Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
TRONARTO: A Randomized, Placebo-Controlled Study of Tiotropium/Olodaterol Delivered via Soft Mist Inhaler in COPD Patients Stratified by Peak Inspiratory Flow
Authors Mahler DA , Ludwig-Sengpiel A, Ferguson GT , de la Hoz A, Ritz J, Shaikh A , Watz H
Received 15 June 2021
Accepted for publication 12 August 2021
Published 28 August 2021 Volume 2021:16 Pages 2455—2465
DOI https://doi.org/10.2147/COPD.S324467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Min Zhang
Donald A Mahler,1,2 Andrea Ludwig-Sengpiel,3 Gary T Ferguson,4 Alberto de la Hoz,5 John Ritz,6 Asif Shaikh,7 Henrik Watz8
1Geisel School of Medicine at Dartmouth, Hanover, NH, USA; 2Section of Pulmonary Medicine, Valley Regional Hospital, Claremont, NH, USA; 3KLB Gesundheitsforschung Lübeck GmbH, Lübeck, Germany; 4Department of Medicine, Pulmonary Research Institute of Southeast Michigan, Farmington Hills, MI, USA; 5Cardio-Metabolism and Respiratory, Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany; 6Biostatistics, Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA; 7Clinical Development & Medical Affairs, Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA; 8Pulmonary Research Institute at LungenClinic Grosshansdorf, Airway Research Center North (ARCN), German Center for Lung Research (DZL), Grosshansdorf, Germany
Correspondence: Donald A Mahler
Medicine, Geisel School of Medicine at Dartmouth, Hanover, NH, USA
Tel +1 603 542-6777
Fax +1 603 543-5613
Email [email protected]
Background: Inhaled bronchodilator therapy is currently the mainstay of treatment for patients with chronic obstructive pulmonary disease (COPD). Some inhalers require patients to achieve certain inhalation efforts either to activate the device or to deliver medication to the site of action. For dry powder inhalers, low peak inspiratory flow (PIF) can result in poor medication delivery but the clinical significance of this is not well understood.
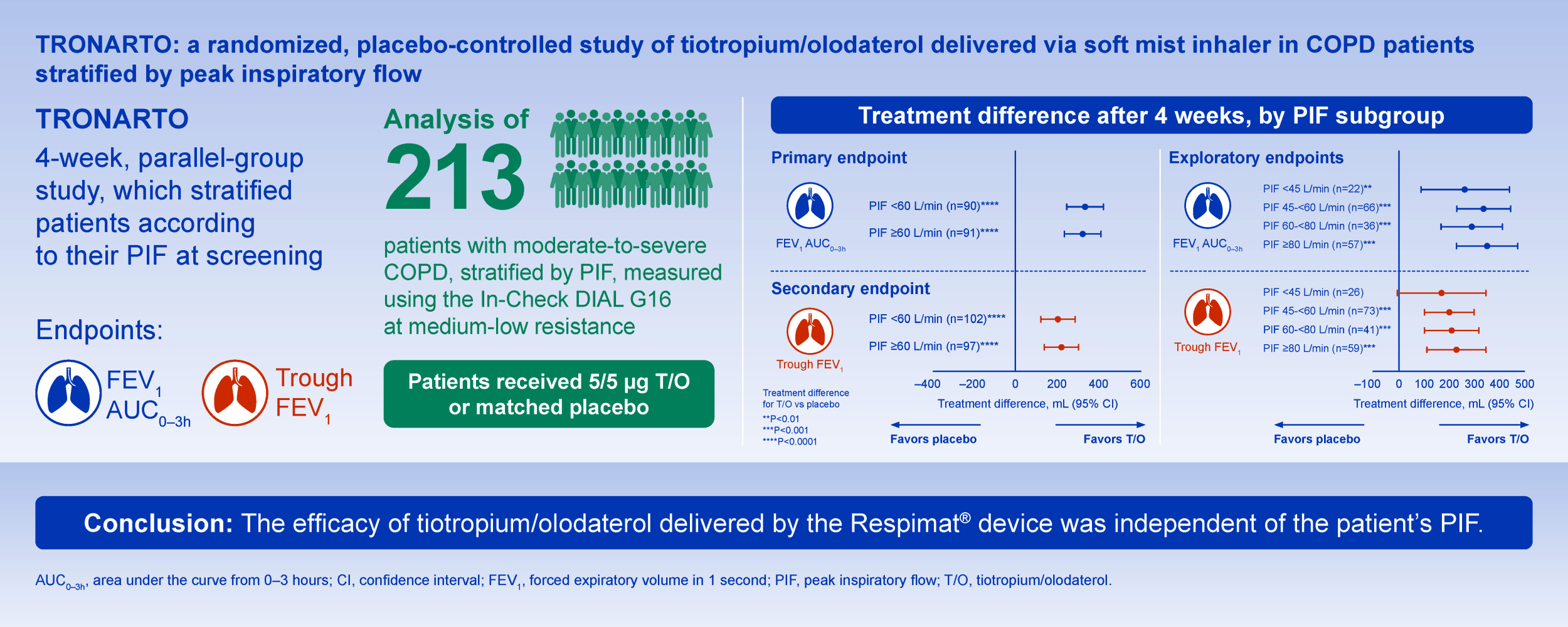
Methods: TRONARTO was a 4-week, randomized, double-blind, placebo-controlled, multicenter, parallel-group study which stratified patients with moderate-to-severe COPD according to their PIF against medium-low resistance at screening. Patients were randomized to receive tiotropium/olodaterol (5 μg/5 μg) or matched placebo delivered via the Respimat® Soft Mist™ inhaler (SMI). After 4 weeks of treatment, we assessed change from baseline in forced expiratory volume in 1 second (FEV1) area under the curve 0– 3 hours (FEV1 AUC0– 3h) and trough FEV1.
