibly related to treatment. Together, these numbers are substantial in terms of adverse events results.
Regarding prophylaxis, of 97 patients on itraconazole with Ctrough 0.25–0.5 mg/L, three patients had abnormal liver function tests possibly related to itraconazole, but no patients were removed from the study because of concerns about hepatotoxicity from itraconazole according to Winston and Busuttil.59 In addition, we discovered from Toubai et al’s study,63 that there were no other obvious side effects caused by prophylactic itraconazole administration. For Ctrough 0.5–1.0 mg/L, Boogaerts et al58 reported three cases of abnormal rise in AST/ALT levels in 17 patients. Serious adverse events were reported in 26 (9%) patients in Harousseau et al’s study.17 Glasmacher et al,60 in their study, claimed that there were no severe adverse effects (necessitating an interruption of prophylaxis) clearly attributable to itraconazole, especially no severe hepatotoxicity events. Lin et al’s study61 showed that drug-related adverse events occurred in 19 (15.7%) of the patients involved, including 15 with gastrointestinal disorders (12.4%), two with abnormal liver function (1.65%), one with hypokalemia (1/121), and one with hydrothorax (0.8%). In Liu’s study there were 16 patients who withdrew from the study due to adverse reactions, including three cases of elevated aminotransferase.65 Adverse events were observed in as many as 42 patients (14.1%) in Liu et al’s study (transaminase elevation in two patients, drug withdrawal in 16, heart dysfunction in one).64 For Ctrough ≥1.0 mg/L, in Lindsay et al’s research, there were eleven mild derangements of liver function tests and two moderate raised bilirubin in 57 patients.62 Meanwhile, Kim et al’s study20 showed adverse events in 67 patients (32.8 %); specifically, hepatotoxicity (n=39, 19.1 %) and nephrotoxicity (n=8, 3.9 %) were common in this study and seven patients discontinued itraconazole therapy due to toxicity.
Considering the myriad and prevalence of adverse effects in all these studies, an itraconazole trough level of 1.0 mg/L is associated with increased hepatotoxicity and other adverse events.
Evaluation when using the bioassay method
Due to the variation in measurements of blood itraconazole using bioassay (measures both itraconazole and hydroxy-itraconazole) vs HPLC (which measures itraconazole separately), it seems that concentrations measured by bioassay are 5-fold higher compared with HPLC/mass spectrometry.67 Few studies have incorporated bioassay, so we excluded these studies from our meta-analysis. Accordingly, we have not identified any recommendations on itraconazole trough level measured by bioassay.
For treatment, Tucker et al68 reported a trough concentration of 6.5±4.2 mg/L in 28 responders and 4.0±3.2 mg/L in eleven non-responders. Another, Sharkey et al’s study, noted no elevations in hepatic enzyme values compared to baseline values in eight patients where two were <2 mg/L and the other six were >5 mg/L.27 Galgiani et al69 found 61 responded to itraconazole treatment in 97 patients with 6–8 mg/L and two adverse events could have been caused by itraconazole including one case of elevated liver enzyme levels and one hypokalemia cased. Denning et al’s study25determined that trough levels <2.5 mg/L in three responders and one non-responder, 2.5–5 mg/L in two responders and one non-responder, 5–10 mg/L in two responders; and finally >10 mg/L in three responders.
For prophylaxis, according to Lestner et al’s study,32 at Ctrough <17.1 mg/L, 47 (31%) patients developed itraconazole-related toxicity; at Ctrough ≥17.1 mg/L, 55 (86%) patients developed toxicity. Wheat et al70 reported that, in their study, 39 of 42 patients with 6.8 mg/L achieved successful suppression, but 37 patients (88%) experienced grade 3 or 4 (severe or life-threatening) adverse events. One patient was removed from the trial entirely because of itraconazole. In addition, no patient receiving prophylaxis developed invasive aspergillosis and withdrew from the trial due to side effects, nor were toxicities attributed to rising cyclosporine levels associated with itraconazole (Patterson et al: 0.5 mg/L, six patients; 3.5 mg/L, six patients).71 Denning’s study also exhibited that patients with serum concentrations of 8 mg/L tended to have better clinical outcomes when using itraconazole for primary treatment of invasive aspergillosis.72
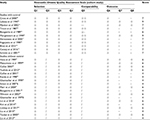
Quality assessment
Using a 9-point scoring system, most studies we analyzed scored between 7 and 9. In a 6-point scoring system, most studies scored between 4 and 6. The result showed that most studies did well in sample selection and comparability but failed in outcome due to short or inadequate follow-ups. Assessment of study-specific quality scores from the Newcastle–Ottawa Scale system is summarized in Table 8.