Results: Overall, 213 patients were randomized, of whom 106 received tiotropium/olodaterol (PIF < 60 L/min, 55; PIF ≥ 60 L/min, 51) and 107 received placebo (PIF < 60 L/min, 55; PIF ≥ 60 L/min, 52). For FEV1 AUC0– 3h, the adjusted mean change from baseline versus placebo was 336 mL (95% confidence interval [CI] 246– 425 mL; P< 0.0001) in the PIF < 60 L/min group and 321 mL (95% CI 233– 409 mL; P< 0.0001) in the PIF ≥ 60 L/min group. For trough FEV1, the adjusted mean change from baseline versus placebo was 201 mL (95% CI 117– 286 mL; P< 0.0001) in the PIF < 60 L/min group and 217 mL (95% CI 135– 299 mL; P< 0.0001) in the PIF ≥ 60 L/min group.
Conclusion: In the TRONARTO study, which included patients with moderate-to-severe COPD and varying inspiratory flow abilities, treatment with tiotropium/olodaterol resulted in significant lung function improvements versus placebo. This SMI can be used irrespective of the PIF that a patient can generate.
Keywords: inhaler, tiotropium/olodaterol, peak inspiratory flow, SMI, lung function
Graphical Abstract:
Plain Language Summary
People with chronic obstructive pulmonary disease (COPD) have difficulty breathing during activities of daily living. They sometimes experience worsening of their symptoms, known as a flare-up.
Inhalers are used to relieve symptoms and reduce the risk of a flare-up in people with COPD. To use a dry powder inhaler, you need to be able to breathe in “hard and fast” to break up the powder within the device. However, not all people with COPD can do this. With the Respimat® Soft Mist™ inhaler (SMI), the person should take a slow, deep breath, and the mechanical energy released by pressing the dose-release button will help release the medication (called tiotropium/olodaterol) as a soft mist.
The TRONARTO study evaluated whether tiotropium/olodaterol SMI is suitable for all patients regardless of their ability to breathe in from an inhaler device. Subjects were given tiotropium/olodaterol or placebo using the SMI for 4 weeks. Changes in lung function were assessed after 4 weeks of treatment.
The results showed that regardless of people’s ability to breathe in strongly, tiotropium/olodaterol treatment delivered using the SMI improved lung function compared with placebo.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive disease that requires maintenance treatment for symptom relief and exacerbation risk.1,2 Inhaled bronchodilator therapy with long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs), alone or in combination, is currently a mainstay of COPD treatment.2–4 Correct use of inhalers and patient adherence to prescribed therapy are critical in order to achieve better clinical control and improved quality of life.5
There are many different inhalers available for the treatment of COPD, and delivery systems vary. The three handheld inhalation devices used in the treatment of COPD are dry powder inhalers (DPIs), pressurized metered-dose inhalers (pMDIs) and soft mist inhalers (SMIs).5,6 The delivery and deposition of medication in the lungs by these devices is affected by both inhaler characteristics and patient-related factors, such as peak inspiratory flow (PIF).6,7 DPIs, for example, require a PIF of >60 L/minute (low to medium-high resistance devices)6,8–10 to overcome the inhaler’s internal resistance and separate the medicine from its carrier particles.11–14 Duarte et al reported that as many as one in five ambulatory patients with COPD have suboptimal PIF.15 pMDIs operate independently of PIF but require the patient to coordinate inhaler activation with intake of breath.5 Furthermore, they can be associated with high oropharyngeal deposition of larger particles.5 SMIs use mechanical energy to generate a slow-moving mist of drug and require slow, coordinated inhalation.5,16,17 Patient and modeled lung deposition profiles have shown that the SMI is associated with lower throat deposition and higher and more uniform deposition in the whole lung compared with DPIs and pMDIs.18–20
Tiotropium, a once-daily LAMA, and olodaterol, a once-daily LABA, are available as a fixed-dose combination delivered via the SMI.21–24 This combination has been shown to reduce the risk of exacerbations and provide long-term improvements in lung function, dyspnea, exercise capacity and quality of life.21,23 Tiotropium/olodaterol has been assessed in patients with different disease severities, demonstrating improvements in lung function, symptoms and quality of life across a broad population of patients with COPD.21,25–27 In vitro/in silico data suggest that the SMI delivers high lung deposition even at low modeled flow rates and across moderate-to-severe COPD inhalation profiles.28 However, there are no data on the efficacy of tiotropium/olodaterol SMI in patients with COPD of different inhalation abilities. We anticipate no difference in outcomes according to PIF status.
The TRONARTO study stratified patients according to their PIF at screening. The aim of the TRONARTO study was to investigate the efficacy of inhaled tiotropium/olodaterol 5 μg/5 μg delivered via SMI on lung function in patients with moderate-to-severe COPD and different inhalation abilities (PIF ≥60 L/min or PIF <60 L/min against a medium-low resistance). Additional post hoc analyses were conducted on PIF subgroups.
Methods
Study Design
The TRONARTO study (NCT04223843) was a Phase IV, 4-week, randomized, double-blind, placebo-controlled, multicenter, parallel-group study of patients receiving tiotropium/olodaterol (5 μg/5 μg) via the SMI.
At screening, patients were stratified by their PIF (PIF <60 L/min or PIF ≥60 L/min) using the In-Check DIAL G16 set at medium-low resistance. Following the screening visit, patients continued to receive their prescribed COPD medication; a 72-hour washout period (during which patients could use salbutamol rescue medication) was then implemented prior to randomization. Patients were randomized (1:1) to tiotropium/olodaterol 5 μg/5 μg or matching placebo using a validated system of pseudo-random number generation (approximately 50 patients per randomization block). Patients attended a clinic visit at Weeks 2 and 4, and a follow-up telephone call was conducted at Week 7.
The study protocol was reviewed and approved by the respective independent review boards and ethics committees of the participating sites: 26 in Germany and the United States of America beginning January 8, 2020 and ending September 29, 2020. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent.
Patients
Patients were included if they were aged 40 years or older with a diagnosis of moderate-to-severe COPD and were current or ex-smokers with a smoking history of >10 pack-years. Patients had a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity of <70% and a post-bronchodilator FEV1 of ≥30–<80% of predicted normal at screening.
Patients were excluded if they had a significant disease other than COPD, defined by the investigator as any disease that could put the patient at risk, influence the results of the trial or raise concerns regarding the patient’s ability to participate in the trial. Patients who had a COPD exacerbation that required treatment with antibiotics, systemic steroids or hospitalization in the 6 weeks prior to Visit 1 or during the screening period were excluded, as were patients who experienced ≥2 moderate exacerbations that required treatment with antibiotics or systemic steroids or ≥1 exacerbation leading to hospitalization within the year prior to Visit 1. Those with a history of asthma or receiving inhaled corticosteroids in the 6 months prior to Visit 1 were also excluded.
Study Outcomes and Assessments
The primary endpoint was the change from baseline in FEV1 area under the curve 0–3 hours (AUC0–3h) at Week 4 for tiotropium/olodaterol vs placebo in each PIF stratum (PIF <60 L/min and PIF ≥60 L/min). The secondary endpoint was the change from baseline in trough FEV1 at Week 4 for tiotropium/olodaterol vs placebo. PIF was measured three times at each clinic visit at both medium-low and high resistance and the highest PIF was used. In addition, patients also measured their PIF against medium-low resistance at home daily.
Post Hoc Analyses
Post hoc analyses were conducted to investigate which baseline patient characteristics showed an association with PIF when conducting a test for difference in patients with PIF <60 L/min and PIF ≥60 L/min.