Discussion
As an antifungal agent, itraconazole has been widely used for the treatment of deep mycoses and prophylaxis of IFIs in patients with profound and prolonged neutropenia, bone marrow transplant recipients, solid organ transplant recipients, and other immunocompromised populations. Although many studies have analyzed itraconazole’s efficacy compared with other antifungal drugs, researchers, so far, have drawn no definitive conclusions on the optimum trough concentration for treatment. Itraconazole exhibits high inter- and intra-patient variability in the pharmacokinetic profile following oral and intravenous doses, so TDM is suggested to optimize the efficacy and avoid toxicity.21 Considering the lower power of individual observational studies and clinical needs, we conducted this systematic review and meta-analysis to provide a more reliable and explicit recommendation on the optimum trough concentration of itraconazole, with less random errors and more precise estimates.
To our knowledge, this is the first meta-analysis focusing on the relationship between itraconazole trough level and the efficacy/safety. After pooling available data from 29 included articles, the meta-analysis revealed that an itraconazole trough concentration of 0.25 mg/L is associated with successful prophylaxis of IFI. This conclusion differs from the 0.5–1.0 mg/L threshold that some publications have suggested, likely due to their limited study subjects.73,74 We conducted a subgroup analysis by study location, thereby demonstrating that the rate of prophylaxis failure significantly increased at a cut-off level of <0.25 mg/L in USA + Australia subgroup patients compared with other concentration regimens. However, subgroup analysis results of the Asia and Europe subgroup were different and insignificant, suggesting a possibility that the concentration–efficacy relationship follows a different profile among different ethnicities. We recognize that the study number was limited and sample size was small in our meta-analysis, therefore further studies are needed to verify the differences in different ethnicities.
Our meta-analysis also demonstrated that a target value of 0.5 mg/L increased treatment success. It is similar to the guideline which recommends itraconazole trough concentration of 0.5–1.0 mg/L as it pertains to treatment of IFIs.21 We divided our subgroups into combination therapy and study location. First, itraconazole inhibits CYP3A4, which leads to a number of clinically relevant drug–drug interactions. Combination therapy with amphotericin B or other drugs may change the profile of drug exposure,14,75 which might explain why the combination therapy subgroup did not show significance at the 0.5 mg/L cut-off level. Second, each cut-off value of Asian location subgroup differed significantly. As there is only one study in every concentration group, we regard these subgroup analysis results as likely to be unreliable. Notably, the small number and size of included studies for treatment success limits the utility of this metric.30,46
During our meta-analysis, we observed heterogeneity in the results, some of which persisted even in subgroup analysis by combination therapy and study location. Nevertheless, sensitivity analysis resulted in a dramatic decrease in I2 through removing method (Table S1 and S2). These results revealed that sample size and the criteria used for assessment could be the sources of heterogeneity. It is also possible that differences in disease, itraconazole dose, age and/or duration of disease or follow-up may be responsible for the heterogeneity we observed. Hence, future studies are needed to explore further causalities. However, though I2 decreased, the 95% CI of each cut-off value only changed insignificantly, indicating the robustness.
There are too little study-contributed data for IFI-related mortality for us to have effectively pooled data in our review.60 The clinical outcomes and definitions of safety in studies were various, wherein some studies reported adverse events grade 3 or 4,66 some reported liver function disorders with different criteria,46,50 some reported adverse reactions related to itraconazole treatment,51,60 and various trials reported the adverse events and abnormal laboratory examination values without consistent standards.17,48,58 Thus we could not combine results to forge a comprehensive meta-analysis. Most patients treated with itraconazole are already immunocompromised and undergoing chemotherapy, which each involves conditions with many adverse effects. The relationship between treatment and adverse events remains unclear, and most guidelines indicate an increased incidence of toxicity at higher itraconazole concentrations without explicitly recommending an optimum trough concentration for safety.21,73 After reviewing many studies, we offer 1.0 mg/L as the cut-off value that is associated with increased hepatotoxicity and other adverse events. To fully elucidate this issue, further studies are needed.