In additional exploratory subgroup analyses, patients were sub-divided into PIF subgroups of PIF <45, PIF ≥45–<60, PIF ≥60–<80 and PIF ≥80 L/min. Analyses of percentage change from baseline for FEV1 AUC0–3h and trough FEV1 were conducted in PIF subgroups PIF <60 L/min and PIF ≥60 L/min and in the PIF subgroups of PIF <45, PIF 45–<60, PIF 60–<80 and PIF ≥80 L/min.
Safety
For this analysis, safety and tolerability were assessed in a descriptive way based on adverse events (AEs), serious AEs and physical examination. All AEs, whether serious or non-serious, that occurred during the course of the clinical trial were documented and reported by the investigators.
Randomization and Blinding
Patients, investigators, and everyone involved in trial conduct or analysis, or with any other interest in this study, were blinded regarding the randomized treatment assignments until after database lock.
Statistical Methods
For the primary endpoint, the adjusted means were calculated using an analysis of covariance model including the fixed categorical effects of treatment and the fixed continuous effect (FEV1) of baseline.
The secondary endpoint was analyzed using the restricted maximum likelihood-based approach using a mixed model with repeated measures. The analysis of the secondary endpoint included the fixed, categorical effect of treatment at each visit and the fixed continuous effect (FEV1) of baseline at each visit.
The study was designed to meet significance for primary and key secondary endpoints if significance was established for each stratum. A formal comparison on the magnitude of response between strata was planned.
The full analysis set (FAS) comprised patients who were randomized, received any dose of trial medication and who had both baseline and any evaluable post-baseline measurement for at least one of the efficacy endpoints, including FEV1 AUC0–3h and trough FEV1. The FAS was used for analysis of both the primary and secondary endpoints within the PIF <60 L/min and PIF ≥60 L/min groups. The TRONARTO study was not designed to detect differences in the primary or secondary endpoints between PIF subgroups. Because the primary endpoint only used baseline and Week 4 data, whereas secondary endpoints used baseline, Week 3 and Week 4, the number of patients in the FAS for the primary and secondary endpoints was different.
A sample size of 200 patients with a 1:1 randomization ratio was considered appropriate to provide adequate power to detect a treatment difference of 260 mL for FEV1 AUC0–3h and to detect a treatment difference of 140 mL for trough FEV1, with a standard deviation of 210 mL. Additional post hoc efficacy sensitivity analyses were conducted to adjust for age, gender and disease severity.
COVID
For patients who were unable to attend follow-up visits due to the COVID-19 pandemic and were thus not included in the efficacy analysis, missing data analysis using multiple imputation was conducted as an additional sensitivity analysis.
Results
Patient Disposition
In total, 213 patients were randomized (106 to tiotropium/olodaterol [PIF <60 L/min, 55; PIF ≥60 L/min, 51] and 107 to placebo [PIF <60 L/min, 55; PIF ≥60 L/min, 52]). At the end of the study period, 203 patients (95.3%) had received the full course of medication; 10 patients prematurely discontinued trial medication.
Of the 10 patients who did not receive the full course of trial medication, two patients withdrew due to an AE, two patients were lost to follow-up, two patients withdrew consent and four patients withdrew for “other” reasons (Figure 1).
Baseline Characteristics
Patient characteristics (by PIF stratum and by treatment) are shown in Table 1. In total, 110 patients were included in the PIF <60 L/min group (51.6% [tiotropium/olodaterol, 55; placebo, 55]) and 103 patients in the PIF ≥60 L/min group (48.4% [tiotropium/olodaterol, 51; placebo, 52]). Some differences in baseline characteristics were noted between PIF strata (Table 1).
 |
Table 1 Patient Characteristics by Treatment and by PIF |
Of the baseline characteristics shown in Table 1, there were differences in disease severity (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage), height, post-bronchodilator and percent predicted FEV1 (all P<0.05) between the PIF <60 L/min and PIF ≥60 L/min groups. Some differences were also apparent for gender and age; we noted more females with PIF <60 L/min and the average age was higher in this group (both not significant).
Primary Endpoint: FEV1 AUC0–3h
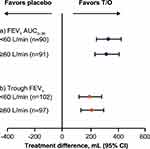
For FEV1 AUC0–3h, 181 patients were included in the FAS. After 4 weeks of treatment with tiotropium/olodaterol, an improvement in adjusted mean FEV1 AUC0–3h was observed in both the PIF <60 L/min (250 ± 33 mL, percentage improvement from baseline 20.3 ± 2.9) and PIF ≥60 L/min (333 ± 32 mL, percentage improvement from baseline 27.2 ± 2.4) PIF groups.