Our meta-analysis and review has the following strengths. First, it is the first to focus on the relationship of itraconazole trough concentration with efficacy and safety, providing certain reference significance to clinical practice. Second, our meta-analysis compared commonly used cut-off levels in a single analysis for individual cut-off levels. Finally, we included Japanese articles in this meta-analysis to maximize the reliability considering the prevalence of the research in Japan, while most English language reviews have not done this until now.
We acknowledge the following limitations to our work. First, the number of studies and sample sizes were relatively small, leading to potentially insufficient power to detect mild differences. Second, we were unable to perform subgroup analysis for the pediatric population because we only identified two studies designed for children. Besides, we could not analyze the influences of different pathogen and infection locations on the results. Third, the use of observational studies in a meta-analysis is prone to biases and confounding factors inherent in the original studies. Finally, although our subgroup analysis and sensitivity analyses explained some heterogeneity in the results, there is a clear need for further study.
Conclusion
In conclusion, our meta-analysis of published studies demonstrates that 0.25 mg/L is the lower threshold of the target itraconazole trough concentration during prophylaxis of fungal infections. Additionally, the target 0.5 mg/L is the lower limit for successful treatment. We have deduced that a trough level of 1.0 mg/L is associated with substantially increased hepatotoxicity and other adverse events (using HPLC).
Acknowledgment
This work was supported by the National Science and Technology Major Project “Creation of major new drugs” (number 2017ZX09304029).
Disclosure
The authors report no conflicts of interest in this work.
References
Chamilos G, Luna M, Lewis RE, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989-2003). Haematologica. 2006;91(7):986–989. | ||
Slobbe L, Polinder S, Doorduijn JK, et al. Outcome and medical costs of patients with invasive aspergillosis and acute myelogenous leukemia-myelodysplastic syndrome treated with intensive chemotherapy: an observational study. Clin Infect Dis. 2008;47(12):1507–1512. | ||
Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48(3):265–273. | ||
Slain D, Rogers PD, Cleary JD, Chapman SW. Intravenous itraconazole. Ann Pharmacother. 2001;35(6):720–729. | ||
Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. 2012;18 Suppl 7:53–67. | ||
Lortholary O, Petrikkos G, Akova M, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: patients with HIV infection or AIDS. Clin Microbiol Infect. 2012;18 Suppl 7:68–77. | ||
Hope WW, Castagnola E, Groll AH, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18 Suppl 7:38–52. | ||
Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–360. | ||
Limper AH, Knox KS, Sarosi GA, et al. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183(1):96–128. | ||
Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):409–417. | ||
Patterson TF, Thompson GR, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–e60. | ||
Maertens J, Marchetti O, Herbrecht R, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3--2009 update. Bone Marrow Transplant. 2011;46(5):709–718. | ||
Allegra S, Fatiguso G, de Francia S, et al. Pharmacokinetic evaluation of oral itraconazole for antifungal prophylaxis in children. Clin Exp Pharmacol Physiol. 2017;44(11):1083–1088. | ||
Morgan J, Wannemuehler KA, Marr KA, et al. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol. 2005;43 Suppl 1:49–58. | ||
Yamagishi Y, Hamada Y, Hagihara M, Mikamo H. Population pharmacokinetics of itraconazole in Japanese patients with invasive fungal peritonitis. Jpn J Antibiot. 2013;66(3):159–168. | ||