The treatment difference between tiotropium/olodaterol and matched placebo for FEV1 AUC0–3h was 336 mL (95% confidence interval [CI] 246–425 mL; percentage improvement from baseline 24.1 ± 3.9) in the PIF <60 L/min group and 321 mL (95% CI 233–409 mL; percentage improvement from baseline 24.4 ± 3.4) in the PIF ≥60 L/min group (both analyses P<0.0001) (Figure 2A).
Secondary Endpoint: Trough FEV1
For trough FEV1, 199 patients were included in the FAS. After 4 weeks of treatment with tiotropium/olodaterol, an improvement in adjusted mean trough FEV1 was observed in patients in both the PIF <60 L/min (95 ± 31 mL, percentage improvement from baseline 8.1 ± 2.7) and PIF ≥60 L/min (177 ± 30 mL, percentage improvement from baseline 15.2 ± 2.1) groups.
The treatment difference between tiotropium/olodaterol and matched placebo was 201 mL (95% CI 117–286 mL; percentage improvement from baseline 13.4 ± 3.8) for the PIF <60 L/min group and 217 mL (95% CI 135–299 mL; percentage improvement from baseline 16.0 ± 2.9) for the PIF ≥60 L/min group (both analyses P<0.0001) (Figure 2B).
Post Hoc Analyses
Baseline Characteristics
A post hoc sensitivity analysis was performed to adjust for age, gender and disease severity as some differences were seen within strata for these variables. The results for FEV1 AUC0–3h and trough FEV1 were consistent with the original efficacy results (Supplementary Table 1).
Exploratory Subgroup Analysis
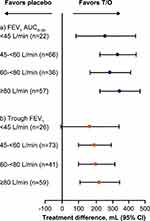
Consistent with findings for PIF subgroups of PIF <60 and PIF ≥60 L/min, we noted an improvement in FEV1 in patients receiving tiotropium/olodaterol compared with placebo when patients were stratified into PIF groups of <45, 45–<60, 60–<80 and ≥80 L/min. In these subgroup analyses for FEV1 AUC0–3h, all PIF subgroups reached P<0.01 (Figure 3A). For trough FEV1, all PIF subgroups reached P<0.001 apart from PIF <45 L/min, which was the smallest subgroup in this analysis (Figure 3B). Further information on percentage change can be found in Supplementary Table 2.
Missing Post-Baseline Measurements
Of the patients who were excluded due to lack of post-baseline efficacy measurements, results from the missing data analysis showed that the results were similar when accounting for the missing data (Supplementary Table 3).
Safety
In total, 30 patients experienced an AE. Four patients experienced investigator-defined drug-related AEs, including dry mouth, dry tongue, cough, rhinitis and COPD, and two patients experienced AEs leading to discontinuation of the trial drug. The most common AEs were grouped under “respiratory, thoracic and mediastinal disorders” (tiotropium/olodaterol, n=5; placebo, n=7). These included COPD, allergic rhinitis, bronchiectasis, cough, dyspnea and epistaxis. AE profiles were similar between the treatment arms. Serious AEs resulting in hospitalization occurred in two patients treated with tiotropium/olodaterol (endometrial cancer and gastroenteritis [1.9%]) and in one patient receiving placebo (necrotizing fasciitis [0.9%]).
Discussion
The TRONARTO study, which included patients with moderate and severe COPD (GOLD 2 and 3), demonstrated that treatment with tiotropium/olodaterol for 4 weeks delivered via SMI resulted in a clinically significant improvement in lung function, irrespective of the PIF that the patient could generate.
In clinical practice, PIF is not routinely measured. The results from the TRONARTO study suggest that, when prescribing SMIs, measurement or consideration of PIF is not necessary. The SMI is an active device that does not rely on patient inhalation effort for activation or release of the drug from the device;29 it also has a very low internal resistance, and in vitro studies have demonstrated optimal lung deposition with the SMI at inspiratory flow rates of 15–30 L/min.6 In this study, clinically significant lung function improvement was seen in all subgroups, from <45 L/min to ≥80 L/min.
Several studies of various inhaler types have extrapolated in vivo and in vitro modeling data to assume improvements in lung function at different inspiratory flow rates,10,28,30,31 but clinical data to support these assumptions are limited. The efficacy of single bronchodilator therapy delivered via a handheld device in patients with different inhalation abilities has previously been reported,32 but to our knowledge, this is the first study to investigate the relationship between PIF and efficacy in the context of dual bronchodilator therapy delivered via a handheld device.
In the current study, all patient subgroups showed clinically important improvements in lung function when treated with tiotropium/olodaterol delivered via Respimat SMI compared with those treated with placebo, which was also delivered via Respimat SMI. Patients with very low PIF may have benefitted from the SMI as this operates independently of PIF, delivering treatment over a longer time period. This supports in vitro data from Ciciliani et al, which found high lung deposition in patients using the Respimat SMI, regardless of PIF.28
Low PIF is a patient-related factor associated with suboptimal use of DPIs,6,8,30,33 but there is limited evidence regarding its effect on lung function in patients with COPD. According to the GOLD 2021 strategy report, regular inhaler assessment is recommended and healthcare professionals should select the inhaler device that matches the individual patient characteristics and ensure that patients continue to use their device correctly.2
In the TRONARTO study, there were numerically more female patients in the PIF <60 L/min cohort than in the PIF ≥60 L/min cohort, and the mean age was slightly higher in the PIF <60 L/min group (neither significant). This supports previous studies which have shown that female patients and older patients tend to have lower PIF.7–9 Additionally, we noted a higher proportion of tall participants (>180 cm) in the PIF ≥60 L/min cohort than the PIF <60 L/min cohort, in line with previous studies that suggest an association between height and PIF.8,34 Of note, there were more patients with severe COPD, according to GOLD classification, or a lower percent predicted FEV1 in the PIF <60 L/min cohort.
The TRONARTO study has several strengths. This multicenter study included a large patient population, across a range of disease severities. The study was randomized, double-blind, placebo-controlled and included a parallel-group design. PIF was measured against a simulated resistance and not modeled or extrapolated from spirometry measurements. At the visits, patients were not informed of their PIF status, reducing potential performance bias. Furthermore, patients were trained in correct inhaler technique at two separate clinic visits, thereby reducing bias according to the patient’s ability to use the SMI.
This study has some limitations. For example, symptom burden was not assessed, so it is unclear to what extent symptoms of COPD were associated with PIF status and the improvements in lung function. Placebo was used as the comparator for this study, which limited inclusion of very severe COPD patients (GOLD 4); additionally, patients with recent exacerbations and those taking inhaled corticosteroids were excluded.
Conclusion
In the TRONARTO study, treatment with tiotropium/olodaterol delivered via the SMI device resulted in significant lung function improvements versus placebo, irrespective of the PIF that a patient can generate. This indicates that PIF should not be a factor for healthcare professionals to consider when prescribing a soft mist inhaler.
Abbreviations
AE, adverse event; AUC0–3h, area under the curve 0–3 hours; CI, confidence interval; COPD, chronic obstructive lung disease; DPI, dry powder inhaler; FAS, full analysis set; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2-agonist; PIF, peak inspiratory flow; pMDI, pressurized metered-dose inhaler; SMI, soft mist inhaler.
Data Sharing Statement
The data set used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study protocol was reviewed and approved by the respective independent review boards and ethics committees of the participating sites: 26 in Germany and the United States of America beginning January 8, 2020 and ending September 29, 2020. A full list of participating sites in the study in this analysis is included in the supplementary file (Supplementary Table 4) and can be found at https://www.clinicaltrials.gov/ct2/show/NCT04223843. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent.
Consent for Publication
All authors provide their consent for publication of this manuscript and all related contents. All patients provided their informed consent when entering the TRONARTO study.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance, in the form of the preparation and revision of the manuscript, was supported financially by Boehringer Ingelheim and provided by Paul Todd, PhD, at MediTech Media, under the authors’ conceptual direction and based on feedback from the authors.
Author Contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. They take full responsibility for the scope, direction, content of, and editorial decisions relating to the manuscript, were involved at all stages of development and have approved the submitted manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work. The authors received no compensation related to the development of the manuscript.
Funding
Support for this project was provided by Boehringer Ingelheim International GmbH.