Myoken Y, Sugata T, Kyo T, Fujihara M, Mikami Y. Itraconazole prophylaxis for invasive gingival aspergillosis in neutropenic patients with acute leukemia. J Periodontol. 2002;73(1):33–38. | ||
Harousseau JL, Dekker AW, Stamatoullas-Bastard A, et al. Itraconazole oral solution for primary prophylaxis of fungal infections in patients with hematological malignancy and profound neutropenia: a randomized, double-blind, double-placebo, multicenter trial comparing itraconazole and amphotericin B. Antimicrob Agents Chemother. 2000;44(7):1887–1893. | ||
Brett J, Chong O, Graham GG, et al. Antifungal use and therapeutic monitoring of plasma concentrations of itraconazole in heart and lung transplantation patients. Ther Drug Monit. 2013;35(1):133–136. | ||
Ceesay MM, Couchman L, Smith M, Wade J, Flanagan RJ, Pagliuca A. Triazole antifungals used for prophylaxis and treatment of invasive fungal disease in adult haematology patients: Trough serum concentrations in relation to outcome. Med Mycol. 2016;54(7):691–698. | ||
Kim JS, Cheong JW, Kim YK, et al. The relationship between the success rate of empirical antifungal therapy with intravenous itraconazole and clinical parameters, including plasma levels of itraconazole, in immunocompromised patients receiving itraconazole oral solution as prophylaxis: a multicenter, prospective, open-label, observational study in Korea. Ann Hematol. 2014;93(1):33–42. | ||
Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother. 2014;69(5):1162–1176. | ||
Boogaerts MA, Verhoef GE, Zachee P, Demuynck H, Verbist L, de Beule K. Antifungal prophylaxis with itraconazole in prolonged neutropenia: correlation with plasma levels. Mycoses. 1989;32 Suppl 1:103–108. | ||
Tricot G, Joosten E, Boogaerts MA, Vande Pitte J, Cauwenbergh G. Ketoconazole vs. itraconazole for antifungal prophylaxis in patients with severe granulocytopenia: preliminary results of two nonrandomized studies. Rev Infect Dis. 1987;9 Suppl 1:S94–99. | ||
Glasmacher A, Hahn C, Leutner C, et al. Breakthrough invasive fungal infections in neutropenic patients after prophylaxis with itraconazole. Mycoses. 1999;42(7-8):443–451. | ||
Denning DW, Tucker RM, Hanson LH, Stevens DA. Treatment of invasive aspergillosis with itraconazole. Am J Med. 1989;86(6 Pt 2):791–800. | ||
Denning DW, Tucker RM, Hanson LH, Hamilton JR, Stevens DA. Itraconazole therapy for cryptococcal meningitis and cryptococcosis. Arch Intern Med. 1989;149(10):2301–2308. | ||
Sharkey PK, Rinaldi MG, Dunn JF, Hardin TC, Fetchick RJ, Graybill JR. High-dose itraconazole in the treatment of severe mycoses. Antimicrob Agents Chemother. 1991;35(4):707–713. | ||
Wheat J, Hafner R, Korzun AH, et al. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. AIDS Clinical Trial Group. Am J Med. 1995;98(4):336–342. | ||
Cartledge JD, Midgely J, Gazzard BG. Itraconazole solution: higher serum drug concentrations and better clinical response rates than the capsule formulation in acquired immunodeficiency syndrome patients with candidosis. J Clin Pathol. 1997;50(6):477–480. | ||
Cross LJ, Bagg J, Oliver D, Warnock D. Serum itraconazole concentrations and clinical responses in Candida-associated denture stomatitis patients treated with itraconazole solution and itraconazole capsules. J Antimicrob Chemother. 2000;45(1):95–99. | ||
Glasmacher A, Prentice A, Gorschlüter M, et al. Itraconazole prevents invasive fungal infections in neutropenic patients treated for hematologic malignancies: evidence from a meta-analysis of 3,597 patients. J Clin Oncol. 2003;21(24):4615–4626. | ||
Lestner JM, Roberts SA, Moore CB, Howard SJ, Denning DW, Hope WW. Toxicodynamics of itraconazole: implications for therapeutic drug monitoring. Clin Infect Dis. 2009;49(6):928–930. | ||
Lestner JM, Denning DW. Tremor: a newly described adverse event with long-term itraconazole therapy. J Neurol Neurosurg Psychiatry. 2010;81(3):327–329. | ||
Vardakas KZ, Michalopoulos A, Falagas ME. Fluconazole versus itraconazole for antifungal prophylaxis in neutropenic patients with haematological malignancies: a meta-analysis of randomised-controlled trials. Br J Haematol. 2005;131(1):22–28. | ||
Wang J, Zhan P, Zhou R, et al. Prophylaxis with itraconazole is more effective than prophylaxis with fluconazole in neutropenic patients with hematological malignancies: a meta-analysis of randomized-controlled trials. Med Oncol. 2010;27(4):1082–1088. | ||
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. | ||
Shokohi T, Badali H, Amirrajab N, Ataollahi MR, Kouhpayeh SA, Afsarian MH. In vitro activity of five antifungal agents against Candida albicans isolates, Sari, Iran. Curr Med Mycol. 2016;2(2):34–39. | ||
Pfaller MA, Diekema DJ, Messer SA, Hollis RJ, Jones RN. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolates. Antimicrob Agents Chemother. 2003;47(3):1068–1071. | ||
Sabatelli F, Patel R, Mann PA, et al. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob Agents Chemother. 2006;50(6):2009–2015. | ||
Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807–825. | ||
Hardin TC, Graybill JR, Fetchick R, Woestenborghs R, Rinaldi MG, Kuhn JG. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob Agents Chemother. 1988;32(9):1310–1313. | ||
Smith D, van de Velde V, Woestenborghs R, Gazzard BG. The pharmacokinetics of oral itraconazole in AIDS patients. J Pharm Pharmacol. 1992;44(7):618–619. | ||
Wells GA, Shea BJ, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Appl Eng Agric. 2014;18:727–734. | ||
Lopez-Olivo MA, Pratt G, Palla SL, Salahudeen A. Rasburicase in tumor lysis syndrome of the adult: a systematic review and meta-analysis. Am J Kidney Dis. 2013;62(3):481–492. | ||
Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. | ||
Lebeau B, Pelloux H, Pinel C, et al. Itraconazole in the treatment of aspergillosis: a study of 16 cases. Mycoses. 1994;37(5-6):171–179. | ||
Havu V, Brandt H, Heikkilä H, et al. Continuous and intermittent itraconazole dosing schedules for the treatment of onychomycosis: a pharmacokinetic comparison. Br J Dermatol. 1999;140(1):96–101. | ||
Matsumoto T, Tanuma H, Nishiyama S. Clinical and pharmacokinetic investigations of oral intraconazole in the treatment of onychomycosis. Mycoses. 1999;42(1-2):79–91. | ||
Caillot D. Intravenous itraconazole followed by oral itraconazole for the treatment of amphotericin-B-refractory invasive pulmonary aspergillosis. Acta Haematol. 2003;109(3):111–118. | ||
Yoshida K, Kurashima A, Kamei K, et al. Efficacy and safety of short- and long-term treatment of itraconazole on chronic necrotizing pulmonary aspergillosis in multicenter study. J Infect Chemother. 2012;18(3):378–385. | ||
Caillot D, Bassaris H, Mcgeer A, et al. Intravenous itraconazole followed by oral itraconazole in the treatment of invasive pulmonary aspergillosis in patients with hematologic malignancies, chronic granulomatous disease, or AIDS. Clin Infect Dis. 2001;33(8):e83–e90. | ||
Morgenstern GR, Prentice AG, Prentice HG, Ropner JE, Schey SA, Warnock DW. A randomized controlled trial of itraconazole versus fluconazole for the prevention of fungal infections in patients with haematological malignancies. U.K. Multicentre Antifungal Prophylaxis Study Group. Br J Haematol. 1999;105(4):901–911. | ||
Kageyama S, Masuya M, Tanaka I, et al. Plasma concentration of itraconazole and its antifungal prophylactic efficacy in patients with neutropenia after chemotherapy for acute leukemia. J Infect Chemother. 1999;5(4):213–216. | ||
Schmitt C, Perel Y, Harousseau JL, et al. Pharmacokinetics of itraconazole oral solution in neutropenic children during long-term prophylaxis. Antimicrob Agents Chemother. 2001;45(5):1561–1564. | ||
Kanda Y, Kami M, Matsuyama T, et al. Plasma concentration of itraconazole in patients receiving chemotherapy for hematological malignancies: the effect of famotidine on the absorption of itraconazole. Hematol Oncol. 1998;16(1):33–37. | ||