Disclosure
Donald A. Mahler, M.D., FCCP reports fees for advisory boards from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Mylan, Teva, Theravance, and Verona; royalties from Salem Media Group – COPD: Answers to Your Questions (2015); work with pharmaceutical companies on the use of BDI/TDI; and participation in speaker’s bureau for AstraZeneca, Boehringer Ingelheim and Teva; and https://www.donaldmahler.com – an educational website for those with COPD and their families. Andrea Ludwig-Sengpiel reports investigator fees from Boehringer Ingelheim, paid to her institution during the conduct of the study; investigator fees from AstraZeneca, GlaxoSmithKline, Sanofi, Teva, Avillion, IONIS, Gossamer, Chiesi, Verona, Insmed and Novartis paid to her institution. Gary T. Ferguson reports grants, personal fees and non-financial support from Boehringer Ingelheim, during the conduct of the study; grants, personal fees and non-financial support from Teva Pharmaceuticals, Novartis, AstraZeneca, Pearl Therapeutics, Sunovion, Verona, Theravance, Mylan, Verona, and GlaxoSmithKline; grants and personal fees from Sanofi, grants from Altavant, Chiesi, and Knopp; personal fees from DevPro, Galderma, Ionis, and Orpheris, outside the submitted work. Alberto de la Hoz, John Ritz and Asif Shaikh are all employees of Boehringer Ingelheim. Henrik Watz reports grants from Boehringer Ingelheim for conductance of the study paid to his institution, consulting fees, honoraria payments, support for attending meetings and participation on a data safety monitoring or advisory board from AstraZeneca, Bayer, Boehringer Ingelheim, Chiesi, Novartis, Takeda, Verona Pharma, and GlaxoSmithKline. The authors report no other conflicts of interest in this work.
References
1. Lopez-Campos JL, Calero C, Quintana-Gallego E. Symptom variability in COPD: a narrative review. Int J Chron Obstruct Pulmon Dis. 2013;8:231–238. doi:10.2147/COPD.S42866
2. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2021 report); 2020. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
3. Lavorini F, Janson C, Braido F, et al. What to consider before prescribing inhaled medications: a pragmatic approach for evaluating the current inhaler landscape. Ther Adv Respir Dis. 2019;13:1753466619884532. doi:10.1177/1753466619884532
4. Miravitlles M, Soler-Cataluña JJ, Alcázar B, et al. Factors affecting the selection of an inhaler device for COPD and the ideal device for different patient profiles. Results of EPOCA Delphi consensus. Pulm Pharmacol Ther. 2018;48:97–103. doi:10.1016/j.pupt.2017.10.006
5. Usmani OS. Choosing the right inhaler for your asthma or COPD patient. Ther Clin Risk Manag. 2019;15:461–472. doi:10.2147/TCRM.S160365
6. Mahler DA. The role of inspiratory flow in selection and use of inhaled therapy for patients with chronic obstructive pulmonary disease. Respir Med. 2020;161:105857. doi:10.1016/j.rmed.2019.105857
7. Represas-Represas C, Aballe-Santos L, Fernández-García A, et al. Evaluation of suboptimal peak inspiratory flow in patients with stable COPD. J Clin Med. 2020;9:3949. doi:10.3390/jcm9123949
8. Ghosh S, Pleasants RA, Ohar JA, et al. Prevalence and factors associated with suboptimal peak inspiratory flow rates in COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:585–595. doi:10.2147/COPD.S195438
9. Loh CH, Peters SP, Lovings TM, et al. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305–1311. doi:10.1513/AnnalsATS.201611-903OC
10. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30(6):381–387. doi:10.1089/jamp.2017.1416
11. Virchow JC, Crompton GK, Dal Negro R, et al. Importance of inhaler devices in the management of airway disease. Respir Med. 2008;102(1):10–19. doi:10.1016/j.rmed.2007.07.031
12. Capstick TG, Clifton IJ. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Rev Respir Med. 2012;6(1):91–101. doi:10.1586/ers.11.89
13. Newman SP. Inhaler treatment options in COPD. Eur Respir Rev. 2005;14(96):102–108. doi:10.1183/09059180.05.00009605
14. Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14:1103–1107.
15. Duarte AG, Tung L, Zhang W, et al. Spirometry measurement of peak inspiratory flow identifies suboptimal use of dry powder inhalers in ambulatory patients with COPD. Chronic Obstr Pulm Dis. 2019;6:246–255.