Simon A, Besuden M, Vezmar S, et al. Itraconazole prophylaxis in pediatric cancer patients receiving conventional chemotherapy or autologous stem cell transplants. Support Care Cancer. 2007;15(2):213–220. | ||
Marr KA, Crippa F, Leisenring W, et al. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood. 2004;103(4):1527–1533. | ||
Boogaerts MA, Maertens J, van der Geest R, et al. Pharmacokinetics and safety of a 7-day administration of intravenous itraconazole followed by a 14-day administration of itraconazole oral solution in patients with hematologic malignancy. Antimicrob Agents Chemother. 2001;45(3):981–985. | ||
Winston DJ, Busuttil RW. Randomized controlled trial of oral itraconazole solution versus intravenous/oral fluconazole for prevention of fungal infections in liver transplant recipients. Transplantation. 2002;74(5):688–695. | ||
Glasmacher A, Molitor E, Hahn C, et al. Antifungal prophylaxis with itraconazole in neutropenic patients with acute leukaemia. Leukemia. 1998;12(9):1338–1343. | ||
Lin R, Xu X, Li Y, et al. Comparison of long-term and short-term administration of itraconazole for primary antifungal prophylaxis in recipients of allogeneic hematopoietic stem cell transplantation: a multicenter, randomized, open-label trial. Transpl Infect Dis. 2014;16(2):286–294. | ||
Lindsay J, Sandaradura I, Wong K, et al. Serum levels, safety and tolerability of new formulation SUBA-itraconazole prophylaxis in patients with haematological malignancy or undergoing allogeneic stem cell transplantation. J Antimicrob Chemother. 2017;72(12):3414-3419. | ||
Toubai T, Tanaka J, Ota S, et al. Itraconazole capsules as antifungal prophylaxis for neutropenic patients with hematological malignancies from a single institution. Jpn J Antibiot. 2005;58(6):507. | ||
Liu X, Huang Y, Yang DL, et al. Prophylaxis of invasive fungal infection with different administration regimens of itraconazole in patients with acute myeloid leukemia: a report from a randomized, controlled trial. Chin J Hematol. 2013;34:502. | ||
Liu WX. Clinical Research on Ltraconazole in Prevention of Pulmonary Invasive Fungal Infection (IFI) in Patients with Hemopathy. Journal of Aerospace Medicine. 2015;26:1318–1320. Available from: http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2015&filename=HKHT201511002&v=MTUyMjJDVVJMS2ZZT2RvRkNqblVyL05MU2JEZXJHNEg5VE5ybzlGWm9SOGVYMUx1eFlTN0RoMVQzcVRyV00xRnI=. | ||
Indiana University. A Pilot Trial of Itraconazole Pharmacokinetics in Patients With Metastatic Breast Cancer. Available from: https://clinicaltrials.gov/ct2/show/results/NCT00798135. NLM identifier: NCT00798135. Accessed June 22, 2018. | ||
Law D, Moore CB, Denning DW. Bioassay for serum itraconazole concentrations using hydroxyitraconazole standards. Antimicrob Agents Chemother. 1994;38(7):1561–1566. | ||
Tucker RM, Denning DW, Arathoon EG, Rinaldi MG, Stevens DA. Itraconazole therapy for nonmeningeal coccidioidomycosis: Clinical and laboratory observations. J Am Acad Dermatol. 1990;23(3):593–601. | ||
Galgiani JN, Catanzaro A, Cloud GA, et al. Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis. A randomized, double-blind trial. Mycoses Study Group. Ann Intern Med. 2000;133:676–686. | ||
Wheat J, Hafner R, Wulfsohn M, et al. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1993;118(8):610–616. | ||
Patterson TF, Peters J, Levine SM, et al. Systemic availability of itraconazole in lung transplantation. Antimicrob Agents Chemother. 1996;40:2217–2220. | ||
Denning DW. Treatment of invasive aspergillosis. J Infect. 1994;28(Suppl 1):25–33. | ||
Goodwin ML, Drew RH. Antifungal serum concentration monitoring: an update. J Antimicrob Chemother. 2008;61(1):17–25. | ||
Andes D, Pascual A, Marchetti O. Antifungal Therapeutic Drug Monitoring: Established and Emerging Indications. Antimicrob Agents Chemother. 2009;53(1):24–34. | ||
Isoherranen N, Kunze KL, Allen KE, Nelson WL, Thummel KE. Role of itraconazole metabolites in CYP3A4 inhibition. Drug Metab Dispos. 2004;32(10):1121–1131. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.