16. Dhand R, Eicher J, Hansel M, et al. Improving usability and maintaining performance: human-factor and aerosol-performance studies evaluating the new reusable Respimat inhaler. Int J Chron Obstruct Pulmon Dis. 2019;14:509–523. doi:10.2147/COPD.S190639
17. Hochrainer D, Holz H, Kreher C, et al. Comparison of the aerosol velocity and spray duration of Respimat Soft Mist inhaler and pressurized metered dose inhalers. J Aerosol Med. 2005;18(3):273–282. doi:10.1089/jam.2005.18.273
18. Ciciliani AM, Langguth P, Wachtel H. In vitro dose comparison of Respimat® inhaler with dry powder inhalers for COPD maintenance therapy. Int J Chron Obstruct Pulmon Dis. 2017;12:1565–1577. doi:10.2147/COPD.S115886
19. Pitcairn G, Reader S, Pavia D, et al. Deposition of corticosteroid aerosol in the human lung by Respimat® Soft Mist™ inhaler compared to deposition by metered dose inhaler or by turbuhaler dry powder inhaler. J Aerosol Med. 2005;18(3):264–272. doi:10.1089/jam.2005.18.264
20. Brand P, Hederer B, Austen G, et al. Higher lung deposition with Respimat® Soft Mist™ inhaler than HFA-MDI in COPD patients with poor technique. Int J Chron Obstruct Pulmon Dis. 2008;3:763–770. doi:10.2147/COPD.S3930
21. Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4). Eur Respir J. 2015;45(4):969–979. doi:10.1183/09031936.00136014
22. Boehringer Ingelheim Limited. Spiolto Respimat 2.5 microgram/2.5 microgram, inhalation solution – summary of product characteristics; 2017. Available from: https://www.medicines.org.uk/emc/medicine/30495.
23. Ferguson GT, Karpel J, Bennett N, et al. Effect of tiotropium and olodaterol on symptoms and patient-reported outcomes in patients with COPD: results from four randomised, double-blind studies. NPJ Prim Care Respir Med. 2017;27(1):7. doi:10.1038/s41533-016-0002-x
24. Boehringer Ingelheim. New advance in COPD maintenance treatment, Spiolto® Respimat®, approved in first European countries; 2015. Available from: https://www.boehringer-ingelheim.com/press-release/new-advance-copd-maintenance-treatment-spiolto-respimat-approved-first-european.
25. Buhl R, Singh D, de la Hoz A, et al. Benefits of tiotropium/olodaterol compared with tiotropium in patients with COPD receiving only LAMA at baseline: pooled analysis of the TONADO® and OTEMTO® studies. Adv Ther. 2020;37(8):3485–3499. doi:10.1007/s12325-020-01373-3
26. Buhl R, de la Hoz A, Xue W, et al. Efficacy of tiotropium/olodaterol compared with tiotropium as a first-line maintenance treatment in patients with COPD who are naïve to LAMA, LABA and ICS: pooled analysis of four clinical trials. Adv Ther. 2020;37(10):4175–4189. doi:10.1007/s12325-020-01411-0
27. Wedzicha JA, Buhl R, Singh D, et al. Tiotropium/olodaterol decreases exacerbation rates compared with tiotropium in a range of patients with COPD: pooled analysis of the TONADO®/DYNAGITO® trials. Adv Ther. 2020;37(10):4266–4279. doi:10.1007/s12325-020-01438-3
28. Ciciliani AM, Denny M, Langguth P, et al. Lung deposition using the Respimat® Soft Mist™ inhaler mono and fixed-dose combination therapies: an in vitro/ in silico analysis. COPD. 2021;18(1):91–100. doi:10.1080/15412555.2020.1853091
29. Dalby RN, Eicher J, Zierenberg B. Development of Respimat® Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Devices (Auckl). 2011;4:145–155.
30. Price DB, Yang S, Ming SWY, et al. Physiological predictors of peak inspiRatory flow using observed lung function resultS (POROS): evaluation at discharge among patients hospitalized for a COPD exacerbation. Int J Chron Obstruct Pulmon Dis. 2018;13:3937–3946. doi:10.2147/COPD.S174371
31. Altman P, Wehbe L, Dederichs J, et al. Comparison of peak inspiratory flow rate via the Breezhaler®, Ellipta® and HandiHaler® dry powder inhalers in patients with moderate to very severe COPD: a randomized cross-over trial. BMC Pulm Med. 2018;18(1):100. doi:10.1186/s12890-018-0662-0
32. Mahler DA, Ohar JA, Barnes CN, et al. Nebulized versus dry powder long-acting muscarinic antagonist bronchodilators in patients with COPD and suboptimal peak inspiratory flow rate. Chronic Obstr Pulm Dis. 2019;6:321–331.
33. Sulaiman I, Cushen B, Greene G, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(10):1333–1343. doi:10.1164/rccm.201604-0733OC
34. Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78. doi:10.1183/09031936.00024807
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.